OX40 (CD134) and OX40 ligand interaction plays an adjuvant role during in vivo Th2 responses
Abstract
The role of OX40-OX40 ligand (OX40L) interaction in Th cell differentiation remains contentious. In vitro studies have revealed a Th2-biased effect by OX40 signals in T cells. However, in vivo studies demonstrated that OX40-OX40L interaction is involved in responses either Th1 or Th2, or both, which appears to be dependent on the experimental conditions used. We document in our report Th cell differentiation in OX40L-deficient and OX40L-transgenic (Tg) mice in response to protein antigens (Ag) and to Leishmania major (L. major) infection. Upon immunization with protein Ag, we demonstrate the adjuvant effect of OX40 signals during in vivo Th2 responses. However, adjuvant treatment to mice ameliorates the Th2-specific effect of OX40-OX40L interaction and rather induces concurrent promotion of both Th1 and Th2 responses via OX40 signals. Thus, previous reports showing promotion of Th1 response by OX40-OX40L interaction may in actual fact be affected by the adjuvant effects mediated by the various experimental conditions. Indeed, constitutive OX40–OX40L interactions in OX40L-Tg mice converted the normally resistant C57BL/6 strain, into a susceptible status following L. major infection due to an extraordinary elevated Th2 response. These results provide convincing evidence demonstrating that the OX40-OX40L interaction is paramount in the development of Th2 responses in vivo.
Abbreviations:
-
- OX40L:
-
OX40 ligand
-
- OX40L-Tg:
-
OX40L-transgenic
-
- Alum:
-
Aluminum hydroxide gel
-
- KLH:
-
Keyhole limpet hemocyanin
-
- L. major:
-
Leishmania major
-
- SLA:
-
Soluble leishmania antigen
1 Introduction
Costimulation provided by APC, is an essential event for most acquired T cell immune responses. For example, interactions between CD28/ICOS and B7 family molecules are well known to be crucialfor Ag-specific T cell responses 1–4. OX40 (CD134), a member of the TNF receptor superfamily, is expressed on activated T cells 5–8 and also plays an important role in providing costimulatory signals to T cells upon interacting with its ligand (OX40L), a TNF family member 8–10 expressedon professional APC 11–14. Recent studies using OX40 or OX40L-deficient mice have demonstrated that the OX40-OX40L signal is indispensable for the generation of CD4+ memory T cells through T cell-APC interactions in several immunological conditions, such as viral infection, contact hypersensitivity and protein-antigenic challenge 14–17.
Controversy still persists however, regarding the in vivo role of OX40-OX40L interactions in regulating Th1 or Th2 responses. When using Th1-specific experimental systems, several reports have shown that blockade of OX40-OX40L interactions ameliorated Th1-specific diseases, such as experimental autoimmune encephalomyelitis (EAE) 18, 19, experimental inflammatory bowel disease 20, 21, and graft-versus-host-disease 22, 23. We have also demonstrated markedly reduced andenhanced EAE symptoms in OX40L-deficient and OX40L-transgenic (OX40L-Tg) mice, respectively 24. In this scenario, EAE was induced using Ag and CFA. On the other hand, in experimental systems with a predominant Th2 bias, it has been reported that OX40-OX40L interaction may be involved mainly in Th2 responses. OX40-deficient mice have been shown to be resistant to the murine Th2-specific allergic disease model of asthma 25. In addition, an inhibitory anti-OX40L mAb treatment significantly inhibited progression of Leishmania major (L. major) infection in susceptible BALB/c strain probably mediated by suppressed Th2 responses 26. However, these in vivo observations may reflect an impairment in the generation of memory T cells. Indeed, upon protein Ag immunization, deficient mice for OX40 or OX40L demonstrated a concurrent reduction in both Th1 and Th2 responses due to a reduced number of Ag-specific memory T cells 14, 17. Several reports support this notion by showing that OX40-OX40L interaction enhances the survival of CD4+ T cells, leading to the generation of CD4+ memory T cells 14, 17, 27–29. These previous findings suggest that OX40-OX40L interaction plays important roles in both Th1 and Th2 responses by promoting memory T cell generation.
In vitro studies, however, clearly demonstrated that interaction between OX40 and OX40L upon Ag-stimulation, affected predominantly Th2 cytokine production by CD4+ T cells, suggesting that signals through OX40 are specifically involved in the development of Th2 responses but not Th1 responses in vitro 30–33. These in vitro experiments were conducted with the aim of assessing T cell priming, and thus the duration of the culture experiments undertaken were too short to allow for the generation of memory T cells. Thus, there seems a discrepancy between the in vivo and in vitro results on the Th cell differentiation through OX40-OX40L interactions.
To assess directly the in vivo role of OX40L in the regulation of Th cell differentiation, we examined immune responses to protein Ag and the physiological consequences of L. major infection in OX40L-deficient and OX40L-Tg mice. Infection using the protozoal parasite L. major provides a useful model for studying the induction of Th1/Th2 responses in vivo 34–36. In resistant mouse strains such as C57BL/6, CBA, and C3H/He, the infection induces parasite-specific Th1 response characterized by the enhanced production of IFN-γ mediated by an increase in IL-12, which results in complete recovery from the infection. In contrast, strains such as BALB/c, are biased predominantly towards a Th2 response, making them susceptible to the infection 34–36.
In the present study, OX40L-Tg mice generated a biased Th2 response to protein Ag only in the absence of adjuvant. Furthermore, OX40L-Tg mice on both the C57BL/6 and BALB/c backgrounds were unable to resolve the L. major infection, during which they displayed markedly enhanced Th2 responses. These results strongly suggest that the interaction between OX40 and OX40L may be a critical costimulatory pathway involved in Th2 differentiation rather than solely a Th1 or mixed response in vivo.
2 Results
2.1 OX40 signaling in the absence of adjuvant induces a marked T cell proliferative response
Most soluble proteins are nonimmunogenic and can induce anergy of T cell in mice in the absence of adjuvant. Thus, the requirement of adjuvant in the induction of robust T cell responses in mice is well documented. Indeed, upon OVA immunization alone to the footpads of mice, negligible CD4+ T cells proliferative responses were provoked in wild-type or OX40L-deficient mice (Fig. 1A). OX40L-Tg mice, on the contrary, were able to induce a robust T cell proliferative response even in the absence of adjuvant, to similar levels as that in the presence of adjuvant (Fig. 1A). This result suggests that OX40 signaling plays an adjuvant role in protein Ag-specific CD4+ T cell responses. Comparing the responses among the three genotypes in the presence of adjuvant, OX40L-Tg mice had markedly increased T cell recall proliferative responses, while a muted response by the CD4+ T cells derived from OX40L-deficient mice when compared to the wild-type mice was observed.
A similar result on proliferative responses was obtained using splenic CD4+ T cells derived from OX40L-Tg mice systemically immunized with KLH either in the presence or absence of adjuvant (Fig. 2A).

Constitutive OX40-OX40L interaction specifically augments Th2 responses of LN CD4+ T cells to OVA in the absence of adjuvant. Eight-week-old OX40L-Tg, OX40L-deficient, and wild-type mice (n=3 in each group) were immunized with 200 μg OVA in PBS, or 200 μg OVA along with Alum (OVA/Alum) or CFA (OVA/CFA) in the footpads. Fourteen days after immunization, CD4+ T cells from draining LN, were purified and subjected to an in vitro challenge of OVA in the presence of APC. After a 48-h culture, their [3H]thymidine uptakes were measured (A). Cytokine production by CD4+ T cells stimulated with 50 μg/ml of OVA was assayed (B). CD4+ T cells from each genotypic mice immunized with OVA alone, OVA/Alum, or OVA/CFA were used. Results are expressed as mean ± SD in each group. Similar results were obtained in three independent experiments. ND; not detectable. *p<0.01, **p<0.001, c.f. wild-type mice.
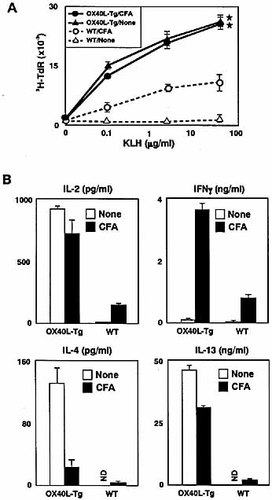
Constitutive OX40-OX40L interaction specifically augments Th2 responses of splenic CD4+ T cells to KLH in the absence of adjuvant. Eight-week-old OX40L-Tg (n=4, black) and wild-type (n=4, white) littermates were intraperitoneally immunized with 1 mg KLH alone (circle), or KLH with CFA (triangle). Fourteen days after immunization, splenic CD4+ T cells were subjected to an in vitro challenge of KLH. After a 48-h culture, their [3H]thymidine uptakes were measured (A). Cytokine production by CD4+ T cells stimulated with 50 μg/ml of KLH was assayed (B). CD4+ T cells from each genotypic mice immunized with KLH alone or KLH with CFA were used. Results are expressed as mean ± SD in each group. Similar results were obtained in three independent experiments. ND; not detectable. *p<0.001, c.f. wild-type mice.
2.2 OX40 signaling plays a role as an adjuvant during Th2 cell differentiation
CD4+ T cells derived from OX40L-Tg mice were analyzed for their recall production of various cytokines upon immunization with OVA alone, OVA/Alum, and OVA/CFA. Interestingly, production levels of IL-2, IL-4 and IL-13 of CD4+ T cells from OX40L-Tg mice were very similar among the three groups (Fig. 1B), indicating the dispensability of adjuvant for Th2 responses in the OX40L-Tg mice. In contrast, when using CFA, IFN-γ production of OX40L-Tg CD4+ T cells was much higher than those from wild-type mice (Fig. 1B). In addition, the presence of Alum, a Th2-prone adjuvant, also significantly induced higher IFN-γ production by OX40L-Tg CD4+ T cells than wild-type CD4+ T cells (Fig. 1B). These results imply that constitutive OX40-OX40L interaction alone is sufficient to induce a Th2 response, while, in the presence of adjuvant, the interaction enhances both Th1 and Th2 responses. On the other hand, when utilizing adjuvants, cytokine productions of CD4+ T cells from OX40L-deficient mice were significantly suppressed as compared with those from wild-type mice (Fig. 1B). This result supports the conclusion from the OX40L-Tg T cell results demonstrating that OX40-OX40L interaction promotes both the Th responses when using adjuvant.
To confirm this adjuvant-independent Th2 promotion by the OX40L-Tg mice, we examined T cell responses using a different immunization protocol through intraperitoneal administration with KLH in the presence or absence of CFA. Recall productions of IL-2, IL-4 and IL-13 from OX40L-Tg CD4+ T cells immunized with KLH alone were similar to or higher than those from KLH/CFA-immunized OX40L-Tg mice (Fig. 2B). In contrast, the level of IFN-γ production from OX40L-Tg mice was elevated only in the presence of CFA. This result is consistent with previous findings that in vivo ligation of OX40 promotes Th1 responses upon Ag immunization with CFA 17, 24, 29. CFA-treatment significantly suppressed IL-4 production by OX40L-Tg CD4+ T cells (Fig. 2B), suggesting that the biased Th2 responses in OX40L-Tg mice may partially shift to a Th1 response by the presence of CFA. Nevertheless, the Th2 cytokine production levels of the CD4+ T cells from OX40L-Tg mice immunized with KLH/CFA were markedly higher than those from wild-type mice immunized with KLH/CFA (Fig. 2B). Taken together, OX40 signaling participates in playing the role as an adjuvant during in vivo Th2 cell differentiation.
2.3 Susceptibility to L. major in OX40L-Tg and OX40L-deficient mice
In light of these results, we next investigated the immune responses to L. major infection in OX40L-deficient, OX40L-Tg and wild-type mice. Experimental leishmaniasis is a well-designed system to determine responsible molecules for Th responses. Normally resistant wild-type C57BL/6 mice displayed an initial period of footpad swelling, and the swelling decreased to near normal levels by 7 weeks (Fig. 3). Consistent with a previous observation using OX40-deficient mice 37, OX40L-deficient mice on the C57BL/6 background showed a comparable resolution of the disease as seen in the wild-type C57BL/6 mice (Fig. 3). In sharp contrast, the footpads of infected OX40L-Tg mice continued to increase in size, leading to severe ulceration and marked deterioration of the integrity of the foot by 4 weeks (Fig. 3). Another independently established line of OX40L-Tg mice on the C57BL/6 strain 29 exhibited similar progressions of disease (data not shown). Next, to more accurately assess the disease presentation, the numbers of L. major parasites in the draining popliteal LN were quantified by using a limiting dilution assay 38. Parasite burdens in the popliteal LN from OX40L-Tg were surprisingly even greater than those from wild-type BALB/c mice (Table 1). These results demonstrated that constitutive OX40-OX40L interactions appear to convert resistant mice to a susceptible phenotype.
We next examined the effect of L. major infection in the OX40L-mutated mice on the well-described susceptible BALB/c background. As expected, OX40L-Tg and wild-type BALB/c mice developed progressive non-healing lesions by 5 weeks (Fig. 3). In contrast, OX40L-deficient mice on the BALB/c background demonstrated a significant reduction in footpad swelling as compared to the wild-type BALB/c mice (Fig. 3). Ulcerations in the footpad of these mice were not seen until at least 8 weeks. Most of the OX40L-deficient mice (83%), however, did not have complete resolution in footpad integrity thereafter as seen in wild-type C57BL/6 mice. Parasitic counts from popliteal LN at 4 weeks post-infection revealed that OX40L-deficient mice had fewer parasites as compared with the wild-type BALB/c mice, although the burden never approached those observed in the resistant C57BL/6 mice (Table 1).
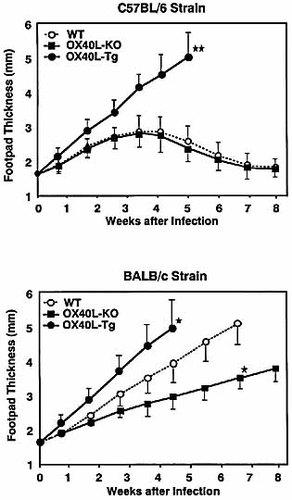
OX40L-Tg mice show susceptibility to L. major infection. OX40L-Tg (n=7), OX40L-deficient (n=7) and wild-type (n=7) mice on the C57BL/6 background were infected with promastigotes in the footpads and the thickness was measured (upper panel). OX40L-Tg (n=6), OX40L-deficient (n=7) and wild-type (n=8) mice on the BALB/c background were infected with promastigotes in the footpads and the thickness was measured (lower panel). Results are expressed as mean ± SD in each group. Data are representative of five separate experiments. *p<0.005, **p<0.001, c.f. wild-type mice.
|
Exp. (days after infection) |
Genotype |
n |
Titer of viable L. Major per 106 LN cells |
|---|---|---|---|
|
1 (30 days) |
B6-WT |
3 |
219 ± 62 ]* |
|
|
B6-OX40L-Tg |
3 |
2,586 ± 583 |
|
|
BALB-WT |
4 |
1,893 ± 229 |
|
|
BALB-OX40L-KO |
3 |
708 ± 240 |
|
2 (42 days) |
B6-WT |
3 |
51 ± 20 ]* |
|
|
B6-OX40L-Tg |
3 |
15,105 ± 3276 |
|
|
B6-OX40L-KO |
3 |
32 ± 4 |
|
|
BALB-WT |
3 |
4,444 ± 896 |
|
|
BALB-OX40L-Tg |
3 |
17,236 ± 4965 |
|
|
BALB-OX40L-KO |
3 |
2,898 ± 1011 |
- a) At the indicated days post-infection draining lymph node cells were collected and assayed for the parasite burden using a limiting dilution assay. *p<0.01, Student's t-test.
2.4 Constitutive OX40-OX40L interaction markedly promotes Th2 response of draining LN-derived CD4+ T cells in the recall response to Leishmania antigen
Th2 responses have been shown to promote susceptibility to L. major infection 34–36. Therefore, we examined the cytokines produced by the draining LN CD4+ T cells of the C57BL/6 strains of mice 21 days after infection. Production of Th2 cytokines, IL-4, IL-5 and IL-13 by the OX40L-Tg CD4+ T cells upon soluble Leishmania antigen (SLA) stimulation was 50–300 times higher than those of the wild-type T cells (Fig. 4A). Similar to cytokine production to protein Ag in the absence of adjuvant (Fig. 1 and 2), SLA-specific IFN-γ production of the OX40-Tg mice was comparable to that of the wild-type littermates (Fig. 4A). This presentation by OX40L-Tg (C57BL/6) mice indicates that constitutive OX40-OX40L interaction strongly enhances Th2 cytokine production of CD4+ T cells in an Ag-specific manner, leading the C57BL/6 strain to susceptibility. It is worth noting that upon examination of the recall responses of the draining LN from OX40L-deficient (C57BL/6) mice, undetectable levels of Th2 cytokines were seen, while IFN-γ production was only half the level of that of wild-type C57BL/6 mice (Fig. 4A).
Similar analyses were performed on the susceptible BALB/c strain. Upon SLA stimulation, the Th2 cytokines, IL-4, IL-5 and IL-13 were significantly increased in the culture supernatant of OX40L-Tg CD4+ T cells as compared with the wild-type CD4+ T cells (Fig. 4B). This increase in Th2 response was mirrored by the severity of L. major infection in the OX40L-Tg mice (Fig. 3). In contrast, OX40L-deficient mice exhibited a marked reduction in Th2 cytokines, but not IFN-γ production in the in vitro recall response to SLA (Fig. 4B).
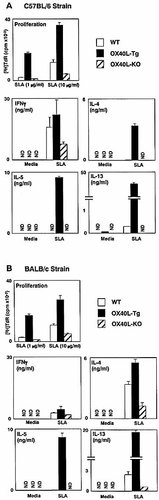
Strong increase in Th2 responses of the draining LN cells from the OX40L-Tg mice during L. major infection. In vitro recall response to SLA of CD4+ T cells from the draining LN in the OX40L-Tg, OX40L-deficient and wild-type mice on the C57BL/6 background (A) and BALB/c background (B) were examined. Twenty-one days after infection, CD4+ T cells were purified from draining LN of three individual mice per group, and stimulated with SLA. After culturing for 48 h, their [3H]thymidine uptakes were measured. Cytokine production by the draining LN CD4+ T cells stimulated with 10 μg/ml of SLA was assayed. Values represent the average of triplicate wells ± SD. Similar results were obtained in four independent experiments. The representative results shown in (A) and (B) were obtained in a simultaneous experiment. ND; not detectable.
2.5 In vivo treatment with the inhibitory anti-OX40L mAb rescues OX40L-Tg C57BL/6 mice from susceptibility to L. major
The failure to control L. major infection mediated by overexpression of OX40L suggests a causative link between constitutive OX40-OX40L interaction and disease susceptibility, probably caused by a biased Th2 response. Thus, we addressed whether administration of inhibitory anti-OX40L mAb, MGP34, could rescue the susceptibility to disease in the OX40L-Tg C57BL/6 mice. Intraperitoneal administration of MGP34 mAb to OX40L-Tg mice provided complete protection from infection (Fig. 5A). Furthermore, the MGP34-treatment strongly reduced the serum level of IgE and IL-13 accompanied with completely suppressed parasite burden in the OX40L-Tg mice (Fig. 5B–D). Recall cytokine production in response to SLA of the CD4+ T cells derived from the draining LN demonstrated that in vivo MGP34-treatment markedly suppressed Th2 cytokine production accompanied with a significant elevation of IFN-γ production (Fig. 5E–H). These results clearly indicate that OX40-OX40L interaction is directly and critically involved in Th2 response in vivo.
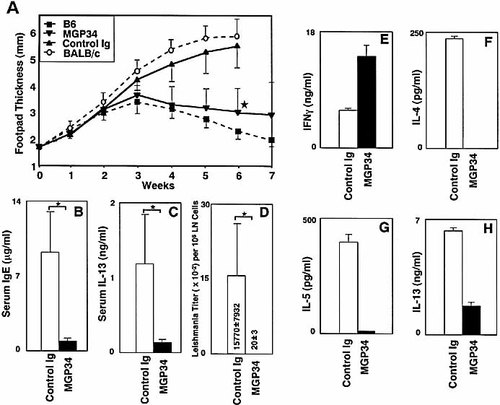
Blockade of the OX40-OX40L interaction rescues the OX40L-Tg mice from their susceptibility to L. major infection. (A) MGP34-treated OX40L-Tg (n=12), control Ig-treated OX40L-Tg (n=10), and wild-type C57BL/6 mice (n=5), and BALB/c wild-type mice (n=5) were infected with promastigotes in the footpads, and the thickness was measured. MGP34 mAb or control Ig (500 μg) was injected intraperitoneally at days –1, 0, and 2 of infection. After the initial injection, 500 μg of the mAb was injected every 3 days for 7 weeks. Results are expressed as mean ± SD in each group. Data are representative of two separate experiments. *p<0.005, c.f. control Ig-treated mice. Serum levels of IgE (B) and IL-13 (C) at 35 days post-infection in the OX40L-Tg mice treated with MGP34 mAb or control Ig are demonstrated. Results are expressed as mean ± SD of four mice in each group (*p<0.05, Student's t-test). (D) Parasite burden per 106 popliteal LN cells in the OX40L-Tg at 35 days post-infection are demonstrated (*p<0.05, Student's t-test). Thirty-five days after infection, CD4+ T cells were purified from draining LN of four individual mice per group, and stimulated with 10 μg/ml of SLA. Supernatants were collected at 48 h for IL-4 (F), and at 72 h for IL-5 (G), IL-13 (H) and IFN-γ (E), and the cytokines were assayed by ELISA. Values represent the average of triplicate ELISA wells ± SD. Similar results were obtained in two independent experiments.
3 Discussion
Several reports have previously demonstrated that OX40-OX40L interactions are critically involved in both Th1 and Th2 responses through their effect on the generation of memory CD4+ T cells 14, 17, 27, 29. To induce effective immune responses to protein Ag in mice, adjuvant/adjuvant-like products are required. Indeed, wild-type and OX40L-deficient mice showed little, if any, responses to protein Ag when immunized in the absence of adjuvant (Fig. 1 and 2). Several studies have revealed the functional involvement of OX40-OX40L interactions in the differentiation of both Th1 and Th2 responses to protein Ag in combination with adjuvant, LPS or adjuvant-like products 14, 15, 17–19, 24, 27, 29. In comparison with the previous observations, OX40L-Tg and OX40L-deficient mice exhibited an enhancement and suppression of both Th1 and Th2 responses to protein Ag, respectively, when the Ag was administrated with adjuvant (Fig. 1 and 2). In contrast, in the absence of adjuvant, constitutive OX40-OX40L interaction was capable of generating a markedly enhanced Th2 but not Th1 response to protein Ag. Interestingly, immunization of Ag with Alum also induced a significant increase in IFN-γ production by the OX40L-Tg T cells in spite of the prominent Th2 response (Fig. 1B). Thus, it appears that Alum, which is a Th2-prone adjuvant, might also affect the Th1 response generated. Predominant production of Th2 cytokines by OX40 signals was not altered when the lower dose of OVA (0.5 μg/ml) was used in the ex vivo responses (data not shown). Collectively, this indicates that promotion of Th1 responses by OX40 signaling requires the presence of adjuvant in primary protein Ag-specific immune responses. Furthermore, the cytokine profiles of the OX40L-mutated mice during L. major infection were very similar to those presented in protein Ag-induced immune responses in the absence of adjuvant (Fig. 1, 2 and 4). Since blockade of OX40-OX40L interaction completely rescued OX40L-Tg mice from their susceptibility, probably due to the reversal of the Th2 response (Fig. 5), OX40-OX40L interaction is directly involved in enhanced Th2 response in L. major infection. However, we were unable to confirm this in the protein Ag-specific immune responses, as administration with an inhibitory anti-OX40L mAb to OX40L-Tg mice immunized with protein Ag in the absence of adjuvant, suppressed T cell cytokine production to undetectable levels (data not shown).
Susceptibility to L. major is characterized by the development of Th2 responses, especially an IL-4 burst immediately after L. major infection, mediated by its suppressive effect on Th1 responses 34–36. In the susceptible BALB/c strain, OX40L-deficient mice demonstrated significantly delayed symptoms, and a less parasitic burden than wild-type mice (Fig. 3 and Table 1). Nevertheless, OX40L-deficient mice on the BALB/c background were unable to recover from L. major infection as revealed by the non-healing footpad lesions, suggesting that the affected Th responses induced by an absence of OX40L were not sufficient to induce resistance in BALB/c mice. With regard to OX40L function in addressing the susceptibility to L. major infection in mice, Akiba et al. 26 reported that anti-OX40L mAb administration abrogated progressive diseases in BALB/c mice. The authors, however, discontinued the mAb treatment by 6 weeks after infection, and mentioned that halting the treatment encouraged footpad swelling. These results suggest that the effect of anti-OX40L mAb on the disease susceptibility in BALB/c mice may be transient. Short-term treatment with the mAb thus seems insufficient to draw a conclusion of complete recovery from disease. We have clearly observed a similar suppression of symptoms in OX40L-deficient mice on the BALB/c strain as seen following the anti-OX40L mAb-treated mice by 6 weeks, However, eventually non-healing footpad lesions appeared. Nevertheless, we cannot exclude the possibility that the association between OX40L function and the disease susceptibility might be mediated by a different mechanism other than the Th1/Th2 paradigm.
In this context, we conclude in the present study that constitutive OX40-OX40L interaction plays an adjuvant role specific for Th2 cell differentiation. Some previous reports have observed conflicting results to our conclusion. Weinberg et al. 39 demonstrated that excessive OX40 signaling enhanced CD4+ T cell mediated-anti-tumor immunity accompanied with an increase in IFN-γ production in tumor transplantation experiments. It is probable that in the presence of tumor cells, OX40 signals are further skewed towards both Th1 and Th2 responses rather than to a predominant Th2 profile. A recent report revealed that in vivo OX40 ligation along with injection of protein Ag-pulsed DC promotes both Th1 and Th2 recall responses, during which no conventional adjuvants was used 40. In this case, the DC used in the experiment were prepared from Flt3 ligand-treated mice. Since in vivo Flt3 ligand treatment has an adjuvant effect to induce DC maturation 41, Flt3-ligand-treated DC might be actually adjuvant-treated DC, which may induce the production of the Th1 cytokine, IL-12. Similarly, we recently demonstrated that constitutive OX40-OX40L interactions enhanced both Th1 and Th2 cytokine production in allergen-induced contact hypersensitivity responses 42 although we used the same OX40L-Tg mice as the present study. Since dinitrofluorobenzene, the allergen used in the study, is known to be a Th1-prone allergen 15, 43, this allergen may affect the OX40-signal-associated Th1 response. In understanding the OX40-OX40L-associated Th1 responses following infections as previously reported 16, it appears that pathogen-derived products may have direct adjuvant-like effects on pathogen-induced immune responses. Similarly, in the experimental inflammatory bowel disease model, intestinal bacterial flora may provoke OX40-induced Th1 responses as reports have shown the essential contribution of intestinal bacteria for disease development 44, 45. Taken together, OX40-OX40L interaction promotes predominantly Th2 responses although enhancement of Th1 responses by OX40-OX40L interaction may further be dependent on the conditions in which Ag is administered, such as the presence of conventional adjuvants, Flt3 ligand-treated DC, allergens, pathogens-derived products, and site of immunization.
4 Materials and methods
4.1 Mice
OX40L-Tg and OX40L-deficient mice have been described previously 14, 29, 42. OX40L-Tg mice which express OX40L mainly on T cells, were constructed by using the proximal promoter from the mouse lck gene 29, 42. OX40L-Tg mice had been backcrossed for 13 generations onto the C57BL/6 strain and 9 generations onto the BALB/c strain. OX40L-deficient mice had been backcrossed onto the C57BL/6 strains 15 times and the BALB/c strains at least 10 times. Age- and sex-linked wild-type littermates of the OX40L-Tg mice were used as control mice accordingly. All mice were bred and maintained under specific-pathogen-free conditions in the Institute for Animal Experimentation, Tohoku University Graduate School of Medicine.
4.2 In vivo T cell priming and recall responses to protein antigens
OX40L-Tg mice, wild-type littermates or OX40L-deficient mice were immunized with 200 μg OVA (Sigma Chemicals Co, MO) in the presence of CFA (Dia-Iatron, Tokyo), aluminum hydroxide gel (Alum; Cosmo Bio, Japan) or in the absence of adjuvant to each hind footpad. Alternatively, OX40L-Tg mice or wild-type littermates were intraperitoneally immunized with 1 mg KLH (Sigma Chemicals Co, MO)in PBS or in the presence of CFA. Fourteen days after, CD4+ T cells (5×104, purity >98%) purified from draining lymph node (LN) or spleen using AutoMACS (Miltenyi Biotec, Gladbach, Germany) were incubated with the indicated dose of the same protein as immunized in the presence of APC (5×105) at 37°C. The APC used were irradiated (3,000 rad) splenocytes derived from unimmunized wild-type littermates. The cultured cells were assayed for [3H]thymidine uptake and cytokine production as described 14, 46. In the cytokine secretion assays, 50 μg/ml of OVA or KLH was added to the cell culture. Culture supernatants were collected at 48 h for IL-2 and IL-4, or at 72 hr for IL-13 and IFN-γ and subjected to ELISA.
4.3 Parasites and infection
L. major (MHOM/SU/73/5ASKH) was maintained by in vivo passage in BALB/c mice. For experimental infection, popliteal LN were extracted from infected mice and cultured in Schneider's medium (Gibco BRL, MD) supplemented with 20% FCS at 26°C. Stationary phase promastigotes were harvested, and used for infection and Ag preparation. Soluble leishmania Ag (SLA) was prepared by repeated, rapid freeze-thaw cycles of a pellet of L. major promastigotes. 8- to 14-week-old mice were infected with one to five million promastigotes in the right hind footpad. Footpad thickness was determined by weekly measurement of the infected footpad with a vernier caliper.
4.4 Ab
Rat mAb, anti-mouse OX40L (MGP34 14) and purified rat IgG (ICN Pharmaceuticals, Inc. CA) as a control Ab were used.
4.5 Preparation of cells from L. major-infected mice
Infected mice were killed and the popliteal LN and blood were collected. CD4+ T cells were purified from popliteal LN using CD4 microbeads (Miltenyi Biotec) by AutoMACS. Purified CD4+ T cells (99% purity; 1.5×105) from three mice in each group were stimulated with SLA (10 μg/ml) in 96-well round-bottom plates containing 200 μl of RPMI 1640 medium supplemented with 10% FCS in the presence of 3×105 cells of irradiated spleen cells as APC from uninfected syngeneic mice. The cultured cells were assayed for [3H]thymidine uptake and cytokine production as described above. In the cytokine secretion assays, 10 μg/ml of SLA was added to the cell culture. Culture supernatants were collected at 48 h for IL-2 and IL-4, orat 72 h for IL-13 and IFN-γ and subjected to ELISA.
4.6 Cytokine ELISA
IL-2, IL-4, IL-5, IFN-γ (PharMingen, CA), and IL-13 (R & D Systems, Minneapolis, MN) ELISA were performed according to the manufacturer's instructions. IgE ELISA was performed by using Yamasa EIA kit (Yamasa Shoyu, Tokyo) according to manufacturer's directions.
4.7 Limiting dilution assay
Parasite numbers in LN of infected mice were measured by limiting dilution cultures as described 38, 47. In brief, serial dilutions of single-cell suspension of LN cells in Schneider's medium supplemented with 20% FCS were distributed to 96-well plates. After 8 days of incubation at 26°C, the assay was read by scoring the number of wells positive for parasite growth, and parasite numbers per 106 LN cells were calculated.
Acknowledgements
We would like to thank Drs. T. Yoshimoto (Hyogo College of Medicine) and F. Sendo (Yamagata University School of Medicine) for providing L. major. This work was supported in part by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan, and a grant-in-aid for scientific research on priority areas from the Japan Society for the Promotion of Science.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




