Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-κB translocation
Abstract
Cholera toxin (CT) is a potent adjuvant; however, the mechanism for its ability to enhance mucosal immunity has not been fully elucidated. We report here that CT exerts its adjuvant propertiesby signaling through the GM1 ganglioside receptor. When ganglioside-defective mice were given the antigen (Ag) ovalbumin (OVA) with CT by the oral route, CT failed to support either OVA-specific antibody or CD4+ T cell responses. In vitro treatment of murine bone marrow-derived dendritic cells (DC) with CT induced full maturation as evidenced by up-regulation of the costimulatory molecules, as well as by an enhanced ability to effectively present OVA for Ag-specific T cell responses. On the other hand, ganglioside-defective DC failed to differentiate to full function as Ag-presenting cells in response to CT. Since ganglioside-defective DC showed a mature phenotype after stimulation with lipopolysaccharide (LPS), the effects of CT on DC was independent of signal transduction through adjuvant receptor for LPS, the Toll-like receptor 4. Furthermore, CT also induced nuclear translocation of nuclear factor (NF)-κB in DC in a GM1-dependent fashion. These results highlight gangliosides expressed by DC for recognition of the non-self protein bacterial enterotoxin, which employ a unique signaling pathway to induce both innate and adaptive immunity.
Abbreviations:
-
- CT:
-
Cholera toxin
-
- GM1:
-
Monosialoganglioside
-
- TLR:
-
Toll-like receptor
-
- β1,4-GalNAc-T:
-
β1,4-N-acetylgalactosaminyltransferase
-
- KO:
-
Knockout
-
- WT:
-
Wild type
1 Introduction
GM1 ganglioside [Gal(β1–3)GalNAc(β1–4)(NeuAc(α2–3)) Gal(β1–4)Glc(β1–1)ceramide] is a glycosphingolipid found ubiquitously on mammalian cells. Intoxication of some bacterialtoxins, including cholera toxin (CT) from Vibrio cholerae and the heat-labile enterotoxin from pathogenic strains of Escherichia coli, occurred following their recognition via ganglioside receptors on epithelial cells 1. These toxins are comprised of a single A-subunit with ADP-ribosyltransferase activity and five B subunits responsible for binding to cellsurface gangliosides 2. After B subunit binding to ganglioside receptors, holotoxins are internalized and cause intracellular increases in cyclic AMP (cAMP) which results in the classic watery diarrhea 3, 4.
The CT molecule is also known to have mucosal adjuvanticity for co-administered protein antigens when given by either the oral or nasal routes 5–8. There have been studies of the effects of CT on APC; however, the mechanism underlying the induction of this activity remains to be precisely elucidated. For example, it was shown that CT, but not CT-B, up-regulates the expression of the costimulatory molecule B7–2, but not B7–1, on murine MΦ and B cells 9–12. In humans, CT induced up-regulation of both B7–1 and B7–2 in blood monocyte-derived DC. Moreover, these CT-treated APC were able to prime T cells 13. Although these previous studies have strongly suggested that the enzyme activity of the A subunit is required for B7 expression and functional costimulatory activity of APC, others showed that mutants of CT (mCT) obtained by a single amino acid substitution (S61F and E112K) in the A subunit lacked ADP-ribosyltransferase activity, yet retained adjuvant activity 14, 15. It was also reported that CT-B alone was associated with potent mucosal adjuvant activity 16–18. Thus, it is not yet clear as to which subunit is actually responsible for the adjuvant activity of CT. Furthermore, whether GM1 gangliosides expressed on APC are required for the adjuvant activity of CT has not yet been determined.
Recently, it was reported that Toll-like receptors (TLR) were expressed on specialized APC, such as DC, and play a critical role in the initiation of the adaptive immune response 19. Triggering TLR on these cells transduces the signal to induce the translocation of NF-κB. As a result of this cascade, the expression of costimulatory molecules and the synthesis of cytokines are up-regulated, which are necessary for the activation of T cells 20. These data suggested that TLR play critical roles as adjuvant receptors. In fact, pathogen-associated molecular patterns (PAMP), including the lipopolysaccharide (LPS), unmethylated CpG motifs, and double-stranded RNA are recognized by a set of pattern-recognition receptors (PRR). The best studied PRR are the TLR, which activate signaling pathways that induce not only antimicrobial effector immune responses but also initiate adaptive immune responses 21, 22. However, whether CT transduces signals, which is the same as PAMP, to elicit mucosal adjuvanticity by triggering GM1 gangliosides expressed on APC has not yet been determined.
In the present study, we show that GM1 gangliosides play critical roles as adjuvant receptors for CT by using β1,4-N-acetylgalactosaminyltransferase (β1,4-GalNAc-T)-deficient mice 23, which lack all complex gangliosides, including GM1. In addition, we show that CT induces nuclear translocation of NF-κB and terminal maturation of DC in a GM1-dependent fashion and this may explain the mechanisms underlying the strong adjuvanticity of CT in vivo.
2 Results and discussion
2.1 Lack of CT adjuvanticity in β1,4-GalNAc-T KO mice
To investigate whether GM1 gangliosides function as adjuvant receptors for CT to enhance the immune response in vivo, we initially analyzed the mucosal adjuvanticity of CT for OVA-specific immune responses in β1,4-N-acetylgalactosaminyltransferase knockout (β1,4-GalNAc-T KO) mice, which lack all complex gangliosides including GM1 23. The OVA-specific Ab response was assessed in β1,4-GalNAc-T KO mice. Oral administration of OVA together with CT as mucosal adjuvant resulted in elevated plasma anti-OVA Ab isotypes as well as fecal S-IgA responses in wild type (WT) mice; however, CT failed to support anti-OVA Ab responses in essentially all β1,4-GalNAc-T KO mice (Fig. 1A and B). Response of plasma Ig in β1,4-GalNAc-T KO mice after subcutaneous immunization with OVA along with CFA as adjuvant was comparable to that seen in WT mice (Fig. 1C). These data revealed that systemic immunological responses in β1,4-GalNAc-T KO mice are not defective and, therefore, lack of gangliosides is principally responsible for decreased immune responses that are induced by CT in β1,4-GalNAc-T KO mice. We then compared β1,4-GalNAc-T KO and WT CD4+ T cell proliferative responses stimulated with anti-CD3/CD28 mAb. There was no significant difference in T cell proliferation in the presence of anti-CD3 and CD28 mAb between WT and β1,4-GalNAc-T KO mice (Fig. 1D). These results indicate that there is no significant defect in β1,4-GalNAc-T KO mice in terms of OVA-specific immune responses. We next examined Ag-specific responses of T cells prepared from mice orally immunized with OVA along with CT as mucosal adjuvant. The CD4+ T cells derived from the Peyer's patches of WT or β1,4-GalNAc-T KO mice that had been given OVA plus CT orally were cultured with splenic APC obtained from naive WT mice. Cocultivation of β1,4-GalNAc-T KO CD4+ T cells and feeder cells derived from WT mice resulted in only minimal levels of OVA-specific proliferation (Fig. 2). Taken together, these results strongly suggest that the response of APC, but not T cells, to orally administered CT was impaired in β1,4-GalNAc-T KO mice.
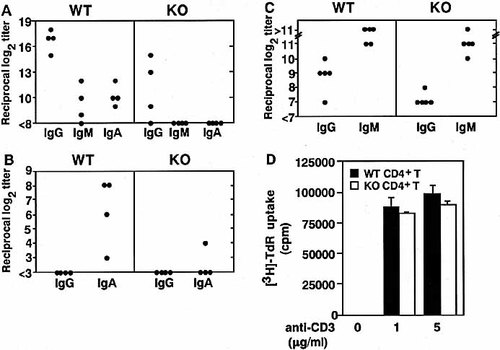
Impaired mucosal adjuvanticity of CT in β1,4-GalNAc-T KO mice. Both WT and β1,4-GalNAc-T KO mice were orally immunized with OVA plus CT as an adjuvant (A and B), or were given OVA in CFA subcutaneously (C). Plasma (A and C) and fecal extracts (B) were collected and examined for OVA-specific Ab responses by ELISA. The results obtained from four mice/group are shown in A and B, and the difference between WT and KO mice in panel A and in panel B for IgA was statistically significant as determined by the Mann-Whitney test (p<0.05). (D) Splenic CD4+ T cells derived from naive WT or β1,4-GalNAc-T KO mice were incubated with anti-CD3 and CD28 mAb. The results are shown as the mean ± one SEM.
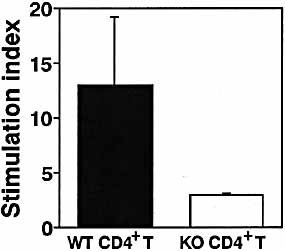
Impaired mucosal OVA-specific T cell responses in β1,4-GalNAc-T KO mice immunized orally with OVA plus CT. Peyer's patch CD4+ T cells derived from mice that had been given OVA plus CT by gavage were incubated with splenic feeder cells generated from naive, WT mice with or without OVA. The results from CD4+ T cells obtained from five mice/group are shown, and the results are representative of two separate experiments. Indicated is the mean ± one SEM.
2.2 CT-induced maturation of DC in a GM1-dependent fashion
We next examined the requirement for GM1 on APC as a major target of CT. It has been reported that DC, which are specialized APC, play a critical role in the initiation of the adaptive immune response. Bone marrow (BM)-derived DC from WT mice clearly showed an ability to bind CT-B. This binding of CT-B was not seen when β1,4-GalNAc-T KO BM-derived DC were incubated with FITC-conjugated CT-B (Fig. 3). We also found that GM1 was the only CT-binding ganglioside in BM-derived DC as determined by a thin-layer chromatography-immunostaining method (data not shown). We prepared recombinant CT, CT-A, and CT-B from Brevibacillus choshinensis to clarify whether CT and its subunits could elicit Ag-specific T cell proliferative responses in BM-derived DC cultures. Because B. choshinensis are Gram-positive bacteria, these recombinant proteins purified from them contain essentially no LPS, which itself has strong costimulatory activity for APC. Cocultivation of CD4+ T cells, taken from OVA-immunized WT mice, with CT-treated WT DC resulted in high OVA-specific T cell proliferative responses, a pattern not seen in cultures of untreated WT DC (Fig. 4). This enhancing effect of CT was dose dependent. Neither CT-A nor CT-B induced OVA-specific T cell responses when tested with WT DC. In addition, treatment of WT DC with a mCT, obtained by a single amino acid substitution (E112K) in the A subunit and which lacked ADP-ribosyltransferase activity, did not elicit Ag-specific T cell responses. The BM-derived DC from β1,4-GalNAc-T mice were capable of differentiating into functional APC when stimulated with LPS (Fig. 4), which indicated that there is no intrinsic defect in KO DC. These data provide in vitro evidence that CT-induced maturation of DC requires interactions with the intact holotoxin through binding to GM1 ganglioside. Thus, in order to activate DC, a specific intracellular transport and delivery of CT-A by CT-B is necessary. Further, CT uses a totally different pathway from LPS for induction of functional differentiation of DC.
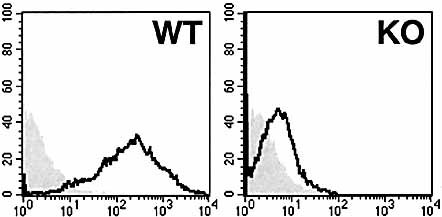
Absence of surface CT-B binding to BM-derived DC taken from β1,4-GalNAc-T KO mice. The BM-derived DC from WT (left panel) and β1,4-GalNAc-T KO (right panel) mice were stained with FITC-conjugated CT-B (solid lines) for 30 min at 4°C. The filled histograms represent untreated samples.
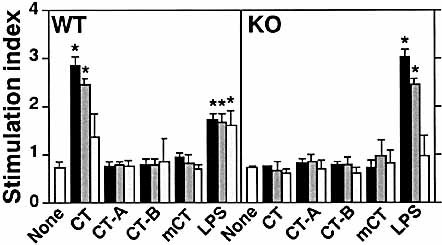
The CT-treated DC from WT but not from β1,4-GalNAc-T KO mice enhanced OVA-specific CD4+ T cell responses. The DC from WT (left panel) and β1,4-GalNAc-T KO (right panel) mice were pretreated with the agents indicated below at a final concentration of 0.1 μg/ml (gray column), 1 μg/ml (dark gray column), or 10 μg/ml (solid column). Pretreated BM-derived DC (1×104 cells) were added to WT CD4+ T cells (1×105 cells / well) with or without OVA. The mean stimulation index ± one SEM from triplicate measurements in one experiment is shown. This experiment is representative of three independent experiments. *;p<0.01, when compared with cultures containing untreated DC.
2.3 The CT-induced expression of DC maturation markers occurs in a GM1-dependent fashion
We next investigated the effects of CT for expression of DC costimulatory molecules. The addition of CT to BM-derived DC resulted in up-regulation of cell surface B7–1, B7–2, and slight up-regulation of MHC class II molecules in a dose-dependent manner. In contrast, BM-derived DC from β1,4-GalNAc-T KO mice failed to up-regulate B7–1, B7–2, and MHC class II expression after stimulation with CT (Fig. 5A and C). Neither CT-A nor CT-B induced changes in the levels of these surface markers on WT or β1,4-GalNAc-T KO DC (data not shown). The addition of LPS to BM-derived DC from β1,4-GalNAc-T KO mice also resulted in up-regulation of cell surface B7–1 and B7–2 molecules, as was seen in WT DC. Since DC from β1,4-GalNAc-T KO mice expressed higher levels of MHC class II molecules when compared with WT DC, the addition of LPS to β1,4-GalNAc-T KO DC resulted in only minimal up-regulation of cell surface MHC class II (Fig. 5B). The basal expression of DC maturation markers was rather high in this experiment. We found this to be a feature of this mouse strain (F1), since less basal expression and remarkable up-regulation of the surface costimulatory molecules was seen in the same experiment using BM-derived DC prepared from BALB/c or from C3H mice (data not shown). Nevertheless, the difference between WT and β1,4-GalNAc-T KO DC was clear, and the changes were indeed dose dependent. Our results indicate that CT-induced up-regulation of B7–1, B7–2, and MHC class II molecules on DC also required interactions with holotoxin binding to GM1 ganglioside.
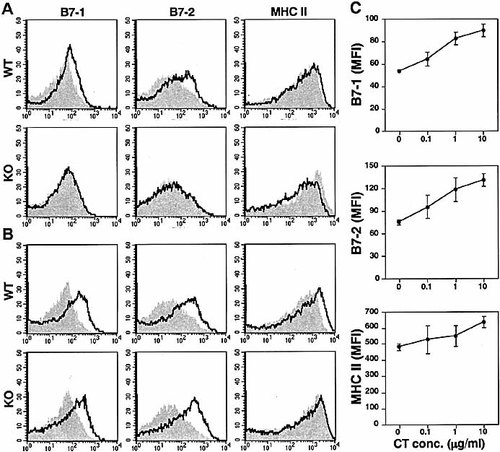
Expression of cell-surface maturation markers by murine BM-derived DC. (A) T and B cell-depleted BM cells were prepared from WT (upper panels) and β1,4-GalNAc-T KO (lower panels) mice and cultured in the presence of mIL-4 and mGM-CSF for 5 days. BM-derived DC were stimulated with 10 μg/ml of CT. After 48 h of incubation, the cells were stained with biotinylated anti-B7–1, anti-B7–2, or anti-MHC class II followed by streptavidin-PE. The filled histograms represent untreated samples. (B) BM-derived DC from WT (upper panels) and β1,4-GalNAc-T KO (lower panels) mice were stimulated with 10 μg/ml of LPS. After 48 h of incubation, the cells were stained with biotinylated anti-B7–1, anti-B7–2, or anti-MHC class II followed by streptavidin-PE. The filled histograms represent untreated samples. (C) Dose dependency of the CT-induced surface marker expression. The BM-derived DC from WT mice were stimulated with 0.1–10 μg/ml of CT and analyzed as in panel A and B. The data are the mean fluorescence intensity of stained cells. The data are representative of 4 separate experiments, which gave essentially identical results.
2.4 CT induces nuclear translocation of NF-κB in a GM1-dependent fashion
It is well known that maturation of cell surface markers on DC and Mϕ activated by LPS are promoted by the activation of NF-κB 19, 24. NF-κB is widely expressed and regulates transcription, particularly of proteins involved in immune and inflammatory responses. To elucidate the signaling requirements, we investigated whether NF-κB was involved in CT-induced DC maturation. Before stimulation, NF-κB was retained in the cytoplasm of both WT and β1,4-GalNAc-T KO DC (data not shown). We found that a 2-h treatment with CT induced massive translocation of the NF-κB to the nuclei, which was not seen in CT-treated β1,4-GalNAc-T KO DC (Fig. 6A and B). In a range of CT-concentrations from 1 μg/ml to 10 μg/ml, clear nuclear translocation of NF-κB was seen. Furthermore, LPS induced NF-κB activation in both WT and KO DC (Fig. 6C and D), indicating no intrinsic defect in the NF-κB transport system in β1,4-GalNAc-T KO DC. Finally, we have reconstituted GM1 on the surface of DC prepared from KO mice. After incubation with GM1, β1,4-GalNAc-T KO DC showed enhanced binding of CT (Fig. 6E). When these GM1-reconstituted DC were stimulated with CT, clear nuclear translocation of NF-κB was seen (Fig. 6F). These results indicated that CT-induced NF-κB translocation is dependent upon interactions with holotoxin binding to GM1 ganglioside.
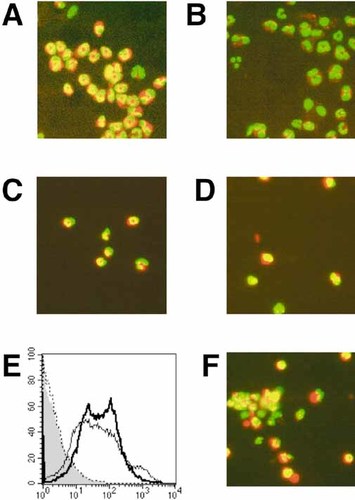
Nuclear translocation NF-κB following CT activation in murine BM-derived DC. The BM-derived DC from WT (A and C) or β1,4-GalNAc-T KO (B and D) mice were incubated with 10 μg/ml of CT (A and B), or LPS (C and D) for 2 h. After incubation, the cells were fixed and stained with anti-NF-κB Ab (red). Nuclei were stained using the Hoechst Dye (green). NF-κB and nuclei are shown merged; the merged signals appear yellow. (E) β1,4-GalNAc-T KO DC reconstituted with GM1 were stained with FITC-conjugated CT-B (solid line). The thin line and dotted line indicate the WT DC and KO DC stained with CT-B, respectively. The filled histogram represents untreated samples. (F) GM1-reconstituted KO DC were stimulated with CT and stained with Ab to NF-κB.
3 Concluding remarks
In this study, our major finding was that CT requires signaling through GM1 gangliosides to exert mucosal adjuvant properties in vivo. Taken together with the results of our in vitro experiments, we consider that our findings are very important to explain the potential of CT itself to induce terminal maturation of DC as APC via GM1 gangliosides, as a pivotal part of the mechanism underlying the strong mucosal adjuvanticity of CT. Furthermore, our results also point out that CT uses a different pathway from LPS to induce maturation of DC but shares the important step of NF-κB activation. Recent reports have shown that triggering of TLR, receptors for pathogen-associated motifs including LPS, expressed on specialized APC play a critical role in the initiation of the adaptive immune response 19. In this study, we showed that triggering GM1 on APC bridges to adoptive immunity following their activation. Although the detailed mechanism resulting in NF-κB activation after triggering of GM1 gangliosides remains to be clarified, our results indicate that GM1 ganglioside functions as PRR in a manner analogous to the TLR.
4 Materials and methods
4.1 Mice
Mice with deletion of β1,4-GalNAc-T (ganglioside KO) were established as previously described 23. Mice were maintained in the Animal Facility under pathogen-free conditions at the International Medical Center of Japan and used between 6 to 10 weeks of age.
4.2 Immunization of mice
For subcutaneous immunization, 100 μg of OVA (Sigma Chemical Co., St. Louis, MO) in CFA (Difco Laboratories, Detroit, MI) was given to mice on days 0 and 7. Three days after the last immunization, the mice were killed and used for preparation of splenic CD4+ T cells. In some experiments, the mice were given OVA in CFA subcutaneously on days 0 and 11. Measurement of Ab responses was performed three days after the last immunization. For oral immunization, 1 mg of OVA with 10 μg of CT (List, Campbell, CA) was given to mice three times at weekly intervals. Cell analysis and measurement of Ab responses were performed 1 week after the third immunization.
4.3 Detection of anti-OVA Ab
Plasma and fecal extracts were collected, and the titers of anti-OVA Ab were determined by an endpoint ELISA 14, 15, 25. Briefly, microtiter plates were coated with OVA (1 mg/ ml), blocked by 1% BSA, and then incubated with appropriately diluted plasma or fecal extracts. For detection, peroxidase-labeled secondary Ab with specificity for mouse IgM, IgG or IgA (Southern Biotechnology Associates Inc., Birmingham, AL) were used. The substrate 3,3′,5,5′-tetramethylbenzidine was used for detection of the peroxidase present. Endpoint titers from individual mice were expressed as the reciprocal log2 of the last dilution that gave an optical density of 0.1 greater than that of the plasma or fecal extract sample taken prior to immunization.
4.4 Recombinant CT, CT-A, and CT-B from Brevibacillus choshinensis
Recombinant CT was prepared by cultivating B. choshinensis bearing pNCMO2-CTB-CTA at 30°C for 3 days 26 and purified from the culture supernatant by affinity chromatography using D-galactose immobilized agarose (Pierce Chemicals, Rockford, IL) 27. For construction of recombinant CT-A and CT-B, a His-tag sequence derived from the pET28 vector (Novagen, Madison, WI) was introduced in front of each CT subunit gene (resulting in pNCMO2 his-CTA and pNCMO2 his-CTB, respectively). A His-Bind column (Novagen) was used for purification of CT-A and CT-B from the B. choshinensis culture supernatants according to the manufacturer's instructions. His-tagged peptides were removed by digestion with thrombin (Amersham-Pharmacia, Piscataway, NJ) and were rechromatographed on the His-Bind column. The flow-through was then loaded onto a Benzamidine Sepharose 6B column (Amersham-Pharmacia) to remove thrombin. The endotoxin levels in purified CT, CT-A and CT-B preparations were checked using an Endospec ES test MK kit (Seikagaku Co., Tokyo, Japan). The concentration of LPS in purified CT, CT-A and CT-B preparations was less than 10 Eu/mg. The purity of proteins was determined by SDS-PAGE. In this regard, the recombinant CT-B contained predominantly a stable pentameric form, and its GM1 binding ability was essentially identical to that of native CT-B 28.
4.5 Generation and analysis of BM-derived DC
BM-derived DC were propagated in vitro by using procedures similar to those reported previously 29. In brief, BM cells were harvested from either WT or KO mice and depleted of lymphocytes with a mAb cocktail consisting of anti-B220 (RA3–3A1/6.1, generated from a hybridoma obtained from the American Type Culture Collection, Rockville, MD); anti-Lyt-2 (YTS 169.4.2.1) and anti-L3T4 (YTS 191.1.1.2), both purified from culture supernatants of hybridomas provided by the European Collection of Cell Culture Collection (Wiltshire, GB); and rabbit complement (Inter-Cell Technologies, Hopewell, NJ). The cocktail-depleted BM cells were then cultured overnight in RPMI 1640 (Sigma) supplemented with 2 mM L-glutamine and 10% v/v FCS. Twenty-four hours later, nonadherent cells were carefully removed added to new dishes at a concentration of 5×105cells/ml containing medium supplemented with 10 ng/ml murine rGM-CSF, 10 ng/ml murine rIL-4 (both purchased from PeproTech, London, GB), and 50 μM 2-ME. The cultures were fed every 2 days by aspirating 75% of the medium, and adding back fresh medium containing GM-CSF, IL-4, and 2-ME. Five days later, the cells were stimulated with CT or LPS at a concentration of 10 μg/ml for 48 h. Cultured DC were then stained with biotinylated antibodies to CD11c (clone HL3), CD80 (clone 16–10A1), CD86 (clone GL1), and MHC class II alloantigens (clone M5/114.15.2), followed by PE-conjugated streptavidin, and analyzed with a FACScan (BD Bioscience, San Jose, CA). All of the mAb were obtained from BD PharMingen (San Diego, CA).
4.6 OVA-specific CD4+ T cell responses
The CD4+ T cells from splenic single-cell suspensions were purified by use of an automated magnetic activated cell separator (AutoMACS; Miltenyi Biotec, Sunnyvale, CA) as described 30. Briefly, mononuclear cells were added to a nylon wool column and incubated at 37°C for one hr to remove adherent cells. The enriched CD4+ T cell population was obtainedby incubation of the flow-through fraction with biotinylated anti-CD4 mAb (BD PharMingen) followed by streptavidin-conjugated microbeads for use with the AutoMACS. Purified splenic CD4+ Tcells (5×105 cells/ml) were cultured with irradiated BM-derived DC (5×103 cells/ml or 5×104 cells/ml) in RPMI1640 supplemented with 2 mM L-glutamine, 10% v/v FCS, 50 μM 2-ME, 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of gentamicin. The CD4+ T cells were cultured for 4 days at 37°C in 5% CO2 in air. To assess T cell proliferation, 1.0 μCi of [3H]thymidine (Perkin-Elmer Life Sciences, Boston, MA) was added to individual culture wells 16 h before termination of the cultures. The uptake of [3H]thymidine by dividing T cells was determined by using a cell harvester and scintillation counting (Packard, Meriden, CT). OVA-specific proliferation was determined by addition of 1 mg/ml of OVA to the cultures 31. The stimulation index was calculated by the following formula: Stimulation index = cpm of the culture with OVA/cpm of the culture without OVA.
4.7 NF-κB nuclear translocation
The BM-derived DC were activated with 10 μg/ml of CT or LPS for 2 h and stained using NF-κB Activation HitKit (Cellomics, Pittsburgh, PA). Briefly, the BM-derived DC were fixed with 3.7% formaldehyde for 10 min at room temperature. After permeabilization, the cells were stained with anti-NF-κB polyclonal Ab. For detection of nuclei, a Hoechst Dye was added to the stainingsolution. In GM1 reconstitution experiments, GM1 was dispersed by sonication in RPMI 1640 medium at a final concentration of 50 μM, and then incubated with BM-derived DC at 37°C for 2 h. After washing to remove excess GM1, the cells were stimulated with CT and stained with antibodies to NF-κB.
4.8 Statistics
The data were expressed as the mean ± one SEM, and the results compared by the two-tailed, unpaired Mann Whitney or Student's t-test. The results were analyzed by using the Statview II statistical program (Abacus Concepts, Berkeley, CA).
Acknowledgements
We thank Dr. Ryuko Fukunaga for technical assistance and Dr. Ayako Ozawa for purification and characterization of the recombinant CT, CT-A, and CT-B. We also thank Drs. Yoshifumi Takeda and Kiyoshi Takeda for their helpful advice and discussions pertaining to this work. This work was supported by grants and contracts from International Health Cooperation Research (11A-1, 14C-10), from the Ministry of Health, Labor, and Welfare; the Ministry of Education, Cultures, Sports, Science, and Technology; the Japan Health Sciences Foundation; and U. S. Public Health Service Grants (AI 18958, AI 43197, DE 12242, DC 04976, DK 44240, and AI 65299).
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




