Stable expression of MHC class I heavy chain/HLA-DO complexes at the plasma membrane
Abstract
Major histocompatibility complex (MHC) class I and II molecules present antigenic fragments to the immune system. MHC-like chaperones, like HLA-DM, HLA-DO and tapasin support peptide loading. HLA class I heavy chains require association with β2-microglobulin and peptide for endoplasmic reticulum (ER) exit. Likewise, HLA-DO is only able to leave the ER by association to DM. Here we show that HLA-DO and free MHC class I heavy chains associate into a stable complex early during biosynthesis and are expressed at the plasma membrane as a complex. These DO/heavy chain complexes are found on DO-transfected cells and on low amounts on human B cells.
Abbreviations:
-
- β2m:
-
β2-microglobulin
-
- ER:
-
Endoplasmic reticulum
1 Introduction
The MHC protein family is a set of highly homologous molecules with diverse functions. Both MHC class I and class II molecules present small peptides. Formation of MHC/peptide complexes is a multi-step process guided by various chaperones. One specialized chaperone, the non-classical MHC class II molecule HLA-DM, exchanges a fragment of the invariant chain from MHC class II molecules forantigenic peptides in the lysosomal-like MHC class II compartment (MIIC). The second non-classical class II molecule, HLA-DO, is primarily expressed by B cells 1, 2 and some thymic epithelial cells 3. HLA-DO modulates the peptide repertoire presented by class II molecules by associating to DM and regulating the catalytic activity of the latter 4, 5.
Many molecules supporting class I, class II and CD1 assembly have been identified. It has been demonstrated that some of these chaperones are shared. For example, β2-microglobulin (β2m) is a structural component of both MHC class I molecules and CD1. The MHC class II associated Ii is able to associate with some MHC class I molecules 6, 7] and influences intracellular trafficking of CD1d [8. Finally, class II molecules have been shown to influence CD1 trafficking 9.
Here we describe another interaction between molecules of the MHC class I and II pathways. HLA-DO stably associates with MHC class I heavy chains. Whereas the separate subunits are retained in the endoplasmic reticulum (ER), the HLA-DO/heavy chain complex is released. The complex is subsequently stably expressed at the plasma membrane of DO-transfected cells and, in low amounts, on human primary B cells.
2 Results
2.1 Association of HLA-DO with MHC class I molecules
HLA-DO associates with HLA-DM and thereby influences the MHC class II peptide repertoire 4, 10, 11. As chaperones of the different antigen presentation pathways are sometimes shared, we analyzed whether the chaperone HLA-DO influences MHC class I expression in DO transfectants. Control Mel JuSo cells or Mel JuSo transfected with DOα βGFP are analyzed by FACS using different MHC class I-specific antibodies. Mel JuSo cells and those expressing DOα βGFP have more or less similar amounts of correctly folded class I molecules (detected by W6/32) and free MHC class I heavy chains recognized by the mAb HC10. The DOα βGFP expressing cells, however, have an increased expression of free heavy chains with the HCA2 epitope (Fig. 1A).
HC10 and HCA2 recognize two distinct epitopes in the MHC class I α1-domain 12 and the one recognized by HCA2 is apparently affected by DO expression. To visualize DO at the plasma membrane, Mel JuSo cells expressing DO-GFP are surface-iodinated, and radio-iodinated proteins are immunoprecipitated with antibodies recognizing the class I complex (W6/32), β2m and associated proteins (BBM.1), free class I heavy chains (αHC), DOβ-GFP (αGFP) and DOα (αDOα). Two prominent proteins of 45 kDa and 32 kDa are isolated with DOβGFP (Fig. 1B, lane 5), the latter corresponding to the size of the DOα chain (Fig. 1B, lane 6). The 45-kDa protein migrates at the same position as the MHC class I heavy chain, precipitated from the same radiolabeled material with various MHC class I-reactive antibodies (Fig. 1B, lanes 2-4). To identify the nature of the two additional bands, the αGFP isolate is denatured, followed by a second immunoprecipitation with anti-heavy chain serum, anti-GFP or anti-DOα. This identifies the MHC class I heavy chain, DOβ-GFP and DOα in the αGFP immunoprecipitate (Fig. 1B, bottom panel). DOα and DOβGFP are not recovered in the W6/32 and BBM.1 isolates (Fig. 1B, upper panel) nor are DM and β2m present in the anti-GFP immunoprecipitations (data not shown). These data indicate that DOα β-GFP (and wild-type DO; data not shown) is expressed at the plasma membrane in association with the MHC class I heavy chain. When αGFP immunoprecipitates are analyzed by one-dimensional isoelectric focussing, all MHC class I alleles expressed by Mel JuSo are recovered (data not shown).
Still, the interaction of DO with the class I heavy chain could be the result of post-lysis association. To exclude this possibility, lysates of cell surface-iodinated Mel JuSo cells are mixed with lysates of non-labeled Mel JuSo DOα βGFP cells followed by immunoprecipitation with αGFP, αHC and BBM.1. As a control, the same immunoprecipitations are performed with cell surface-iodinated Mel JuSo DOα βGFP cells. Whereas iodinated class I heavy chains are recovered with the αHC serum or BBM.1 from the mixed lysate, no class I heavy chains are observed in the αGFP immunoprecipitate (Fig. 1C), excluding post-lysis association.
The DO/heavy chain complex is expressed at the plasma membrane of DO transfectants. To test whether the DO/ heavy chain complex is expressed at the cell surface under physiological conditions, isolated human resting primary B cells are cell surface-iodinated followed by immunoprecipitation with αDOβ or αHC serum. A 45-kDa protein, co-migrating with the free class I heavy chain (αHC) and the class I heavy chain of class I complexes (W6/32), is recovered with the isolated DOβ chain (Fig. 1D). The iodination of this DO/heavy chain complex is considerably less efficient than observed in the DO transfectant, suggesting that it is expressed at low amounts on resting human B cells.
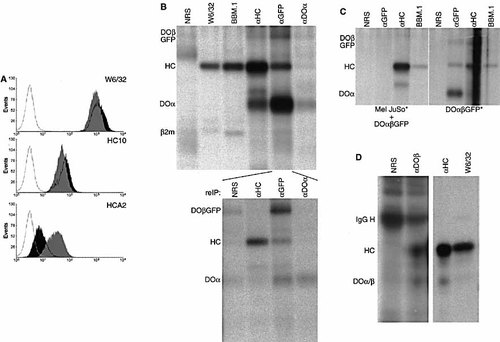
HLA-DO stably associates with the MHC class I heavy chain. (A) FACS analysis of Mel JuSo (black) and Mel JuSo DOα βGFP (gray) cells using the monoclonal antibodies HC10, HCA2 and W6/32. The FACS plots are representative for four independent experiments. Gray line, secondary antibody only. (B) Mel JuSo cells expressing DOα βGFP are iodinated, lysed and proteins are subsequently isolated with the antibodies indicated. The anti-GFP isolate is split, and one half is denatured, followed by immunoprecipitation with the antibodies indicated (reIP). The bands corresponding to DOβGFP, class I heavy chain, DOα and β2m are indicated. (C) Mel JuSo cells are cell surface-iodinated (*), and lysates are mixed with non-radioactive Mel JuSo cells expressing DOα βGFP followed by immunoprecipitation with the antibodies indicated (left panel). As a control, Mel JuSo cells expressing DOα βGFP are radiolabeled (*) and immunoprecipitated with the same antibodies. The position of DOβGFP, class I heavy chain and DOα are indicated. (D) Human B cells are cell surface-iodinated followed by sequential immunoprecipitation with NRS, anti-DOβ, anti-HC and W6/32. The position of the Ig heavy chain, class I heavy chain and DOα/β is indicated. Right panel is exposed for 1 day, left panel for 18 days.
2.2 HLA-DO associates with MHC class I heavy chains during biosynthesis
Free DO molecules 2 as well as free MHC class I heavy chains 13 are usually retained in the ER. The association of DO and class I heavy chain might promote their egress from the ER resulting in plasma membrane appearance. To analyze this, Mel JuSo wtDO cells are metabolically labeled for 15 min and chased for up to 4 h. DO/heavy chain complexes are immunoprecipitated with anti-DOβ serum or anti-heavy chain serum at various chase time points and analyzed by SDS-PAGE. DO/ heavy chain complexes can be recovered immediately after the pulse labeling (Fig. 2), indicating that these complexes are formed rapidly after biosynthesis. These DO/heavy chains can still be recovered after 4 h of chase.
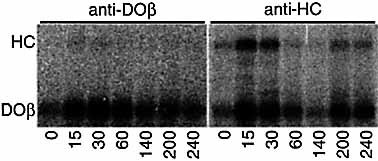
Stable DO/heavy chain complexes are formed shortly after biosynthesis. Mel JuSo cells transfected with wtDOα β are pulse-labeled and chased for the times (minutes) indicated, followed by immunoprecipitation of DOβ and MHC class I heavy chain. The positions of the MHC class I heavy chain and DOβ are indicated.
2.3 The effect of DO on the cell surface half-life of class I heavy chains
To determine whether DO/heavy chain complexes are transiently exposed to the cell surface or are stably expressed, Mel JuSo DOα βGFP cells are surface-iodinated followed by culturing. At several time points, cells are either left untreated or are treated with neuraminidase (NANase) at 0°C to remove cell surface exposed sialic acids. Removal of sialic acids is visualized by SDS-PAGE analysis. The DOα chain in the immunoprecipitated DOα βGFP/heavy chain complexes remains sensitive to NANase treatment for more than 2 h (Fig. 3A), indicating that DO/heavy chain complexes are stably expressed at the plasma membrane. The cell surface half-life is comparable to that of MHC class I complexes (W6/32) and MHC class II (1B5; Fig. 3A). Note that the migration of the DO-associated heavy chain is not affected by NANase treatment in contrast to the heavy chain immunoprecipitated by W6/32, possibly due to differential accessibility to NANase activity.
To assess the half-life of free versus DOα βGFP-associated class I heavy chains, Mel JuSo cells and Mel JuSo DOα βGFP cells are cell surface-iodinated and cultured for various times. Free class I heavy chains (αHC, Mel JuSo) or DOα βGFP-associated class I heavy chains (αGFP, Mel JuSo DOα βGFP) are immunoprecipitated together with the transferrin receptor to control for labeling efficiency (Fig. 3B). The class I heavy chain bands are quantified by PhosphoImager and corrected for labeling efficiency using the transferrin receptor (Fig. 3C). The half-life of the three subunits of the DO/ heavy chain complex is similar. Comparing the half-life of free versus DOα βGFP-associated class I heavy chains suggests that the interaction with DO molecules does not markedly affect the half-life of the class I heavy chains.
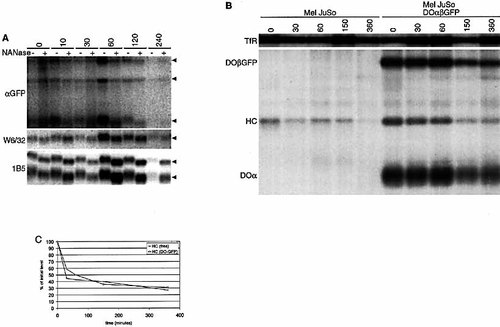
The stability of free and DO-associated MHC class I heavy chains. (A) Mel JuSo cells expressing DOα βGFP are cell surface-iodinated and recultured at 37°C for up to 4 h. At various time points, cells are left untreated or are treated with NANase prior to lysis and immunoprecipitation with the antibodies indicated. The immunoprecipitated DOβGFP, heavy chain and DOα (αGFP), MHC class I (W6/32) and DRα and DR3β (1B5) are indicated by arrows. (B) Mel JuSo or Mel JuSo DOα βGFP cells are cell surface-iodinated and cultured for the times indicated. After lysis, the transferrin receptor (TfR) and free class I heavy chains from Mel JuSo or DOα βGFP-associated class I heavy chains from Mel JuSo DOα βGFP cells are isolated. The position of the respective proteins is indicated. (C) Quantification of the bands in Fig. 3B by phosphoimager. Background-subtracted class I heavy chain intensities are corrected for the efficiency of labeling as deduced from the intensities of the transferrin receptor. Maximal intensity is set to 100% to allow direct comparison.
2.4 DO/heavy chain complexes are stable
To analyze whether DO-associated class I heavy chains can be refolded into mature class I/β2m/peptide complexes, DO/heavy chain complexes are immunoprecipitated with anti-GFP from iodinated Mel JuSo DOα βGFP cells. The isolates are incubated with different combinations of 0.1% SDS, peptide or recombinant human β2m and either left at room temperature or boiled for 5 min. The class I molecules are allowed to refold at 37°C for 12 h. Since addition of an HLA-A1 binding peptide or β2m did not improve the recovery of W6/32-reactive material (Fig. 4, middle panel), class I heavy chains are not folded into peptide-loaded functional class I complexes. Instead, the small amount of released class I heavy chains (Fig. 4, top panel) is recovered with the αHC serum (Fig. 4, bottom panel). Note that the incubations mainly released DOα, suggesting a direct interaction between DOβGFP and class I heavy chains. Other refolding conditions excluding SDS and varying pH in the absence or presence of peptides and β2m also failed to dissociate the DO/heavy chain complex to form MHC class I complexes (data not shown).
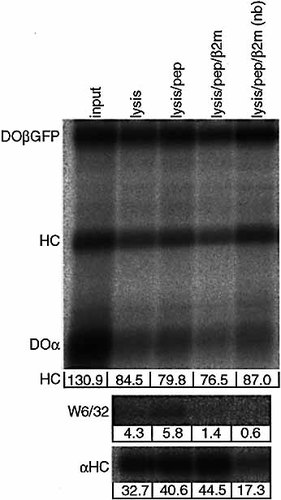
DO-associated class I molecules do not bind peptides in vitro. DO/heavy chain complexes are immunoprecipitated with αGFP serum from surface-iodinated DOα βGFP transfectants. The immunoprecipitate is left untreated (lane 1) or incubated in the presence of 0.1% SDS, peptide and β2m, and either left at room temperature (nb, lane 5) or boiled for 5 min as indicated. MHC class I molecules are subsequently allowed to refold at 37°C for 12 h. The anti-GFP immunoprecipitates are analyzed by 10% SDS-PAGE. The supernatants are immunoprecipitated with W6/32 followed by anti-HC serum. The signals derived from the DOβGFP and heavy chain bands are quantified by phosphoimager analysis. The numbers below the top figure refer to the amount of co-immunoprecipitated heavy chain corrected for the amount of immunoprecipitated DOβGFP. The numbers below the two bottom figures refer to intensities with background intensity subtracted (W6/32 and αHC).
3 Discussion
The MHC class I and class II antigen-presentation pathways have their own sets of chaperones that prevent aggregation and support binding of peptides. The two pathways do not intermingle with some exceptions. Some class I alleles and CD1d can make use of the invariant chain for intracellular trafficking 6–8. In addition, class II molecules may alter intracellular trafficking of CD1d molecules 9.
Here, we report another interaction between two molecules of the class I and class II pathway. HLA-DO associates with MHC class I heavy chains in DO transfectants as well as human primary B cells. The cell surface expression of heavy chain/DO complexes is surprising as class I heavy chains and DO are themselves retained in the ER in the absence of their normal associating partners, β2m and DM 2, 13. Apparently, the class I heavy chain and DO associate into a novel MHC complex that is allowed to pass the ER quality control mechanism. Dissociation experiments, incubating DO/heavy chain isolates under mild denaturing conditions, showed selective loss of the DOα chain suggesting that the class I heavy chain directly interacts with the DOβ chain. DM is not recovered in the DO immunoprecipitations from surface-labeled cells, probably because DO/DM complexes are rapidly internalized upon cell surface deposition 14. In contrast, DO is stably expressed at the cell surface in complex with the class I heavy chain and is efficiently recovered. The association of class I heavy chains with DO does not markedly affect the surface half-life of class I heavy chains. This suggests that DO does not act as a chaperone at the plasma membrane stabilizing class I heavy chains nor does it induce internalization and degradation of class I heavy chains.
MHC class II molecules and HLA-DM are always co-expressed on professional antigen-presenting cells, while HLA-DO is primarily expressed in B cells 15. In the absence of DM, DO molecules are retained in the ER 2. Therefore, DO always seems to function in conjunction with DM. In human B cells the expression level of the DO/heavy chain complex is apparently very low, probably because DO molecules prefer association to HLA-DM over class I heavy chains.
Although the cytoplasmic domain of DO contains lysosomal targeting information, intracellular trafficking of DO is mainly controlled by DM 14. Still, the lysosomal targeting information of DO might target the class I heavy chain into the endosomal pathway allowing class I to interact with peptides and β2m. Whether DO-associated class I heavy chains can refold into a functional class I complex is unclear. The failure of DO-associated MHC class I heavy chains to refold in the presence of peptide and β2m in in vitro assays suggests that it probably does not act as a peptide-presenting device.
Whether the low amount of DO/heavy chain complexes at the cell surface of B cells has a function is unclear. DO molecules that have not been able to associate with DM may find in free MHC class I molecules another partner to form a stable complex that is able to pass the ER quality control mechanisms.
4 Materials and methods
4.1 Cell lines and antibodies
The Mel JuSo cell lines stably expressing HLA-DOα with either wild-type DOβ or DOβGFP have been described previously 14, 16. The following antibodies were used: rabbit anti-DOβ cytoplasmic tail, rabbit anti-GFP, rabbit anti-MHC class I heavy chain, monoclonal antibody 1B5, anti-human transferrin receptor 66Ig10, MHC class I complex-specific W6/32, the rabbit anti-DOα 14, HC10, HCA2 12.
4.2 Isolation of human B cells
Buffy-coats from healthy donors were obtained from the Sanquin Blood Supply Foundation (CLB, Amsterdam, The Netherlands). Mononuclear cells were isolated by centrifugation on Ficoll Hypaque gradient (Pharmacia, Sweden), and B lymphocytes were purified using anti-CD19 Dynabeads and DETACHaBEAD (Dynal, Norway). The isolated B lymphocytes remained quiescent, and cell purity and viability was >99% as determined by cytofluorometric analysis (data not shown).
4.3 Biochemical analysis
Cell surface proteins are labeled by lactoperoxidase-catalyzed iodination using 400-600 μCi [125I]/107 human B cells or 2×106 Mel JuSo transfectants as described 17. Upon lysis in NP40 lysis buffer proteins are isolated with the antibodies coupled to protein G-Sepharose beads. Where indicated, primary immuno-isolates are denatured by boiling in 50 μl 0.2% SDS, diluted to 1 ml with lysis buffer, followed by immunoprecipitation and analysis by SDS-PAGE.
NP40 lysates from iodinated Mel JuSo cells were immediately mixed with the lysates from Mel JuSo DOα βGFP cells. The mixed lysate was incubated at 4 °C for 1 h followed immunoprecipitation and analysis by 12% SDS-PAGE.
Human B cells were frozen in DMSO after isolation and recultured with PBS at 4 °C immediately followed by lactoperoxidase-catalyzed iodination. Cells were washed with PBS followed by lysis in NP40 lysis mixture and four preclears with NRS. Subsequently, immunoprecipitations were sequentially performed with NRS, anti-DOβ serum, anti-HC serum and W6/32 before analysis by 10% SDS-PAGE.
To determine the stability of cell surface proteins, cells are surface-iodinated at 0°C, washed in PBS and recultured in DMEM with 10% FCS at 37°C. Samples taken at several time points of culturing are either left untreated or are incubated with 1 U/ml neuraminidase (NANase, type V; Sigma) at 0°C for 1 h as described 17. After lysis and removal of insoluble material, proteins are isolated with αGFP, W6/32 or 1B5 (anti-DRα) and analyzed by 10% (W6/32) or 12% (αGFP and 1B5) SDS-PAGE.
Alternatively, Mel JuSo cells or Mel JuSo DOα βGFP cells were cell surface-iodinated at 0 °C using lactoperoxidase-catalyzed iodination. The cells were cultured for the times indicatedfollowed by immunoisolation of the transferrin receptor with 66 Ig10. Subsequently, free class I heavy chains were immunoprecipitated with the rabbit anti-HC serum from lysates of Mel JuSo control cells or DOα βGFP complexes with an anti-GFP serum from lysates of DOα βGFP transfectants. The immunoprecipitations were analyzed by 12% SDS-PAGE. Bands were quantified using TINA software (Raytest) after reading by a Fujix BAS 2000 phosphoimager. Background-subtracted class I heavy chain intensities were corrected for labeling differences between various dishes through the intensity of the transferrin receptor. Maximal intensity for the corrected bands was set at 100%.
For pulse-chase analysis, cells grown to confluency in 10-cm2 dishes are starved in methionine/cysteine (Met/Cys)-free RPMI 1640 medium containing 10% FCS for 30 min followed by labeling with 80 μCi [35S]Met/Cys per dish for 15 min. Cells are chased in the presence of 1 mM non-radioactive Met/Cys for the indicated times. After lysis in NP40 lysis buffer, DO and class I heavy chains are isolated with DOβ and αHC serum and analyzed by 12% SDS-PAGE.
For the peptide-binding assays, αGFP immunoprecipitates from cell surface-iodinated Mel JuSo DOα βGFP cells are incubated in the presence of 0.1% SDS, 50 μM HLA-A1-binding peptide (YSDSLVQKGY), 10 μM human β2m and boiled for 5 min or left at room temperature. MHC class I complexes are allowed to refold at 37°C for 12 h. DO-GFP/heavy chain complexes are recovered with αGFP followed by isolation of MHC class I complexes with W6/32 and analysis by 10% SDS-PAGE. Quantification is done by phosphoimager analysis.
Acknowledgements
We thank M. Toebes for recombinant human β2m and Y. Souwer for isolation of the human B lymphocytes. This research was supported by NKB grant 2001-2415.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




