Mini-review SAP: a molecular switch regulating the immune response through a unique signaling mechanism
Abbreviations:
-
- EBV:
-
Epstein–Barr virus
-
- SAP:
-
SLAM-associated protein
-
- SH2:
-
Src-homology 2
-
- SLAM:
-
Signaling lymphocyte activation molecule
-
- XLP:
-
X-linked lymphoproliferative disease
Introduction
Antigen-induced immune cell activation is initiated by the interaction of MHC-bound antigens with a clonotypic TCR. This response is dependent on concomitant engagement of other receptors, including co-receptors (CD4 and CD8) and costimulatory molecules (such as CD28 and ICOS). When properly stimulated, CD4+ T cells secrete cytokines such as IFN-γ and IL-4, which permit recruitment and activation of other immune cells such as macrophages and B cells. By contrast, CD8+ T cells mediate cytotoxicity, in particular against virus-infected target cells. To avoid immune dysfunctions that lead to immunodeficiencies or autoimmunity, the duration and extent of involvement of these various cell types require exquisite regulation.
SAP [signaling lymphocyte activation molecule (SLAM)-associated protein] or SH2D1A is a small cytosolic adaptor-like molecule 1, 2. It is composed only of aSrc-homology 2 (SH2) domain (a phosphotyrosine-binding module), in addition to a short carboxy-terminal extension of undetermined function. SAP is expressed in T cells, NK cells and, perhaps, some B cells. A close relative, EAT-2, accumulates in NK cells, macrophages and, possibly, in DC.
An important clue regarding the function of SAP was obtained by the finding that its gene is mutated or deleted in a large subset of patients with X-linked lymphoproliferative disease (XLP), asevere human immunodeficiency characterized by an inappropriate response to Epstein–Barr virus (EBV) infection 3–5. Usually, boys with XLP exhibit uncontrolled polyclonal lymphoproliferation after EBV infection, leading to massive tissue necrosis and tissue death. In some cases, XLP patients develop progressive hypogammaglobulinemia or malignant lymphomas. Several immune cell types were shown to exhibit functional alterations in XLP, including CD4+ T cells, CD8+ T cells, NK cells and B cells 6. However, given the severe clinical manifestations of EBV infection in these patients, it is unclear whether these various defects are primary or secondary to the disease.
The critical importance of SAP in immune modulation was also demonstrated by the creation of SAP-deficient mice 7–9. It was reported that these animals manifest increased mortality following infection with lymphocytic choriomeningitis virus (LCMV). Alterations of antiviral CD8+ T cell responses were found in these animals. In vitro studies of CD4+ T cells isolated from SAP-deficient mice also revealed diminished IL-4 production and increased IFN-γ production in response to activation with anti-CD3 antibodies, suggesting additional alterations in CD4+ T cell responses. Lastly, production of antibodies, especially IgG2a, is reduced in LCMV-infected SAP-deficient animals, implying that SAP is required for appropriate B cell reactions as well. As is the case for XLP, however, it is unclear whether these various immune abnormalities are direct or indirect consequences of SAP deficiency.
A primary defect in late CD4+ T cell help in SAP-deficient mice
Recently, in a study reported in Nature, evidence was provided that a primary defect in CD4+ T cell functions is implicated in SAP-dependent immune dysfunctions 9. The authors found that, although SAP-deficient mice have normal levels of IgM and IgG as well as normal numbers of antibody-secreting cells (ASC) soon after LCMV infection, these animals demonstrate a severe defect in the ability to maintain antiviral IgG levels and ASC. These abnormalities are observed as early as two weeks post-infection. The capacity to form germinal centers is also drastically diminished. Importantly, adoptive transfer experiments indicated that these alterations are not due to an intrinsic B cell abnormality, but rather are caused by a defect in long-term CD4+ T cell help. Although the precise mechanism involved was not established, this defect does not appear to result from diminished numbers of IL-4-producing CD4+ T cells, or from a decrease in the expression of CD40 ligand on CD4+ T cells. Whether the CD4+ T cell function that explains this phenomenon promotes expansion, maturation and/or survival of memory Bcells and ASC remains to be clarified.
Although these data provide a compelling indication that XLP involves a primary defect in the CD4+ T cell response to EBV infection, it is likely that other cell types, in particular CD8+ T cells and NK cells, also contribute to the pathophysiology of the disease 6. Furthermore, since SAP can be detected in some B cells, a primary B cell dysfunction may also participate in the dysgammaglobulinemias seen in XLP patients. Unfortunately, the SAP-deficient mouse model may be less useful to test some of these possibilities, as mice are not susceptible to EBV infection. However, it is conceivable that alternative viruses, capable of leading to pathologies similar to EBV-induced infectious mononucleosis in mice, could be used to address the roleof cell types other than CD4+ T cells in the disease.
SAP regulates immune cell receptor signaling by a unique mechanism
Since SAP contains an SH2 domain, it is likely to be involved in phosphotyrosine-mediated signal transduction events. In support of this idea, SAP associates by way of its SH2 domain with a unique tyrosine-based motif — T/IxYxxV/I; where T is threonine, I is isoleucine, Y is tyrosine, V is valine and × is any residue — found in the cytoplasmic region of members of the SLAM family of receptors such as SLAM (CD150), 2B4 (CD244), NTB-A/Ly-108, CD84 and Ly-9 1–3, 10. Although little is known about the physiological functions provided by these receptors, they are known to be expressed in diverse immune cell types. Furthermore, their engagement by antibodies or recombinant ligands can cause striking alterations in immune cellresponses in vitro, in particular causing modulation of the types of cytokines produced by activated T cells and induction of NK cell mediated cytotoxicity.
In the light of its single-module composition, SAP was proposed to act by blocking the ability of SLAM-related receptors to interact with other SH2-domain-mediated proteins 3. In keeping with this notion, it was shown that SAP was able to prevent the association of SLAM family members with the SH2-domain-containing protein tyrosine phosphatase SHP-2. However, this effect was observed only in transiently transfected non-immune cells, raising questions regarding its physiological pertinence. A report by Latour et al. 10 provided evidence for a more active role of SAP in SLAM-related receptor signaling. This group showed that SAP is required for the ability of SLAM to mediate an intracellular protein tyrosine phosphorylation signal in T cells, and that this property is a consequence of the capacity of SAP to mediate selective recruitment and activation of the Src-related protein tyrosine kinase (PTK) FynT. This mechanism leads to tyrosine phosphorylation of the cytoplasmic domain of SLAM, thereby causing recruitment of SH2-domain-bearing effectors such as the inositol phosphatase SHIP-1, and causing modulation of IFN-γ productionby activated T cells.
Two recent studies published in Nature Cell Biology unraveled the elegant mechanism by which SAP couples FynT to the SLAM receptors 11, 12. These studies provided biochemical, crystallographic and functional evidence that SAP recruits FynT as a result of a direct interaction between the SAP SH2 domain and the FynT SH3 domain (Fig. 1). Interestingly, this association implicates a non-proline-based surface located in the SAP SH2 domain, centered on arginine 78 of SAP. Since this region is structurally independent of the phosphotyrosine-binding fold in the SAP SH2 domain, it permits simultaneous association of SAP with SLAM and FynT. Hence, through the bifunctional nature of its SH2 domain, SAP is capable of recruiting the PTK needed for SLAM-mediated signal transduction. It is likely that a similar function is provided for other SLAM-related receptors present in T cells, including NTB-A, CD84 and Ly-9. Furthermore, because SAP and FynT are also co-expressed in NK cells, SAP probably carries out a related function in these cells as well. Although a similar purpose may be served by the SAP relative, EAT-2, it should be pointed out that EAT-2 does not share the arginine-78-based motif of SAP and, as a result, does not seem able to bind FynT 11. Perhaps, EAT-2 interacts with one or more other members of the Src family by a distinct mechanism.

SAP is critical for SLAM-mediated protein tyrosine phosphorylation by mediating recruitment of FynT. (A) In the absence of SAP, SLAM (pink) may not be able to mediate an intracellular protein tyrosine phosphorylation signal in T cells, or the signal may be modified. (B) SAP (orange) simultaneously binds via its SH2 domain to SLAM and to the SH3 domain of the Src-related protein tyrosine kinase FynT (green) and thus mediates the selective recruitment and activation of FynT. This mechanism leads to tyrosine phosphorylation of the cytoplasmic domain of SLAM, thereby causingrecruitment of SH2-domain-bearing effectors.
4 Conclusion
On the basis of the available evidence, SAP appears to operate as a molecular switch enabling the diverse SLAM-related receptors to mediate specific intracellular protein tyrosine phosphorylation signals that modulate immune cell functions (Fig. 2). In the absence of SAP, the functions of the SLAM family would be compromised or perhaps qualitatively modified, explaining the pleiotropic consequences of SAP deficiency in humans and mice. In agreement with this idea, it has already been shown that the ability of 2B4 and NTB-A to trigger cytotoxicity is drastically reduced in NK cell cultures isolated from XLP patients 13–18. Furthermore, it is plausible that the late CD4+ T cell help defect observed in SAP-deficient mice (that may also explain the hypogammaglobulinemia seen in XLP patients) is the effect of altered functions of SLAM and, perhaps, NTB-A, Ly-9 and CD84. Future experiments, including the generation of mice lacking the various members of the SLAM family, are required to test this model.
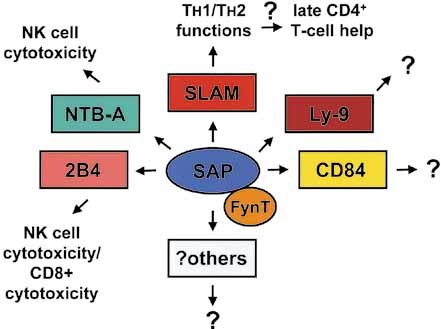
Pleiotropic effects of SAP are mediated (via FynT) by the SLAM-related receptors.
Acknowledgements
The author thanks the members of his laboratory for comments on the manuscript. Work in his laboratory is supported by the National Cancer Institute of Canada, the Canadian Institutes of Health Research, the CANVAC Network of Excellence and Valorisation Recherche Québec. The author is a Senior Scientist of the Canadian Institutes of Health Research, and holds the Canada Research Chair in Signalling in the Immune System.
- WILEY-VCH
- WILEY-VCH




