TGF-β1 modulates Fas (APO-1/CD95)-mediated apoptosis of human pre-B cell lines
Abstract
We have previously shown that Fas-induced apoptosis is markedly enhanced by IL-7 in human pre-B but not pro-B cell lines. In addition, pre-B cell receptor (pre-BCR) ligation significantly potentiates the IL-7 effects on Fas-triggered pre-B cell death. We show herein that transforming growth factor (TGF)-β1 sharply reduces Fas-induced death rate of pre-B but not pro-B cells. TGF-β1 causes inhibition of Fas-mediated disruption of mitochondrial transmembrane potential and cleavage of caspase 8, Bid and caspase 3. Bcl2 expression is markedly increased in TGF-β1-treated pre-B cells, whereas cellular FLICE-like inhibitory protein long (c-FLIPL), Bcl-XL, Bax, and Bad expression remains unchanged. TGF-β1 causes a selective growth arrest of pre-B cells in G0/G1 phase of the cell cycle and induces a partial down-modulation of both Fas and pre-BCR expression. All TGF-β1-mediated effects, but Bcl2 up-regulation, can be reproduced by the LY294002 phosphatidylinositol 3-kinase (PI3K)/Akt inhibitor but not by inhibitors of the MAPK/ERK (MEK) and Janus kinase (Jak)/STAT pathways, which promote cell death. Akt phosphorylation is strongly inhibited by TGF-β1 in pre-B but not pro-B cells and is not modified by Fas engagement. Altogether, our findings suggest that TGF-β1 prevents Fas-induced apoptosis of pre-B lines by inhibiting PI3K pathway and by enhancing expression of Bcl2. They also suggest that the PI3K/Akt pathway is involved in the control of Fas and pre-BCR expression, a checkpoint in B cell development.
Abbreviations:
-
- cFLIPL:
-
Cellular FLICE-like inhibitory protein long (splice form)
-
- Jak:
-
Janus kinase
-
- STAT:
-
Signal transducers and activators of transcription
-
- pre-BCR:
-
Pre-B cell receptor
-
- PI3K:
-
Phosphatidylinositol 3-kinase
-
- EMSA:
-
Electrophoretic mobility shift assay
-
- Δ Ψm:
-
Mitochondrial transmembrane potential
-
- μHC:
-
μ Heavy chain
1 Introduction
Fas (APO-1/CD95) is a member of the TNF receptor superfamily 1–3. Engagement of Fas leads to formation of the death-inducing signaling complex (DISC), whichstarts with the recruitment of the Fas-associated death domain-containing protein (FADD) followed by caspase 8 or its enzymatically inactive homologue, cellular FLICE-like inhibitory protein long (c-FLIPL) 1–5.
It is assumed that two major types of death pathways can be connected to the Fas receptor, and both of them lead to activation of caspase 8 and caspase 3, the initiator and the effector of programmed cell death, respectively 2, 6. In type 1 cells, cleavage of caspase 8 occurs at the level of DISC in large quantities, resulting in direct processing of caspase 3. This step is independent of mitochondrial activation, whereas in type 2 cells apoptosis proceeds through mitochondrial perturbations and involves the proteins Bid, Bax and Bak, which translocation to the outer mitochondrial membrane induces mitochondrial transmembrane potential (Δ Ψm) changes and leads to formation of apoptosome 6–8. In type 2 cells but not type 1 cells, Bcl2 overexpression blocks activation of both caspase 3 and caspase 8. Other mechanisms that do not involve DISC formation and activity can be used by Fas to induce cell death 9.
Fas is expressed on a large variety of cells including hematopoietic cells 1, 2. Expression and function of the Fas receptor depend on the state of cell activation and, in particular, on the costimuli provided to the cells on which Fas receptor has been engaged 10–14. Fas is involved in the homeostasis of the immune system, mainly by inducing cell death of activated B and T lymphocytes. However, its role in peripheral B cell development is uncertain 3, 15, 16. The role of Fas in the earliest steps of B lymphopoiesis is still unclear. Several studies have demonstrated that bone marrow immature B lymphocytes may express significant levels of surface Fas antigen 17, 18, but expression of Fas on unstimulated or activated B cell precursors has been poorly investigated and remains controversial. Recent studies suggested that Fas may play a role in the homeostasis of earliest stages of B cell development 19.
We have previously shown that programmed cell death of human pre-B cell lines can be triggered by cross-linking of membrane Fas protein and that IL-7 enhances Fas-induced apoptosis of pre-B but not pro-B cell lines 14. In addition, pre-B cell receptor (pre-BCR) ligation significantly potentiates IL-7 effects on Fas-triggered pre-B cell death. In this study, we addressed the issue on whether TGF-β1 might interfere with Fas signaling pathway in human pre-B cell lines. We demonstrate herein that TGF-β1 prevents Fas-mediated apoptosis of human neoplastic pre-B cells, but not pro-B cells, by inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and up-regulating Bcl2 expression.
2 Results
2.1 TGF-β1 abrogates anti-CD95-induced pre-B cell apoptosis
The Nalm6 and 697 cell lines were cultured for 48 h in culture medium supplemented or not with TGF-β1 (0.02–20 ng/ml), then stimulated or not with anti-CD95 (1 μg/ml) or isotype-matched control mAb (1 μg/ml) for 48 h. Cell viability was assessed by trypan blue dye exclusion, annexin V staining and in-situ end-labeling assay (not shown).
TGF-β1 (up to 20 ng/ml) did not alter the spontaneous rate of pre-B cell death (Fig. 1A). As previously reported 14, in the absence of TGF-β1, Fas ligation on 697 and Nalm6 pre-B cells caused the death of 25±6.5% and 36±4% of the cells, respectively. However, addition of TGF-β1 induced a dose-dependent inhibition of Fas-mediated pre-B cell death (Fig. 1A). A maximum rescue activity (16±2% and 6.1±1%, respectively) was reached at the concentration of 20 ng/ml. Enhancement of Fas-mediated apoptosis by IL-7 (10 ng/ml) was in a large part prevented by TGF-β1 at 20 ng/ml.
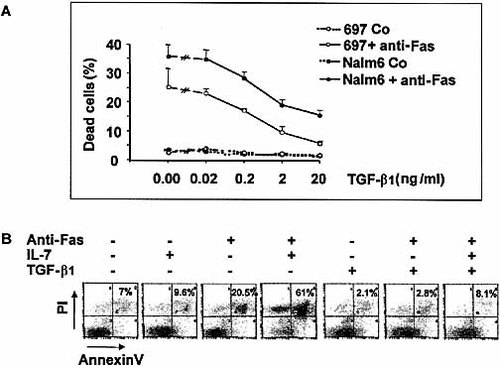
TGF-β1 inhibits Fas-mediated apoptosis of pre-B cells. (A) The 697 and Nalm6 cells were cultured for 48 h in the presence of increasing concentrations of TGF-β1 (0–20 ng/ml), then stimulated or not with anti-Fas (1 μg/ml) mAb for 48 h. Death cell rate was estimated by trypan blue dye exclusion. (B) The 697 cells were first cultured in the presence of medium, IL-7 (10 ng/ml), TGF-β1 (10 ng/ml) and IL-7 + TGF-β1 for 48 h, then incubated or not with anti-Fas (1 μg/ml) mAb for additional 48 h. Apoptotic cells were estimated by annexin V-FITC staining.
2.2 TGF-β1 inhibits pre-B cell proliferation and causes a growth arrest at the G0/G1 phase of cell cycle
TGF-β1 partially depressed DNA synthesis and cell proliferation of the two pre-B cell lines as assessed by [3H]dThd uptake (Fig. 2A for 697 cells) and cell counting (not shown). The inhibition of DNA synthesis was dose-dependent and could be only partly counteracted by IL-7. Incorporation of [3H]dThd was significantly higher in the presence of TGF-β1 + IL-7 than with TGF-β1 alone (Fig. 2A). FCM analysis of DNA content of 697 cells revealed that the relative number of cells in G0/G1 phase increased paralleling a diminution of the percentage of cells in S and G2+M phases after incubation with TGF-β1 for 48 h (Fig. 2B).
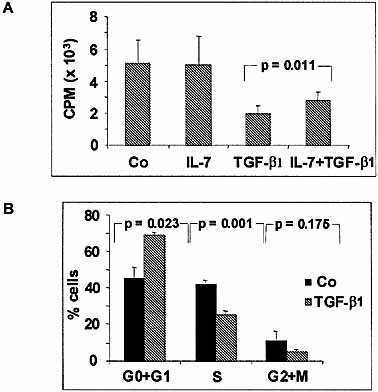
TGF-β1 inhibits pre-B cell growth and induces a blockade of the cell cycle. (A) The 697 cells were cultured in the presence or absence of IL-7 (10 ng/ml) and/or TGF-β1 (20 ng/ml) for 48 h, then pulsed with 1 μCi [3H]dThd for 6 h. (B) The 697 cells were cultured for 48 h in the presence of medium or TGF-β1 (20 ng/ml). Cell cycle was analyzed by measuring cellular DNA content.
2.3 TGF-β1 alters surface expression of Fas protein and pre-B cell receptor on pre-B cells
Cells cultured for 48 h in medium alone or in the presence of TGF-β1 were analyzed by FCM for surface expression of Fas, CD19, CD38, IL-7Rα and pre-BCR components (Fig. 3). TGF-β1 down-modulated surface expression of CD95 on the Nalm6 and 697 cells with a decrease of the mean fluorescence intensity about 59±20% (as compared to untreated cells). Surface expression of μ heavy chain (μHC), Igβ, and surrogate light chain was also decreased (about 80±5% for μHC) in a dose-dependent manner (not shown), whereas expression of CD19, IL-7Rα and CD38 (not shown) was unaffected by TGF-β1 treatment.
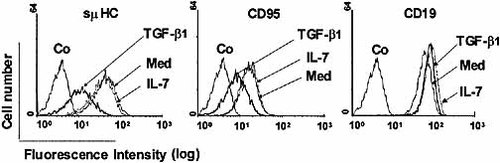
TGF-β1 down-modulates Fas and pre-BCR surface expression. The 697 cells were cultured for 48 h with medium, IL-7 or TGF-β1, incubated with purified anti-μHC, CD19 and Fas (CD95) mAb, then stained with anti-mouse PE-conjugated goat Ab. Stained cells were analyzed by FCM.
2.4 TGF-β1 alters the mitochondrial pathway of the Fas-mediated pre-B cell apoptosis
To determine whether Fas-mediated apoptosis proceeds through mitochondrial perturbations, we monitored the ability of Fas engagement to change the Δ Ψm by staining the 697 cells with the Δ Ψm-sensitive dye DiOC6(3) after incubation with culture medium or anti-Fas mAb (1 μg/ml) for 48 h. We found that the percentage of 697 cells exhibiting a low Δ Ψm (16.5%) was rapidly increased following Fas engagement (51.9%) and that prior incubation with TGF-β1 (20 ng/ml) completely abrogated the fall of Δ Ψm (21.4%) (Fig. 4A).
Caspase 8 cleavage was assessed by Western blotting using a specific mAb (Fig. 4B), whereas caspase 3 cleavage was assessed by a fluorimetric assay (Fig. 4E). Fas ligation on the 697 cells induced a slight cleavage of caspase 8, as shown by the generation of the p18 subunit, and activation of caspase 3. Fas-induced activation of both caspases 3 and 8 was strongly potentiated by IL-7. The Z-VAD large-spectrum inhibitor of caspases completely inhibited the Fas-mediated cell death and its potentiation with IL-7 (not shown). By contrast, treatment with TGF-β1 caused a marked inhibition of Fas-induced caspase 8 cleavage and caspase 3 activity, both of which were completely abrogated with 20 ng/ml of TGF-β1. In addition, TGF-β1 strongly prevented the enhancement of caspase 8 activation by IL-7. Expression of c-FLIPL, a caspase homologue that negatively regulates Fas activity, was unchanged in TGF-β1-stimulated cells as compared to unstimulated cells (Fig. 4C).
As caspase 8 may induce a cleavage of Bid protein, generating a truncated form of the molecule (tBid), we investigated herein by Western blot the capacity of Fas antigen to cleave Bid in the 697 cells (Fig. 4D). We found that Fas engagement induced generation of tBid (15 kDa), which was more abundant in IL-7-stimulated cells. By contrast, when cells were incubated for 48 h with TGF-β1, prior to Fas stimulation, expression of tBid returned to baseline levels. Intermediate levels of tBid were generated in cells incubated with both IL-7 and TGF-β1 as compared to IL-7- or TGF-β1-stimulated cells (Fig. 4D).
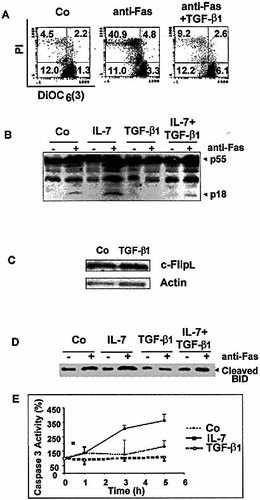
Effects of TGF-β1 on Fas-induced Δ Ψm disruption, caspase 8, c-FLIPL and Bid cleavage, and caspase 3 activation. (B) The 697 cells were incubated for 48 h with anti-Fas mAb (1 μg/ml) or with medium alone, then stained with the Δ Ψm-sensitive dye DiOC6(3) and PI. (B) Fas-induced caspase 8 cleavage was evaluated by Western blot using unstimulated and TGF-β1-(20 ng/ml) and/or IL-7- (10 ng/ml) stimulated 697 cells. (C) Expression of c-FLIPL was analyzed in unstimulated and TGF-β1– (20 ng/ml) stimulated 697 pre-B cells. (D) Fas-induced Bid cleavage was analyzed in unstimulated, IL-7- (10 ng/ml) and/or TGF-β1- (20 ng/ml) stimulated 697 cells. (E) Fas-induced caspase 3 activation was measured using a fluorimetric assay in unstimulated and TGF-β1- (20 ng/ml) or IL-7- (10 ng/ml) stimulated 697 cells.
2.5 TGF-β1 enhances expression of Bcl2 in pre-B cell lines
We then investigated the contribution of some members of the Bcl2 family proteins to the regulation of Fas-mediated cell death. We found that expression of Bcl2 (but not Bcl-XL, Bax and Bad) protein, as estimated by Western blot (Fig. 5A), was only increased in TGF-β1-treated pre-B cells and not in control cells.
Expression of Bcl2, Bcl-XL, and Bax transcripts was then analyzed using an RNase protection assay (Fig. 5B). Bcl2 mRNA were found to be expressed at low levels in untreated 697 and Nalm6 pre-B cells but were strongly enhanced (tenfold increase) in TGF-β1-treated pre-B cells and unchanged in IL-7-treated cells. Expression of Bcl-X transcripts (almost exclusively of the Bcl-XL type) was not affected by IL-7 nor by TGF-β1 treatment.
Incubation of pre-B cells for 48 h with TGF-β1 (20 ng/ml) and IL-7 (10 ng/ml) resulted in a fourfold and a 2.5-fold increase in Bax transcript levels, respectively. No significant quantitative variation of Mdm2 mRNA was noted in cells cultured with TGF-β1 (not shown).
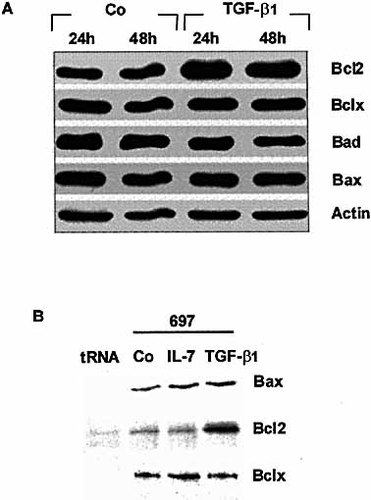
TGF-β1 up-regulates Bcl2 expression in 697 pre-B cells. (A) Lysates from 697 cells treated or not with TGF-β1 (20 ng/ml) were analyzed by Western blot for the expression of Bcl2, Bcl-XL, Bad and Bax, using specific Ab. (B) Unstimulated and TGF-β1-stimulated 697 cells were analyzed by RNase protection assay for the expression of Bcl2, Bcl-XL and Bax.
2.6 TGF-β1 inhibits PI3K/Akt activation
To identify the molecular mechanisms by which TGF-β1 modulates Fas expression and function, we examined the implication of three major signaling pathways, PI3K/Akt, Janus kinase (Jak)/Signal transducers and activators of transcription (STAT), and MAPK/ERK kinase (MEK), in the TGF-β1-mediated effects. For this purpose, cells were first cultured in the presence of specific pharmacological inhibitors (LY294002, AG490 and U0126, respectively) and tested for their sensitivity to Fas-induced apoptosis. The LY294002 PI3K/Akt inhibitor (10 μM) completely protected 697 pre-B cells from Fas-mediated cell death (Fig. 6A), whereas the AG490 (10 μM) Jak/STAT inhibitor and the U0126 (10 μM) MEK inhibitor enhanced Fas-mediated cell death, and did not abrogate the TGF-β1 protective effects. LY294002 down-modulated both pre-BCR and Fas surface expression but not CD19 expression, and this decrease was less important than with TGF-β1 (Fig. 6B). LY294002 also abrogated disruption of Fas-mediated Δ Ψm (Fig. 6C). However, it did not induce changes in Bcl2 expression (Fig. 6D). Altogether, these results suggest that the PI3K/ Akt pathway might be involved in the negative effects of TGF-β1 on Fas expression and function.
We therefore examined whether TGF-β1 might modulate phosphorylation of Akt in 697 cells. Cell extracts from 697 cells, stimulated or not with TGF-β1 for 5 min, 15 min, 1 h, 24 h (not shown) and 48 h, were analyzed by Western blot for Akt phosphorylation (Thr-308 and Ser-473) using specific Ab (Fig. 7A–C). We found that Akt was constitutively phosphorylated on both residues in 697 cells and that TGF-β1 induced a sharp decrease in the phosphorylation of the Thr-308 residue (Fig. 7A) and to a lesser extent of the Ser-473 residue (Fig. 7B). However, phosphorylation of Akt was not modified upon ligation of Fas antigen on the 697 pre-B cells for 3 h up to 24 h, indicating that PI3K is not required for Fas-mediated apoptosis of these cells (Fig. 7D).
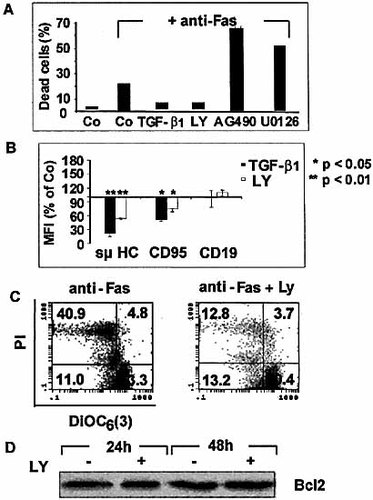
LY294002 reproduces some of TGF-β1 effects on pre-B cells. (A) Viability of 697 cells was assessed after their incubation with either control medium, LY294002 (10 μM), AG490 (10 μM) and U0126 (10 μM), followed by cross-linking of Fas using specific mAb (1 μg/ml). (B) The 697 cells were treated for 48 h with TGF-β1, LY294002 or culture medium and analyzed for expression of surface μHC, Fas and CD19. Results are expressed as variations for the mean fluorescence intensity (MFI) between treated and untreated cells (Co). (C) The 697 cells were incubated for 48 h with culture medium or LY294002, then with anti-Fas mAb (1 μg/ml) for additional 48 h, and stained with the Δ Ψm-sensitive dye DiOC6(3). (D) Expression of Bcl2 was estimated by Western blot in unstimulated and LY294002-stimulated 697 cells.
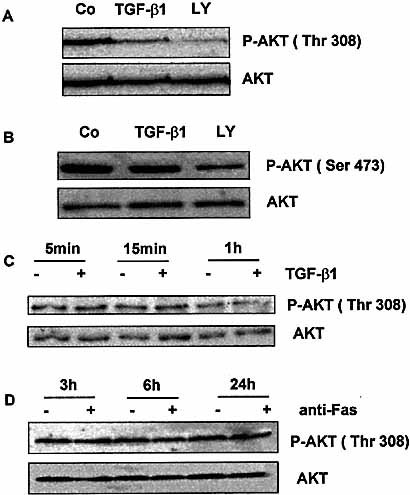
Effects of TFG-β1, LY294002 and Fas receptor ligation on Akt phosphorylation in 697 cells. Cells stimulated for 48 h or not with TGF-β1 (20 ng/ml) or LY294002 (10 μM) were analyzed by Western blot for the expression of phospho-Thr-308 (A) and phospho-Ser-473 Akt (B). Cells were incubated in the presence of TGF-β1 for 5 min, 15 min and 1 h and analyzed by Western blot for the expression of phospho-Thr-308 Akt (C). Cells were incubated in the presence of anti-Fas mAb for 3, 6 or 24 h and analyzed by Western blot for the expression of phospho-Thr-308 Akt (D). In all experiments, the blot was reprobed with an Ab that recognizes both phosphorylated and non-phosphorylated Akt to verify equal loading.
2.7 TGF-β1 did not alter Fas-mediated apoptosis of pro-B cell lines
We have previously shown that the Nalm16 and RS4; 11 pro-B cells express equal amounts of surface Fas molecule as compared to the Nalm6 and 697 pre-B cells, and that a fraction of these cells undergo apoptosis upon Fas cross-linking 14. However, IL-7 did not enhance Fas-mediated apoptosis of pro-B cells, though they express comparable amounts of surface CD127 14. We show herein that TGF-β1 (up to 20 ng/ml) was unable to prevent Fas-induced apoptosis of pro-B cells (Fig. 8A), to affect pro-B cell growth (18±4% decrease, p=0.17 for Nalm16 cells) and cell cycle, and to inhibit phosphorylation of Akt (Fig. 8B).
To explain the discrepancies between the responses of pro-B and pre-B cells to TGF-β1, we tested the hypothesis that they could be due to a differential capacity to activate TGF-β1 signaling pathway. It has previously been demonstrated that TGF-β1 directly binds type 2 TGF-βR (TGF-βR2) unit which recruits TGF-βR1, resulting in the activation of a signal transduction cascade initiated by Smad2 and Smad3 molecules which then associate with Smad4 for nuclear translocation 20. We therefore compared the ability of TGF-β1 to activate Smad3 and Smad4 molecules in pro-B and pre-B cells. Whole-cell extracts from IL-7- (used as a control) and TGF-β1-stimulated 697 (pre-B) and Nalm16 (pro-B) cells were analyzed by electrophoretic mobility shift assay (EMSA) with the specific Smad binding sequence from the plasminogen activator inhibitor type 1 (PAI-1) gene as probe (Fig. 8C). DNA binding complexes were only detected after TGF-β1 but not following IL-7 stimulation of the 697 pre-B and the Nalm16 pro-B cells. To identify these complexes, we employed specific Ab raised against Smad proteins which elicited a supershift of these complexes in both cell lines.
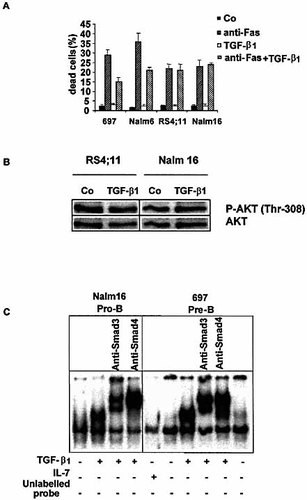
Comparative analysis of TFG-β1 effects on pre-B and pro-B cells. (A) The 697 and Nalm6 pre-B cells and the RS4;11 and Nalm16 pro-B cells were first cultured in the presence of medium or TGF-β1 (20 ng/ml) for 48 h, then incubated or not with anti-Fas (1 μg/ml) mAb for additional 48 h. Dead cells were estimated by trypan blue exclusion. (B) Pro-B cells stimulated for 48 h or not with TGF-β1 (20 ng/ml) were analyzed by Western blot for the expression of phospho-Thr-308 Akt. The blot was reprobed with an Ab that recognizes both phosphorylated and non-phosphorylated Akt to verify equal loading. (C) Whole-cell extracts from IL-7- (used as a control) and TGF-β1-stimulated 697 (pre-B) and Nalm16 (pro-B) cells were analyzed by EMSA using a probe containing a specific Smad binding sequence. DNA binding complexes were only detected after TGF-β1 (20 ng/ml) but not after IL-7 (10 ng/ml) stimulation of the 697 pre-B and the Nalm16 pro-B cells. Anti-Smad3 and -Smad4 Ab elicited a supershift of these complexes.
3 Discussion
We have previously shown that engagement of Fas triggers apoptosis of a fraction of neoplastic human pre-B cells, and that Fas-induced apoptosis can be enhanced by IL-7 in pre-B but not pro-B cells 14. In addition, IL-7 effects were found to be potentiated by pre-BCR cross-linking. In the present work we aimed to examine whether TGF-β1 may alter Fas-mediated apoptosis in such cells. We show herein that TGF-β1 does not affect the survival of unstimulated pre-B cells, but protects these cells from the Fas-mediated apoptosis. We also show that this cytokine can abolish the enhancement of Fas-mediated apoptosis by IL-7; and that in contrast to IL-7, TGF-β1 effects are not affected by pre-BCR cross-linking (not shown).
TGF-β1 is a pleiotropic cytokine that usually exerts inhibitory effects on mesenchymal cells, including hematopoietic stem and progenitor cells, and induces apoptosis of a large variety of cells 21, 22. However, this cytokine can also protect various malignant and normal cells, including T lymphocytes, from apoptosis, mediated or not by Fas 23. Consistent with these findings, in TGF-β1–/– mice both thymic and peripheral T cell apoptosis is increased, and engagement of TCR, Fas or TNF-α receptor enhances this aberrant T cell apoptosis. It has been suggested that in thymocytes TGF-β1 maintains the integrity of mitochondria without interfering with Fas or Bcl2 expression 23. We demonstrate herein that TGF-β1 abrogates Fas-induced Δ Ψm disruption, causes inhibition of both Fas-mediated caspase 8 and Bid cleavage, and significantly enhances Bcl2 transcript and protein expression in 697 pre-B cells. Although stimulation with TGF-β1 results in a fourfold increase in Bax transcript levels, it does not induce detectable changes in Bax protein expression, neither affects Bad nor Bcl-XL expression. Altogether these findings suggest that TGF-β1 abrogates the pre-mitochondrial steps of type 2 Fas death pathway, and thereby inhibits Fas-mediated caspase 3 cleavage and Fas-induced cell death.
In microglia, TGF-β1 interferes with the earliest steps of Fas receptor signaling by a mitogen-activated protein kinase (MAPK) kinase (MKK)-dependent induction of c-FLIPL expression 24. In our pre-B cell lines, neither the MAPK nor the Jak/STAT pathways seem to be involved in the TGF-β1 protective effects. By contrast, incubation of pre-B cells with TGF-β1 strongly inhibits Akt phosphorylation, and most of TGF-β1 effects described herein can be reproduced by the LY294002 Akt inhibitor. Both TGF-β1 and LY294002 abolish Fas-induced apoptosis andΔ Ψm disruption in leukemic pre-B cells. They also cause a cell growth arrest at the G0/G1 phase of the cell cycle as well as a partial down-modulation of Fas antigen expression. In addition and unexpectedly, incubation with either TGF-β1 or LY294002 causes a partial down-modulation of surface pre-BCR but not of CD19, CD38 or CD127.
PI3K is implicated in the growth and survival of a wide range of cell types 25. Akt typically enhances cell survival by interfering with Forkhead, Bim, Bad, Bax, caspase 9 and/or NF-κB activities 26–30, and in most cases, the PI3K/Akt pathway is considered to be dispensable to the Fas-mediated apoptosis 31, 32. Some studies suggest that this pathway can negatively modulate Fas receptor signaling in various cells 33–39 by up-regulating c-FLIP expression or by activating NF-κB. However, in some cells such as neutrophils, PI3K activation seems to be required for Fas-mediated apoptosis 40. We found herein that Akt was not involved in Fas-induced pre-B cell death and that c-FLIP was not implicated in the TGF-β1 protective effects. The mechanisms by which TGF-β1 inhibits Akt activation and results in resistance to Fas need to be elucidated.
Fas antigen down-modulation caused by TGF-β 1 or LY294002 might also play a role in their protective effects, although surface Fas expression was only partially reduced on pre-B cells. Inhibitory effects of TGF-β1 on Fas expression have previously been described in neoplastic cells 41. In a more recent work, the PI3K/Akt pathway was shown to up-regulate Fas receptor expression on human tumors and to induce sensitization of malignant cells to Fas ligand-mediated apoptosis by inhibition of PI3K effects on cooperation between c-jun and STAT3, which is required for silencing Fas promoter 42.
That Bcl2 expression was up-regulated by TGF-β1 but not LY294002, suggests that some of the TGF-β1 protective effects of pre-B cells were not due to inhibition of PI3K/Akt. Further studies are required to determine the respective roles of the PI3K-dependent and -independent mechanisms, by which TGF-β1 protects from Fas-induced pre-B cell death.
Strinkingly, TGF-β1 protective effects were not observed with pro-B cell lines. This is reminiscent of the differential response of Fas-stimulated pre-B and pro-B cells to IL-7 14. We found that TGF-βR1 and TGF-βR2 were similarly expressed on all four pro-B and pre-B cell lines studied herein (not shown) and exhibited similar capabilities to activate Smad3 and Smad4. Interestingly, TGF-β1 was not able to inhibit Akt phosphorylation in both Nalm16 and RS4;11 pro-B cells, strengthening the idea that inhibition of the PI3K/Akt pathway plays a major role in the TGF-β1 protective effects against Fas-mediated apoptosis. This indicates that downstream factors or unidentified Smad-independent mechanisms might be involved in the discrepancies between both types of cells.
Some recent studies indicate that TGF-β1 significantly enhances the survival of hematopoietic precursor cells 43, 44 and protects them from Fas-mediatedapoptosis, presumably by enhancing Bcl2 expression, which is reminiscent of our findings in pre-B cell lines. This strongly suggests that the sensitivity of hematopoietic and B cell progenitors to Fas engagement is finely tuned by various cytokines and highly depends on the state of cell activation, as it was also reported with more mature lymphocytes.
Our data indicate that TGF-β1 might also regulate surface expression of pre-BCR, a checkpoint in B cell development 45, and that this effect could be partly mediated viainhibition of Akt. Conversely, PI3K/Akt is known to be activated upon engagement of pre-BCR 46 or other receptors involved in B lymphopoiesis, such as IL-7R 47, highlighting the critical role of PI3K/Akt in the earliest steps of B lymphopoiesis 48. We believe that the use of our cell lines would be helpful to analyze many effects mediated by TGF-β1 in pre-B cells.
4 Materials and methods
4.1 Cell lines and culture conditions
The human pro-B cell lines Nalm16 and RS4;11 and the human pre-B cell lines 697 and Nalm6 49 were cultured as previously 14. For viability and proliferation studies, triplicate samples of 2×105 cells were cultured overnight in flat-bottom well microculture plates (Becton Dickinson, Mountain View, CA). In other experiments cells were cultured in the presence of LY294002 (Sigma), AG490, and U0126.
4.2 Reagents
The following Ab were used: anti-CD19 (BC3; a kind gift of Dr. Wigdenes, Innothérapie, Besançon, France); anti-human μHC (SA-DA 4) and anti-Igβ (CB3–1; Southern Biotechnology Associate, Birmingham, AL); anti-surrogate light chains (SLC1 49); anti-APO-1 (a kind gift of Dr. P. Krammer, Heidelberg, Germany); anti-Fas/CD95 (CH11); anti-human IL-7R/CD127 (R34,34);anti-CD38 (T16 or LS198–4–3; Immunotech, Marseille, France); anti-phospho-Akt (Thr-308, Ser-473) and anti-Bid (Cell Signaling, Beverly, MA); anti-c-FLIPL (Calbiochem), anti-caspase 8 (Immunotech); isotype-matched control mAb included anti-chicken CD4 (CT4) and anti-chicken MHC class II 50; anti-Bcl2 (Zymed, San Francisco, CA), anti-Bad (Transduction Lab, Lexington, KY ), anti-Bax and anti-Bcl-XL; goat anti-actin Ab, anti-Smad3 and anti-Smad4, anti-TGF-βR1 (V-22) and anti-TGF-βR2 (H-567) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-conjugated Ab specific for rabbit or mouse IgG were from Amersham Pharmacia Biotech (GB) and for goat IgG from Jackson ImmunoResearch Laboratories (PA). PE-conjugated goat anti-mouse Ig was obtained from Dako (Trappes, France). IL-7 and TGF-β1 were purchased from Innotest (Besançon, France).
4.3 Flow cytometry
Cell surface antigen analysis was performed as previously described 14. Stained cells were analyzed by FCM (EPICS Elite Cytometer, Coulter Corp.). For measurement of cellular DNA content, 106 cells were washed in cold PBS/5 mM EDTA and fixed in 1 ml of a mixture of 70% ethanol-PBS at –20°C for 15 min. After centrifugation, cells were incubated for 30 min at room temperature with RNase A (Roche Diagnostics; 10 mg/ml) and propidium iodide (PI; Sigma, France; 100 μg/ml). Samples were analyzed using the Cychred sofware (University of Wales College of Medicine, Cardiff, Wales).
Annexin V-FITC staining was performed according to the manufacturer's instructions (Boehringer, Mannheim, Germany). The percentage of apoptotic cells was analyzed by FCM. For Δ Ψmanalysis, cells were washed in PBS/5 mM EDTA, incubated for 20 min with 40 nM DiOC6(3) (Calbiochem, France) and 5 μg/ml of PI at room temperature, and then immediately analyzed by FCM.
4.4 [3H]dThd incorporation assays
Cells were cultured in flat-bottom plates in culture medium supplemented or not with IL-7 (10 ng/ml) or TGF-β1 (20 ng/ml), for 48 h. Six hours of pulse with 1 μCi per well of [3H]dThd (Amersham Pharmacia Biotech) were subsequently applied.
4.5 Western blot
Cells (106) were lysed in Laemmli's buffer (30 μl) and 20 μl of whole lysates were resolved by either SDS-10%-PAGE or SDS-12%-PAGE, blotted onto nitrocellulose membrane (Bio-Rad, France), and probed with various Ab. The blots were developed with the ECL chemiluminescence system (Amersham Pharmacia Biotech) using specific peroxidase-conjugated anti-IgG Ab.
4.6 Caspase 3 assays
The enzymatic activity was measured with a fluorogenic substrate as previously described 51.
4.7 RNase protection assay
bax, bcl2, and bcl-x cDNA or segments of the respective genes as well as control human gadph cDNA were inserted into Bluescript vectors. Labeled antisense RNA were synthesized by T3, SP6 or T7RNA polymerases using [32P]UTP and purified on polyacrylamide gels. RNA probes (5×105 cpm) were hybridized during 12 h at 45°C with 10 μg total RNA from B cell lines or 10 μg tRNA as control. These mixtures were digested with RNase T1 and RNase A (30 min, 30°C), then with proteinase K (30 min, 37°C), extracted with phenol-chloroform and ethanol-precipitated. Recovered RNA were analyzed on 6% or 8% acrylamide/7 M urea gels. On autoradiographic images, the intensity of the protected bands was monitored using the Bioimage® IQ program. Alternatively, phosphorimager files were analyzed using the Molecular Dynamics ImageQuaNT software.
4.8 Electrophoretic mobility shift assay
Whole-cell extracts were prepared as previously described 52. The specific smad binding site from the PAI-1 gene promoter (5′-TCGAGAGCCAGACAAGGAGCCAGACAAGCAGCCAGACAC-3′) was end-labeled with polynucleotide kinase and used as a probe. Anti-Smad3 and -Smad4 specific Ab were used in band shift experiments.
4.9 Statistical analysis
The paired Student's t-test was used for statistical analysis.
Acknowledgements
We wish to thank B. Arnulf for the gift of PAI-1 probe. This work was supported by grants from ARC (Association pour la Recherche contre le Cancer), FRM (Fondation pour la recherche Médicale), FDF (Fondation de France) and ARES-VERRE.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




