Localization of peptide/MHC class II complexes in macrophages following antigen processing of viable Streptococcus pyogenes
Abstract
The subcellular localization of peptide/MHC complexes was investigated during processing of the surface M5 protein from Streptococcus pyogenes. Bone marrow-derived macrophages were pulsed with viable S. pyogenes for 20 min followed by various periods of chase. T hybridoma cells detected complexes of one epitope, M517–31 with Ed on the surface of macrophages within 30 min of chase. In contrast, complexes with another epitope, M5308–319 with Ad peaked later. Intracellular localization of peptide/MHC-II complexes was studied by subcellular fractionation and detection of complexes in fractions by T hybridoma cells. M517–31/Ed complexes were detected in light membrane fractions containing plasma membrane and early endosomes by 10–30 min. M5308–319/Ad complexes were detected in these light membranes after 3 h of chase. Thus, the time course of M5308–319/Ad presentation was delayed relative to M517–31/Ed. However, neither type of complex was detected at any time in fractions containing phagosomes. Both species of peptide/MHC complexes localized to endocytic compartments, indicating a role for endosomes in presentation of antigens from phagocytosed bacteria.
Abbreviations:
-
- HRP:
-
Horseradish peroxidase
-
- MIIC:
-
MHC class II compartment
1 Introduction
Newly-synthesized class II major histocompatibility (MHC-II) molecules present antigenic peptides to CD4+ T cells following uptake and proteolytic degradation of soluble antigen in late endosomal/lysosomal compartments constituting the classical MHC-II antigen presentation pathway 1–3. In this pathway, trafficking and peptide-loading of MHC-II molecules is controlled by invariant chain and H2-DM 4. Several studies suggest that antigenic peptides can also bind MHC-II molecules recycled from the cell-surface in early endosomes ina recycling MHC-II antigen presentation pathway, which is less dependent on invariant chain and H2-DM 5, 6.
Particulate antigens are internalized by phagocytosis and processed for presentation by MHC-II molecules. Phagosomes contribute to antigen processing 7, but it is still unclear whether partially processed antigen can also be transferred from phagosomes to endocytic compartments for additional processing and formation of peptide/MHC-II complexes. Previous studies have analyzed phagocytosis leading to antigen processing of ovalbumin (OVA)-coupled latex beads, erythrocytes, necrotic and apoptotic cells, and microbial pathogens such as Mycobacterium tuberculosis, Escherichia coli, Salmonella typhimurium, Leishmania donovani, Listeria monocytogenes or Streptococcus gordonii, which are internalized by macrophages 2 ,7–14 and dendritic cells 15, 16. In experiments with OVA-coupled latex beads and Mycobacterium tuberculosis, T cell hybridomas were used to detect specific peptide/MHC-II complexes in subcellular fractions 7, 13. Peptide/MHC-II complexes appeared initially in phagosomes and subsequently on the plasma membrane, but were not found within endosomal compartments including MHC class II compartment (MIIC), within 30 min of phagocytosis 13. However, the localization of peptide/MHC-II complexes after longer periods of bacterial antigen processing was not studied, and it is not clear whether all phagocytosed pathogens are processed in the same way.
In this report, we used kinetic and subcellular fractionation techniques to identify endosomal and phagosomal compartments engaged in processing of two epitopes of the streptococcal surface M protein from viable Streptococcus pyogenes in mouse macrophages. Peptide/MHC-II complexes were detected in light membrane fractions corresponding to plasma membranes and early endosomes and dense fractions containing late endocytic compartments and MIIC, but not in phagosomal fractions. The results suggest that streptococcal M proteins or peptide/MHC-II complexes are transported from phagosomes to plasma membrane or endosomes in the course of antigen presentation.
2 Results
2.1 Kinetics of expression of M5-specific peptide/MHC-II complexes at the macrophage surface
Following our previous demonstration that M517–31/Ed and M5308–319/Ad complexes are generated by different mechanisms during processing of viable S. pyogenes 17–20, we assessed the kinetics of cell-surface expression of these epitopes. Macrophages were pulsed with S. pyogenes for 20 min, washed and chased for various periods, fixed and incubated with T hybridoma cells to detect surface expression of streptococcal peptide/MHC-II complexes. M517–31/Ed complexes appeared rapidly, peaked at 30 min of chase, rapidly declined in expression from 30 min to 5 h and were not detected at 6 h (Fig. 1). In contrast, M5308–319/Ad complexes appeared more slowly, peaking at 2–3 h after bacterial challenge, and then declined from 3 to 7 h of chase (Fig. 1). Thus, M517–31/Ed and M5308–319/Ad complexes were processed and expressed with different kinetics, with M517–31/Ed complexes exhibiting rapid expression and decay, and M5308–319/Ad complexes exhibiting slower expression and decay.
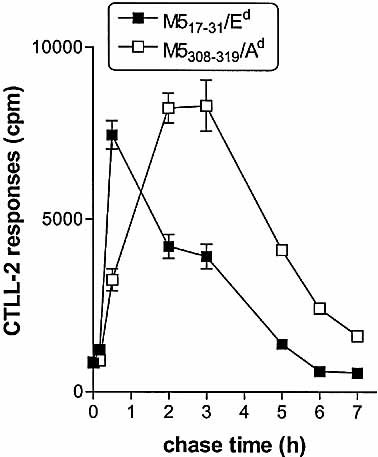
Presentation of streptococcal M5-specific T cell epitopes on the cell surface of bone-marrow macrophages in whole-cell antigen presentation assay. IFN-γ-stimulated macrophages were pulsed with S. pyogenes for 20 min, chased for the times shown, fixed and incubated with HX17 T hybridoma cells (specific for M517–31, closed symbols) or HY2 T hybridoma cells (specific for M5308–319, empty symbols). IL-2 production by T cell hybridomas was tested by CTLL-2 [3H]thymidine incorporation assay, and the results are presented as mean cpm ± SEM.
2.2 Detection of endosomal and phagosomal compartments in Percoll fractions
Subcellular fractionation was used to study the compartments involved in processing of different M5 epitopes after phagocytosis of viable S. pyogenes. Macrophages were homogenized and post-nuclear membranes subjected to subcellular fractionation on 27% Percoll gradients. The resulting fractions were analyzed for markers of plasma membrane, endosomes and phagosomes. Previous studies have shown that fractions 8–13 were the light membrane fractions containing both plasma membranes (detected by radioactive labeling of cell surface MHC-II molecules) and early endosomes 6, 13. In this study the light membrane fractions were identified by the position of early endosomes, which were labeled by incubating macrophages with horseradish peroxidase (HRP) for 5 min. HRP activity was detected in fractions 8–13 (Fig. 2) 21. To identify fractions containing lysosomes and phagolysosomes, IFN-γ-stimulated macrophages were pulsed for 20 min with viable S. pyogenes and chased for various chase periods. Lysosomal enzyme distribution was identified by measuring the activity of β-hexosaminidase 22, which appeared in fractions 23–30 (Fig. 2B). To identify the position of phagosomes, macrophages were pulsed with FLUOS-labeled streptococci for 20 min, washed, chased for various periods and fractionated. Subcellular fractions were tested by fluorometry to detect phagosomal compartments containing fluorescent bacteria, which were consistently detected in dense fractions (26–30) (Fig. 2C).
Macrophages incubated with soluble antigen have been shown to accumulate specific peptide/MHC-II complexes in endosomes (including MIIC) and on the plasma membrane 6. To detect the distribution of endocytic antigen processing compartments, IFN-γ-stimulated macrophages were pulsed with soluble OVA for 1.5 h and fractionated. Subcellular fractions were incubated with DOBW T hybridoma cells. DOBW cells detected the presence of OVA323–339/Ad complexes in fractions 8–13 (containing plasma membrane and early endosomes) and 15–23 (containing late endocytic compartments, e.g. MIIC) (Fig. 2D). These results are consistent with previous findings showing these compartments localized to the same regions of the Percoll gradients 6, 13. Thus, late endocytic compartments (MIIC) are localized to fractions 15–23, distinct from the distribution of phagosomes (fractions 26–30) and light membranes (plasma membrane and early endosomes, fractions 8–13).
To localize the distribution of MHC-II molecules available to load peptides within Percoll gradients, fractions were incubated with the OVA peptide p323–339 and assayed for antigen presentation with the DOBW T hybridoma (Fig. 2D). The data suggest that MHC-II molecules capable of binding the peptide were present in light membrane fractions corresponding to plasma membranes and early endosomes, as well as in dense membrane fractions that included late endosomes, MIIC and lysosomes.
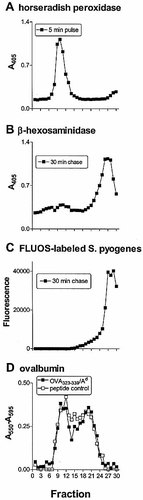
Characterization of subcellular compartments after Percoll density gradient centrifugation. (A) Early endosomal compartments were detected following incubation of macrophages with HRP for 5 min. (B) Macrophages were pulsed with S. pyogenes for 20 min, followed by a chase incubation of 30 min (data for other chase periods not shown). β-hexosaminidase activity was used as a marker for lysosomes. (C) Macrophages were incubated with FLUOS-labeled streptococci for 20 min followed by a chase incubation of 30 min (data for other chase periods not shown). Fractions containing phagosomes were localized by fluorometry. (D) Localization of antigen-presenting compartments after subcellular fractionation of macrophages. Macrophages were incubated with OVA (3 mg/ml) for 1.5 h, fractionated and assayed directly with OVA332–339-specific DOBW T cell hybridoma cells (filled symbols). In control experiments, peptide p323–339 was added to all fractions (empty symbols). Antigen-specific IL-2 responses were measured by a spectrophotometric CTLL-2 assay using Alamar Blue (results plotted as the difference in absorbance between A550 and A595 nm). Background absorbance of 27% Percoll solution was 0.03±0.02.
2.3 Subcellular distribution of M517–31/Ed complexes after challenge with S. pyogenes
To study the production and subcellular distribution of peptide/MHC-II complexes, macrophages were pulsed with viable S. pyogenes for various periods and fractionated. T cell hybridoma HX17 was used to detect M517–31/ Ed complexes in subcellular fractions. No complexes were detected immediately after 20 min of pulse (0-min chase, Fig. 3A), but after 10 min of chase M517–31/Ed complexes were detected at low levels in light membranes (fractions 7–13), with a marginal signal in late endocytic fractions (Fig. 3B). M517–31/Ed complexes increased in light membrane fractions to a peak by 30 min, consistent with transport of complexes to the plasma membrane (Fig. 3C), and thereafter stabilized or declined slowly (Fig. 3D). The level of presentation by later endocytic fractions, however, remained at very low levels at all time points (Fig. 3B–D).
In contrast to findings in other systems 7, 13, 23, M517–31/Ed complexes were not detected in phagosomal fractions (fractions 26–30), suggesting that M517–31/Ed complexes were not formed in phagosomes but were formed in endocytic compartments after export of antigen from S. pyogenes-containing phagosomes. It is possible that early endosomes contributed to the formation of M517–31/Ed complexes, since these complexes were detected in abundance in light membrane fractions (containing early endosomes and plasma membrane), but were never present at more than minimal levels in late endocytic fractions.
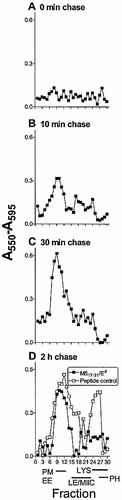
Localization of M517–31/Ed complexes in subcellular fractions. Macrophages were pulsed with viable S. pyogenes for 20 min and chased for 0 min (A), 10 min (B), 30 min (C) and 2 h (D). Fractions were directly assayed with the M517–31-specific HX17 T cell hybridoma (filled symbols). In control experiments, peptide p14–33 was added to all fractions (empty symbols). At the bottom of the figure, bars indicate fractions containing plasma membranes (PM), early endosomes (EE), late endosomes (LE), MHC class II compartments (MIIC), lysosomes (LYS) and phagosomes (PH), as identified in Fig. 2. Background = 0.07±0.03.
2.4 Subcellular distribution of M5308–319/Ad complexes after challenge with S. pyogenes
The distribution of M5308–319/Ad complexes was similarly assessed after incubation of macrophages with viable S. pyogenes for 20 min followed by various chase periods. HY2 T hybridoma cells did not detect M5308–319/Ad complexes at significant levels in fractions until 3 h of chase incubation, when these complexes appeared in light membrane fractions (7–13, corresponding to plasma membranes/early endosomes) (Fig. 4). Dense fractions (15–23, containing late endocytic compartments and MIIC) stimulated little or no specific T cell response, although M5308–319/Ad complexes did appear in some assays at late time points (e.g. 5 h of chase, data not shown), and may have been expressed below the sensitivity of our assay. At no time point were M5308–319/Ad complexes detected in fractions 26–30, corresponding to phagosomal compartments, suggesting that M5308–319/ Ad complexes were not formed in phagosomes.
The distribution of MHC-II molecules available to load peptides in macrophages that have phagocytosed bacteria was studied by incubating fractions with exogenous synthetic peptides p14–33 and p300–319 and measuring antigen presentation with T hybridoma cells (Fig. 3D and 4C). The data shows that MHC-II molecules were available for peptide loading throughout the endosomal pathway, but not in phagosomes.
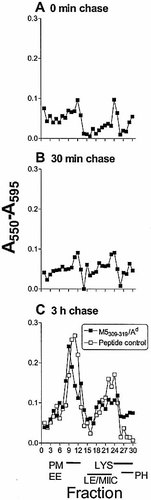
Localization of M5308–319/Ad complexes in subcellular fractions. Macrophages were pulsed with viable S. pyogenes for 20 min and chased for 0 min (A), 30 min (B), 3 h (C). Fractions were directly assayed with the M5308–319-specific HY2 T cell hybridoma (filled symbols). In control experiments, peptide p300–319 was added to all fractions (empty symbols).. The identity of the fractions is shown as described in Fig. 3. Background = 0.04±0.01.
2.5 Subcellular distribution of MHC-II molecules
The distribution of MHC-II molecules within subcellular fractions from macrophages with or without bacterial challenge was determined by ELISA (Fig. 5). The data shows that MHC-II molecules were detected throughout the endosomal system (fractions 5–30) in untreated macrophages, consistent with the data shown in Fig. 2D. However, in macrophages pulse-chased with S. pyogenes for 3 h, MHC-II molecules were found predominantly in light membrane fractions corresponding to plasma membranes and early endosomes and at lower levels in dense fractions including those containing phagosomes.
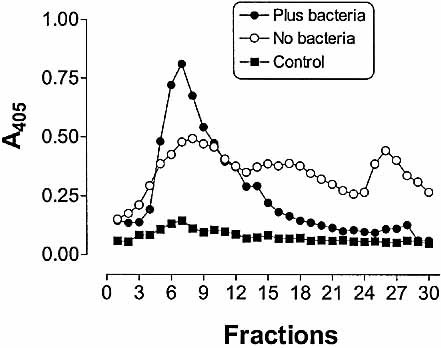
Distribution of MHC-II molecules in macrophages. Macrophages were not pulsed (empty circles) or pulsed (filled circles) with viable S. pyogenes for 20 min and chased for 3 h. Fractions were assayed for the presence of MHC-II molecules by ELISA using anti MHC class II-specific mAb. Fractions from macrophages not pulsed with S. pyogenes were also treated with normal rat serum instead of anti MHC class II-specific mAb (filled squares) as a specificity control.
3 Discussion
We have previously shown that processing of the CD4 T cell epitope M517–31, unlike M5308–319, from viable S. pyogenes was independent of newly-synthesized MHC-II molecules, phagosomal/endosomal acidification and the activity of lysosomal cysteine and aspartic proteinases 19. These results suggested that M517–31/Ed and M5308–319/Ad complexes were generated by the recycling and classical MHC-II pathways, respectively 17, 19. In the present study, presentation of M517–31 from bacteria occurred within 30 min after bacterial challenge, as manifested by the appearance of M517–31/Ed complexes on the cell surface. In contrast, initial appearance of M5308–319/Ad complexes was detected at later time points. These kinetic observations are consistent with our previous results showing processing of the streptococcal M5-specific T cell epitopes by distinct MHC-II antigen-processing mechanisms 17.
Subcellular fractionation of bone-marrow macrophages was applied to detect the localization of M517–31/Ed and M5308–319/Ad complexes following phagosomal processing of Streptococcus pyogenes. Specifically, we studied the kinetics of appearance and subcellular localization of M5308–319/Ad and M517–31/Ed complexes. After phagocytosis of viable S. pyogenes, M517–31/Ed complexes were detected in light membrane fractions (containing plasma membrane/early endosomes) with a marginalsignal in dense fractions (containing late endosomes/MIIC) by 10 min of chase, and by 30 min were at peak level in light membranes with low expression in dense fractions. In contrast, M5308–319/Ad complexes were detected in light membranes by 3 h of chase. Neither M517–31/Ed nor M5308–319/Ad complexes were detected in phagosomal fractions at any time studied, which is consistent with our finding that MHC-II molecules were not detected at substantial levels in phagosomal compartments by ELISA or T cell assay. This observation suggests that peptide/MHC-II complexes may have been generated after export of M5 protein or fragments of M5 protein from phagosomes to endocytic antigen processing compartments.
The present study further develops our understanding of phagosomal processing of bacteria for MHC-II presentation. Latex-bead phagosomes were recently shown to be fully competent antigen presenting compartments 13. Flow organellometry and subcellular fractionation revealed that latex-bead phagosomes acquire MHC-II molecules, invariant chain and DM in the course of antigen processing of particulate antigens 7. Approximately two thirds of phagosomal MHC-II was shown to be newly synthesized and one third recycled from the cell surface, and phagosomal antigen processing was shown to engage primarily newly-synthesized MHC-II molecules following phagocytosis of antigen-coupled latex beads 23. During processing of OVA-coupled latex beads, OVA323–339/Ad complexes were generated directly within phagosomes detected by T hybridoma responses to subcellular fractions 7. Also, upon infectionof macrophages with Mycobacteria tuberculosis, Mycobacteria-specific peptide/MHC-II complexes were formed initially in phagosomes and subsequently transported to the plasma membrane. Mycobacteria-specific peptide/MHC-II complexes were not identified in dense membrane fractions containing late endosomes and MIIC at any time during the study period of 30 min 13. Collectively, these data suggested that phagosomes can process particulate antigens, mediate formation of complexes of processed peptides predominantly with newly-synthesized MHC-II molecules, and facilitate their transport to the plasma membrane.
The data reported here suggest that S. pyogenes-containing phagosomes differ from phagosomes containing latex beads or M. tuberculosis in that they contain lower levels of MHC-II molecules or MHC-II/peptide complexes. In our case it was shown that endocytic compartments contributed to the formation of peptide/MHC-II complexes during bacterial processing. S. pyogenes M5-specific peptide/MHC-II complexes localized to endosomal compartments, but, unlike Mycobacteria-specific peptide/MHC-II complexes, were not detected in phagosomes. The ability of M. tuberculosis to block transport between phagosomes and late endocytic compartments 13, 24, 25 may cause Mycobacteria-specificpeptide/MHC-II complexes to accumulate in phagosomes and decrease the transfer of either mycobacterial antigen or peptide/MHC-II complexes to endocytic compartments. However, published studies of subcellular distribution of peptide/MHC-II complexes during M. tuberculosis processing examined only much earlier time points, and unpublished observations (L. Ramachandra) indicate that M. tuberculosis–specific peptide/MHC-II complexes do appear in endocytic/MIIC fractions at later time points. During processing of S. pyogenes streptococcal M proteins or antigen/MHC-II complexes formed during phagosomal processing may be rapidly transported from phagosomes to plasma membranes or early endocytic compartments. If peptide/MHC-II complexes are formed in phagosomesin this system, their rapid transfer to plasma membrane or endocytic compartments may render them undetectable in phagosomes under our experimental conditions. Alternatively, the ability to transfer antigens from S. pyogenes phagosomes to endocytic compartments may allow endocytic compartments to contribute to processing of these antigens and formation of peptide/MHC-II complexes.
4 Materials and methods
4.1 Antigens
Manfredo strain of S. pyogenes was grown overnight in Todd-Hewitt medium, resuspended in PBS and the concentration adjusted spectrophotometrically to 3×108/ml (A600=0.6). In some experiments, bacteria were labeled with 5(6)-carboxyfluorescein-N-hydroxysuccinimide ester (FLUOS, Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. Synthetic peptides p14–33 (14KEALDKYELENHDLKTKNEG33) and p300–319 (300DASREAKKQVEKALEEANSK319) contain epitopes M517–31 and M5308–319 (underlined) of the type 5 streptococcal M protein, respectively. Peptide p323–339 (323ISQAVHAAHAEINEAGR339) corresponds to the OVA323–339 epitope (underlined).
4.2 Cells
Macrophages were grown from femoral bone marrow cells of BALB/c mice (Jackson Laboratories, Bar Harbor, ME) to establish a monolayer in 6-well plates using DMEM medium (Life Technologies, Grand Island, NY) supplemented with 0.05 mM 2-ME, 10% fetal bovine serum (FBS), 1.0 mM sodium pyruvate, 10 mM HEPES buffer, antibiotics and 20% LADMAC-conditioned medium 13. S. pyogenes type 5M protein-specific T cell hybridomas HX17 (specific for M517–31/Ed) and HY2 (specific for M5308–319/Ad), as well as the OVA-specific T cell hybridoma DOBW (specific for OVA323–339/Ad) were generated and described previously 19, 26.
4.3 Subcellular fractionation
Subcellular fractionation was performed as described 6, 13 with the following modifications. Macrophages grown on three 6-well pates (total number of cells,mean 17.1±5.2×106) were stimulated with 10 U/ml recombinant IFN-γ (Genzyme, Cambridge, MA) for 24 h and washed with PBS. Viable S. pyogenes were added (mean 77±18 bacteria per macrophage), followed by centrifugation (900×g, 10 min, 37°C). Plates were incubated for additional 10 min at 37°C in a 5% CO2 incubator (total 20 min pulse period), extensively washed to remove extracellular bacteria and chased for various periods. In some experiments, macrophages were pulsed with 3 mg/ml ovalbumin (OVA, Sigma, St. Louis, MO) for 90 min. After the chase period, plates were placed on ice and washed three times with ice-cold DMEM and then with PBS. Macrophages were detached and homogenized in homogenization buffer (0.25 M sucrose, 10 mM HEPES, pH 7.4) in a Dounce tissue homogenizer (Wheaton, Millville, NJ) to obtain 80–85% cell lysis. Intact cells and nuclei were removed by centrifugation (200×g, 5 min, 4°C). The supernatants containing post-nuclear membranes were layered on 27% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ) in homogenization buffer, followed by centrifugation in a Sorvall type 40 or A-1256 fixed angle rotors at (36,000×g, 60 min, 4°C). Thirty fractions were collected manually from the top of the gradient and stored at –80°C.
4.4 Characterization of Percoll fractions
Percoll fractions were tested for β-hexosaminidase activity as a marker for lysosomal enzymes using the chromogenic substrate p-nitrophenyl-N-acetyl-β-D-glucosaminide(Sigma, formulated fresh at 1.37 mg/ml in water). The reaction mixture containing 50 μl sample, 150 μl assay buffer [0.1 M 2-(N-Morpholino) ethanesulfonic acid (Sigma), 0.2% Triton X-100, pH 6.5] and 50 μl substrate was incubated for 90 min at 37°C. The reaction was stopped with 0.5 M glycine, pH 10, and absorbance was measured at 405 nM.
For identification of fractions containing phagosomes, macrophages were pulsed with FLUOS-labeled bacteria for 20 min, chased for various periods and subjected to subcellular fractionation. Percoll fractions were transferred to 96-well clear-bottom black plates (Costar, Cambridge, MA), and fluorescence was measured with a SpectraFluor Plus fluorimeter (Tecan, Reading, GB) using a 485-nm excitation filter and 535-nm emission filter. To identify the location of early endosomes on the gradient, unstimulated macrophages were pulsed with 1 mg/ml horseradish peroxidase (HRP, 1,100 units/mg, Sigma) for 5 min at 37°C, as described 21. Percoll fractions were screened for peroxidase activity using 4.5 nM p–nitrophenylphosphate disodium hexahydrate in substrate buffer (0.1 M NaHCO3, 3.3 mM MgCl2·6H2O, 0.01% NaN3, pH 10.3), and absorbance was measured at 405 nm.
4.5 Detection of MHC-II molecules in ELISA
To detect the presence of MHC-II molecules in subcellular fractions, 50 μl of Percoll fractions were added to 96-well Microtiter® Immunoassays plates (Immulon® 1, flat bottom, Dynatech Laboratories, Inc. Chantilly, USA), dried in a constant flow cabinet and stored at –30°C. Plates were washed in washing buffer (PBS containing 0.05% Tween 20) and blocked in washing buffer supplemented with 10% FBS and unlabeled anti-mouse mAb specific for FcγIIR and FcγIIIR (1:200, clone 2.4G2, Fc Block®, PharMingen, Oxford, GB) for 1 h at room temperature. After washing, the plates were incubated for 1 h with rat anti-Aβb,d,q, Ed,k MHC-II-specific mAb (1:200, clone M5/114.15.2, ATTC, TIB120) diluted in washing buffer containing2% FBS. Normal rat serum (Sigma) was used as a control for antibody specificity. Plates were incubated for 1 h with goat anti-rat IgG (1:1,000, Sigma) peroxidase conjugate, washed and the reaction was developed with the liquid substrate system for ELISA [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), Sigma] and absorbance was measured at 405 nm.
4.6 Antigen-presentation assay
Whole-cell antigen presentation assays were performed as described 17 with the following modifications. Viable S. pyogenes (3×106/well) were added to IFN-γ-activated macrophages (105/well, 48-well plates, Bibby Sterilin, Staffordshire, GB) and pelleted by centrifugation. Cells were washed to remove extracellular bacteria, chased for various periods, fixed with 1% paraformaldehyde and washed. M5-specific T cell hybridomas were added at 2×104/well for 24 h. IL-2 production was measured as proliferation of CTLL-2 cells in the presence of 14.8 kBq of [3H]thymidine (TRA310, specific activity 74 GBq/mmol; Amersham International plc, Buckinghamshire, GB).
For subcellular antigen-presentation assays, vesicular compartments present in the Percoll fractions were disrupted by three freeze-thaw cycles to expose lumenal peptide/MHC-II complexes. Relevant synthetic peptides (at 1 μM) were added to aliquots of fractions as a positive control for T cell hybridoma responsiveness, which remained positive in all experiments. T cell hybridomas were added to the Percoll fractions at 104/well (HX17), 1.5×104/well (HY2) or 105/well (DOBW) for 24 h, and the supernatants were collected and frozen. IL-2 production by T cell hybridomas in response to Percoll fractions was measured as proliferation of CTLL-2 cells by a spectrophotometric assay with Alamar Blue indicator dye (Alamar Biosciences, Sacramento, CA). The response was measured as the difference between absorbance at 550 nm and 595 nm.
Acknowledgements
We are grateful to Dr. M. Torres for advice throughout this work. We thank K. Kovalski, M. Convery and N. Nagy for technical assistance. This project was supported by Grant 058930/Z/99/Z from The Wellcome Trust to A.v.D. and J.H.R., and NIH grants AI35726 and AI34343 to C.V.H.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




