Visualization of peptide presentation following oral application of antigen in normal and Peyer's patches-deficient mice
Abstract
Orally applied antigens generate systemic unresponsiveness by induction of anergy and deletion of specific T cells at high antigen doses, and induction of regulatory T cells at low doses of antigen. These different immune reactions have been attributed to different types of antigen-presenting cells (APC) and/or different secondary lymphoid organs participating in the induction of the immune response. We used high-sensitivity immunofluorescence to directly identify for the first time the cells presenting orally applied antigen in vivo. At low peptide doses (<1 mg) peptide presentation was exclusively detected on dendritic cells (DC) of the Peyer's patches (PP) and mesenteric lymph nodes (mLN). At high doses (>1 mg) peptides were presented systemically and by all types of APC but presentation was still maximal on DC of the PP (up to 65%). Nevertheless, at limiting antigen doses T cell activation in the gut-associated lymphoid tissue occurs preferentially in the mLN but not in PP. PP-deficient mice have the same frequencies of peptide-presenting cells in mLN, peripheral lymph nodes and spleen and activation of naive T cells in vivo is not affected. Therefore, PP are not critical for antigen presentation as well as for T cell activation in response to orally applied soluble antigens.
Abbreviations:
-
- FOva:
-
FITC-labeled Ova-peptide
-
- PP:
-
Peyer's patches
-
- pLN:
-
Peripheral lymph nodes
-
- mLN:
-
Mesenteric lymph nodes
-
- GALT:
-
Gut-associated lymphoid tissue
-
- Dig:
-
Digoxigenin
1 Introduction
Oral administration of proteins, peptides or low-molecular weight haptens in the presence of appropriate adjuvants, e.g. cholera toxin, leads to the induction of an active immune response in the gut 1. In contrast, application of soluble antigens alone induces systemic unresponsiveness, termed oral tolerance, which is characterized by deletion or anergy of antigen-specific T cells or induction of suppressive T cells depending on the antigen dose and the duration of antigen administration 2. The unique features of immune responses in the gut have been attributed to the microenvironment and specialized APC populations of the gut-associated lymphoid tissue (GALT). Peyer's patches (PP) are generally regarded as the most important organized lymphoid structure of the GALT and they are thought to be important for antigen uptake from the intestine 3, 4. In mice and humans the follicle-associated epithelium (FAE) which separates the PP from the intestinal lumen, contains up to 10% of specialized M cells 3, which have been shown to take up and transport antigen efficiently from the gut lumen into the underlying lymphoid tissues 3, 4. However, little is known about the relative contribution of the various lymphoid organs to the presentation of orally applied antigens, and the phenotype and frequency of APC involved in that process. Antigen presentation has been demonstrated by analyzing in vivo activation of specific Tcells following oral antigen application. Activated T cells were found in GALT as well as peripheral lymphoid organs 5. Liu and MacPherson 6 have shown that migrating DC recovered from the thoracic duct can present orally applied ovalbumin. The frequency and type of APC in the individual lymphoid organs that present orally applied antigens as well as the influence of time and antigen dose have not been addressed yet. Direct monitoring and quantification of peptide presentation by immunofluorescent techniques is limited by the low number of specific MHC/peptide ligands in vivo. T cells are able to recognize a few hundred specific MHC/peptide complexes on the APC surface 7, 8 which is far below the detection limit of fluorescent antibody staining 9. To detect antigenic peptide presentation at physiologically relevant quantities we have used a sensitive immunofluorescent technique to directly identify APC ex vivo on the single-cell level according to the presentation of less than 100 specific peptides 9. Here, we have identified and quantitated the cells that present orally applied antigens in gut-associated and peripheral lymphoid organs. We show that orally applied peptides are maximally presented in PP but T cell activation occurs preferentially in mesenteric lymph nodes (mLN). Using PP-deficient mice, we have also analyzed the contribution of PP to uptake and presentation of orally applied antigens as well as T cell activation. PP-deficient mice were generated by in utero treatment with an anti-IL-7 receptor antibody. Blocking IL-7Rα during day 13.5–15.5 of gestation selectively blocks the formation of the PP anlage but not other lymphoid tissues 10.
2 Results
2.1 Identification of APC that present orally applied antigen
For the identification of cells that present the antigenic fluorescein-labeled OVA peptide (FOva) following its oral application, we used high-sensitivity immunofluorescent staining by magnetofluorescent liposomes. In comparison to conventional staining, which has a detection limit of 2,000–3,000 antigens per cell, magnetofluorescent liposomes have a detection limit of less than 100 molecules per cell 9. The detection limit for presented peptide by magnetofluorescent liposomes correlates well with the capacity of the very same APC to stimulate specific T cells in vitro and has been estimated to be below 100 peptides per cell (Kunkel et al., Cytometry 2003, in press). To determine the time course of peptide presentation, 1 mg FOva was orally applied to BALB/c mice and at various time points after feeding, the lymphoid organs were removed and the cells were stained for the specific peptide on the surface. The cells were stained for B220 or CD11c, respectively, to identify B cells and dendritic cells and costained for MHC class II. MHC class II– cells of FOva-fed mice and cells from PBS-fed mice were used as negative controls (Fig. 1). Staining with conventional fluorochrome-conjugated anti-FITC antibodies did not result in a detectable signal (data not shown). As shown in Fig. 1, staining with liposomes clearly identified a significant population of peptide-presenting cells in various lymphoid organs of FOva-fed mice. Liposome staining in FOva-fed mice was restricted to MHC class II expressing cells, confirming its specificity. The maximal frequency of peptide-presenting cells was detectable 4–8 h after feeding (Fig. 1 and data not shown). At this time, 33% of DC and 13% of the B cell population of the PP presented the specific peptide. Peptide-presenting cells were also present in mLN (10% DC, 3% B cells at 6 h) spleen (8% DC, 2% B cells) and peripheral lymph nodes (pLN; 3% of DC). The kinetics of the appearance of peptide-presenting cells in all lymphoid organs analyzed was similar. Eighteen hours after feeding, peptide presentation by DC and B cells was still clearly detectable in PP, mLN and spleen, but the frequency of positive cells was already lower than at the 6 h time point (PP: 22% DC, 3% B cells; mLN: 4% DC, 1% B cells). Thirty-six hours after feeding only a few peptide-presenting cells were detectable within the DC fraction of PP (about 5%).
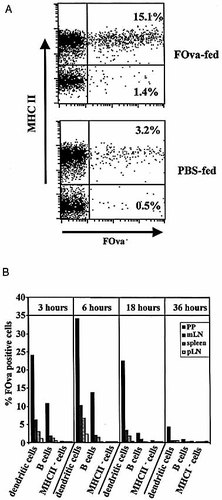
(A) Analysis of peptide presentation after oral application. Mice were fed 1 mg FOva or PBS only and presentation of FOva was analyzed in PP 4 h after antigen application. Cell suspensions were stained with anti-MHC II and for FOva on the cell surface using anti-FITC-Dig/anti-Dig-liposomes. The indicated frequencies are given as the percentage of FOva+ cells within the MHCII+ or MHCII– population, respectively. (B). Kinetics of peptide presentation after oral application. Mice were fed 1 mg FOva and presentation of FOva was analyzed in spleen, pLN, mLN and PP at various time points after application as indicated. Cell suspensions were stained with anti-B220, anti-CD11c, anti-MHC II and for FOva on the cell surface. The frequency of FOva+ B cells and FOva+ DC was assessed by gating for B220+ cells and CD11c+ cells. Background staining on cells of control mice (PBS-fed mice) was subtracted. The data are representative of two separate experiments with three mice in each experimental group.
2.2 The antigen dose determines the frequency of peptide-presenting cells and the kind of lymphoid organs involved in antigen presentation
It has been suggested that high and low doses of orally applied antigen induce different states of tolerance, i.e. induction of anergy or deletion versus generation of CD4 regulatory T cells 11, 12. To clarify the influence of the antigen dose on the frequency of peptide-presenting cells as well as on the kind of lymphoid organs, where peptide-presenting cells occur, we analyzed peptide presentation at the maximum time point of presentation, 6 h following feeding of 1, 0.3 and 0.1 mg of FOva. Since DC are regarded as the main APC population for activation of naive T cells in vivo and in vitro 13, we decided to focus our attention on this population. Due to the low frequency of these cells in the lymphoid organs (0.3–3%), we magnetically enriched the CD11c-positive DC population previous to analysis. As shown in Fig. 2, the frequency of peptide-presenting cells directly correlates to the antigen dose. Feeding 1 mg, the frequency of peptide-presenting cells is highest in PP (up to 75%) followed by mLN (20–65%), spleen and pLN (both about 5–10%). Feeding 0.3 and 0.1 mg of FOva, no peptide-presenting DC were detectable in spleen and pLN, whereas in PP, peptide-presenting DC could still be detected at frequencies of 30% and 20%, respectively. In the mLN, about 10% peptide-presenting DC were detectable at 0.3 mg but none at 0.1 mg. Thus at lower antigen doses the presentation of orally applied peptide is more restricted to the local immune organs, i. e. PP and mLN, whereas at higher doses the antigen is distributed systemically and is also presented in spleen and pLN. In any case, in terms of frequencies of presenting cells, the PP are the major site for presentation of orally applied antigen.
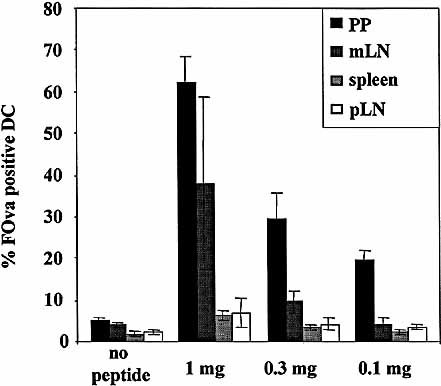
Organ distribution of peptide presentation after oral application. Mice were fed 0.1–1 mg FOva and presentation of FOva was analyzed in spleen, pLN, mLN and PP 4 h after application. DC were enriched by magnetic sorting for CD11c+ cells. Cell suspensions were stained for FOva on the cell surface and the frequency of FOva+ DC was assessed by gating on CD11c+ cells. Frequencies show the average of three separately analyzed animals per group, error bars represent the standard deviation. The results are representative of two separate experiments.
2.3 Naive T cells are preferentially activated in mLN at limiting doses of antigen
To assess, whether the frequency of APC which present the orally applied antigen correlates with T cell stimulatory capacity of the respective organ, we analyzed naive T cell activation in vivo following feeding of different doses of ovalbumin. Naive T cells were isolated from OVA TCR-transgenic mice by magnetic cell separation according to expression of CD62L. The cells were labeled with CFSE and transferred into normal BALB/c mice. Following transfer, mice were fed 20 mg or 0.5 mg of OVA in PBS or just PBS. It has been reported that 20 mg of OVA result in T cell activation restricted to mLN and PP 14. The lowest dose which in our own titration experiments resulted in a detectable response of OVA-specific T cells in vivo was 0.5 mg (data not shown). Fourty-eight hours after feeding 20 mg of OVA proliferating OVA-specific T cells with up to two cell divisions could be detected in PP and mLN but not in spleen or inguinal LN (Fig. 3A) as has been reported 14. Feeding of 0.5 mg induced T cell proliferation only in the mLN but not in the PP, inguinal LN or spleen. At day 3 proliferating T cells could also be observed in spleen and inguinal LN after feeding of both antigen doses (Fig. 3B). However, most of these cells have undergone >two-to-three cell divisions and have already lost the activation marker CD69 (data not shown), indicating that they have been primed probably in the GALT and subsequently migrated to the peripheral lymphoid organs. Similar results were obtained after feeding of OVA-peptide (data not shown). These data show that following feeding of soluble antigen (up to 20 mg of OVA) T cells are mainly activated in the GALT. However, at limiting doses of antigen naive T cell proliferation is mainly induced in the mLN rather than in the PP.
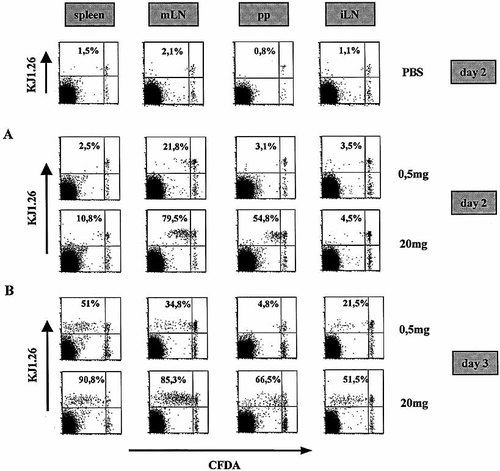
T cell priming to orally applied antigen preferentially occurs in mLN and/or PP. CFDA-SE-labeled CD62L+ CD4+ OVA-specific cells (2×106) were adoptively transferred into BALB/c mice. Twenty-four hours after transfer mice were fed 0.5 or 20 mg OVA. Two days (A) or three days (B) after protein application proliferation of specific T cells was analyzed in spleen, inguinal LN and mLN by flow cytometry. Cell suspensions were stained with anti-CD4 and anti-OVA TCR and the frequency of cells that have proliferated was assessed by gating for CD4+ OVA TCR+ cells. PBS-fed mice were taken as control (only day 2 is shown). The data are representative of two experiments and three pooled mice per group.
2.4 Antigen presentation in the absence of PP
The PP-associated epithelium contains M cells that are considered to be the main entry site of gut-derived antigens into the body. Consistent with this we observed the highest frequency of peptide-presenting cells within the PP. Via the efferent lymphatics of the PP soluble antigens can reach the mLN and eventually the blood via the thoracic duct. Alternatively, protein antigens can directly be taken up via the gut epithelium and transported to the mLN. To directly estimate the contribution of PP to the presentation of soluble antigens in mLN and other peripheral lymphoid organs, we generated mice that were selectively deficient for PP. Such mice were created by in utero treatment with an anti-IL-7Rα antibody as described 10. The absence of PP was confirmed by visual inspection of every single mouse. The presence of peripheral (inguinal, brachial, axillar, popliteal) and mesenteric lymph nodes was not affected by this treatment and PP-deficient mice contained normal numbers of lymphocytes. The phenotypic analysis of lymphocyte populations, (B cells, naive and memory T cells, macrophages) revealed no significant differences when compared to normal mice (data not shown).
PP-deficient and normal mice were fed 1 mg of FOva. After 4 h peptide presentation was analyzed in the mLN and pLN and in the spleen. Cells were counterstained for MHC class II to verify presentation and also for B220, CD11c and CD11b to identify B cells, DC and macrophages. As shown in Fig. 4, we could not detect any differences in the frequencies of peptide-presenting cells between normal and PP-deficient mice with respect to the three types of APC analyzed. The mean fluorescence intensity of peptide staining was also equivalent, indicating that there was no difference in the amount of presented peptide per cell (data not shown).
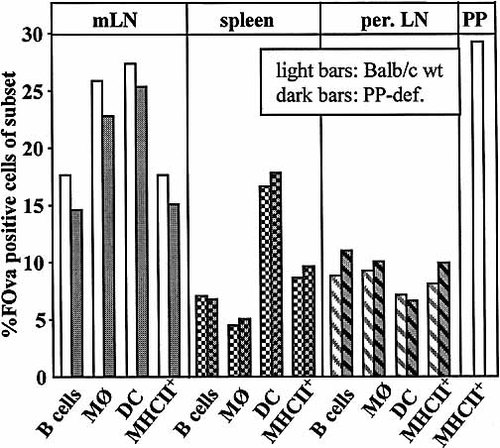
Presentation of orally applied peptide in normal versus PP-deficient mice. Normal (dark bars) and PP-deficient (light bars) mice were fed 1 mg FOva and presentation of FOva was analyzed in spleen, pLN, mLN and PP 4 h after application. Cell suspensions were stained with anti-B220, anti-CD11c, anti-CD11b, anti-MHC II and for FOva on the cell surface. The frequency of FOva+ B cells, FOva+ DC and FOva+ macrophages (MØ) was assessed by gating for B220+ cells, CD11c+ cells and CD11b+ cells. Background staining on cells of control mice (PBS-fed mice) was subtracted. The data are representative of two experiments with three mice in each experimental group.
2.5 Orally applied protein antigens are presented to naive T cells in PP-deficient mice
The experiments described above showed that the presentation of antigenic peptides is not altered in the absence of PP. We wanted to determine whether the T cell response to orally applied antigen was quantitatively affected by the absence of PP. Naive cells were isolated from OVA TCR-transgenic mice by magnetic cell separation according to expression of CD62L. The cells were labeled with CFSE and transferred into normal or PP-deficient BALB/c mice. Following transfer, mice were fed 20 mg of OVA in PBS or just PBS. The dose of 20 mg of OVA was used because we have previously shown that at this dose T cells are activated in both, mLN and PP (Fig. 3). For positive control, another group of mice was immunized subcutaneously with ovalbumin in complete Freund's adjuvant (CFA). To estimate the total proliferative T cell response in the OVA-fed mice we analyzed the proliferation 4 days after the application of antigen, when activated T cells are already distributed to all lymphoid organs. As is evident from Fig. 5, no proliferation of specific T cells can be observed in PBS-fed control mice. As expected the highest frequencies of proliferating T cells, i.e. about 90% in all lymphoid organs, as well as the highest average number of cell divisions, i.e. >three-to-four, were observed in subcutaneously immunized mice. OVA-fed mice showed reduced frequencies of proliferating T cells (45–70%) with less cell divisions per cell. However, no differences were observed between PP-deficient and normal BALB/c mice regarding both, the frequencies of proliferating OVA-specific T cells as well as the average number of cell divisions (Fig. 5). In pLN of PP-deficient mice, the frequency of proliferating T cells (69%) as well as the average number of cell divisions per cell was even slightly increased compared to normal controls (45%). This was probably due to the fact that in normal mice many specific T cells are trapped in the PP and therefore their numbers and frequencies are reduced in the periphery. Similar results were obtained following the application of FOva (data not shown). These data show, that following the oral application of soluble antigens to PP deficient mice the total amount of proliferating T cells is not reduced compared to normal mice, indicating that the PP do not quantitatively contribute to T cell activation in response to orally applied antigens.
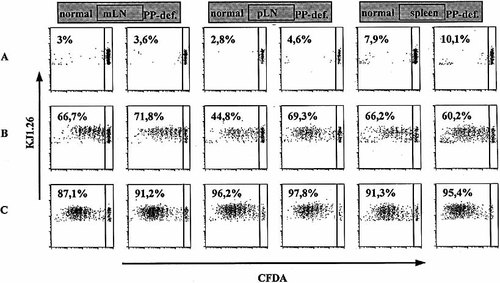
In vivo antigen-specific proliferation of CD4+ T cells in normal and PP-deficient mice. CFDA-SE-labeled CD62L+ CD4+ OVA-specific cells (2×106) were adoptively transferred into normal and PP-deficient mice. Twenty-four hours after transfer mice were fed 20 mg OVA (B) or injected subcutaneously with 100 μg of OVA in CFA (C). Four days after protein application proliferation of specific T cells was analyzed in spleen, pLN and mLN by flow cytometry. Cell suspensions were stained with anti-CD4 and anti-OVA TCR and the frequency of cells that have proliferated was assessed by gating for CD4+ OVATCR+ cells. PBS-fed mice were taken as control (A). The data are representative of two (oral) or three (control, s.c./CFA) separately analyzed mice per group.
3 Discussion
3.1 APC involved in the induction of tolerance versus immunity
The presentation of antigenic peptides in vivo is the key event for the initiation of an immunogenic or tolerogenic T cell response. We have used here a sensitive assay to directly identify and characterize by flow cytometry those APC, which present peptides in vivo at physiologically relevant quantities. It is generally accepted that the induction of T cell immunity requires effective costimulation, e. g. via CD28, most efficiently provided by mature DC 15. The nature of the tolerogenic APC in vivo is less clear. Recently, immature DC have been considered as potential candidates for tolerance induction 16, 17. Oral application of antigen is particularly efficient in generating T cell tolerance. High doses of orally applied soluble antigen induce deletion or anergy of specific T cells and low doses generate T cells with suppressive potential 12. Here, we have identified for the first time the APC involved in the presentation of orally applied peptide in various lymphoid organs and have analyzed the contribution of PP to uptake of orally applied antigens and activation of specific T helper lymphocytes. We have shown that PP are the main lymphoid organ where orally applied peptides are presented. At the lowest antigen dose tested (0.1 mg) peptide presentation was restricted to a subset of DC within the PP. However, T cell activation at limiting antigen doses was mainly observed in mLN rather than in PP. At higher antigen doses peptide-presenting cells were detected also in mLN, pLN and spleen where they appeared with similar kinetics as in PP. Under these conditions, DC, B cells and macrophages presenting the antigen were detectable. By staining for peptide presentation we demonstrated that orally applied peptides are presented equally well in the mLN, pLN and spleen of PP-deficient mice compared to normal mice. Furthermore the overall T cell proliferative response to orally applied ovalbumin protein was not altered in PP-deficient animals.
3.2 Flow-cytometric detection of antigen presentation
Magnetofluorescent liposomes result in 100–1,000-fold increased signal intensity compared to conventional fluorochrome-labeled antibodies and allow the detection of less than 100 antigens per cell 9. The detection limit for peptide presentation corresponds with the in vitro activation threshold of specific T cells (Kunkel et al., Cytometry 2003, in press). The high signal intensity allows clear-cut identification of cells labeled with as few as one liposome. Thus, below the detection threshold of 50–100 molecules no liposomes are bound to the cell. At increasing peptide doses the frequency of positive cells increases as more cells reach the threshold level. At the same time the signal intensity of positive cells is increased only modestly. Therefore we have used the frequency of positive cells rather than the signal intensity as a measure of the amount of peptide presentation in a given APC population. We observed variations in the frequency of peptide presenting cells between different experiments, e. g. Fig. 1 versus Fig. 2 and 4. This is due to the use of different batches of FOva peptide and different analysis time points (3 and 6 h after feeding in Fig. 1 versus 4 h in Fig. 2 and 4). Different batches of FOva occasionally resulted in different frequencies of peptide-presenting cells probably due to variations in the peptide concentration and the efficiency of labeling with FITC. However, the described differences in peptide presentation between the various lymphoid organs and APC populations were the same with all FOva preparations although at different absolute levels.
Since peptides do not require antigen processing the detection method used here might result in a higher frequency of antigen presenting cells as compared to presentation of processing dependent antigens, due to external loading of MHC class II molecules with the peptide. In addition, peptides do also have shorter half-life in vivo compared to proteins, which might lead to different kinetics of antigen presentation and T cell activation.
3.3 The influence of antigen dose
The distribution and the type of peptide-presenting APC was strongly dependent on the antigen dose. It has been postulated that low-dose oral tolerance is characterized by generation of a unique population of regulatory T cells (Treg or Th3 cells), expressing TGF-β, IL-10 or CD25 18, 19. Here we find, that at the lower peptide doses tested, DC of PP and mLN constitute the main type of peptide-presenting APC and therefore might play a role for induction of Th3 or Treg cells. PP DC in contrast to splenic DC have been described to produce IL-10 following in vitro activation and to induce Th2/3 cells rather than Th1 cells in vitro 20. The induction of high dose oral tolerance results in anergy or deletion of specific T cells. As we show here, high doses of orally applied peptide result in peptide presentation by all of the major APC populations in all lymphoid organs. This resembles the situation following intravenous application of antigen, when systemic distribution of the antigen and presentation by all types of APC subsets is observed (21 and Kunkel et al., Cytometry 2003, in press). Although the highest frequencies of peptide loaded cells can be found in the DC population, due to the higher absolute number peptide presenting B cells are the major antigen presenting cells at high peptide doses. It has been suggested that antigen presentation by B cells after systemic antigen application leads to tolerance induction 21, 22.However, it has also been shown that in B cell deficient mice tolerance induction by oral or intravenous application of soluble antigen is not affected 23, 24.
We have shown that at low antigen doses (<1 mg of OVA peptide or 20 mg of OVA protein) antigen presentation and T cell priming is restricted to PP and/or mLN where a subset of DC may induce regulatory T cells. At high doses of peptide (1 mg) PP and mLN still show the highest frequencies of peptide presenting APC. However, the higher absolute number of peptide presenting APC in all other peripheral lymphoid organs may dictate the T cell response by recruiting circulating naive specific T cells into these peripheral organs. We have not examined T cell priming in the periphery athigh antigen doses (>20 mg) but this has been shown by others before 14. This systemic antigen presentation eventually may lead to anergy or deletion of specific T cells as described for tolerance induction by intravenous application of soluble antigen 25.
3.4 The contribution of PP to T cell activation and induction of oral tolerance
PP have been regarded as the main site for the induction of immune responses to intestinal antigens. We show here that APC in PP preferentially present orally administered antigen followed by mLN APC. In contrast, at limiting antigen doses (0.5–5 mg) activation of naive T cells as analyzed at day 2 after feeding predominantly occurred in mLN and only at higher doses (20 mg) in the PP butnot in spleen or inguinal LN (Fig. 3, and data not shown). However, the overall proliferative response as analyzed on day 4 after feeding of 20 mg of antigen was not reduced in the absence of PP (Fig. 5). These data suggest that PP despite a high level of antigen presentation do not significantly contribute to T cell activation in response to soluble antigen and might in fact provide a suppressive environment as has been reported recently 26. It has also been shown that DC from PP but not splenic DC produce the immunosuppressive cytokine IL-10 20. This could influence T cell activation requirements in that either higher antigen doses or additional co-stimulatory signals are required for activation. This suppressive environment may in fact be a strategy of the immune system to prevent inappropriate immune reactions to the large number of non-pathogenic antigens present in the gut.
These data challenge the view that PP play a critical role for the activation of naive T cells in response to orally applied soluble antigens. This is supported by recent functional studies analyzing the induction of oral tolerance in PP deficient mice 23, 27–29. In these studies the absence of PP either only marginally or not at all affected the immunogenic as well as tolerogenic reactions to orally applied antigens except in a single study where complete abrogation of oral tolerance to protein but not to a low-molecular-wight hapten was observed 30. However, in LT-α-deficient mice, which lack both PP and mLN, oral tolerance as well as the induction of specific immunity to oral antigen was completelyabrogated 28, 29. Finally, the reconstitution of mLN in LT-α-deficient mice restored the potential for oral tolerance induction 31. In summary, these functional data and our results indicate that the mLN are the main site for the induction of oral tolerance following feeding of low doses of soluble antigens.
PP might indirectly contribute to oral tolerance induction because antigen is efficiently taken up into PP and can be transported via the efferent lymphatics to the draining mLN and eventuallyto the periphery. Here, we could clearly show that at high antigen doses peptide presentation in mLN, pLN and spleen as well as T cell activation is not quantitatively altered in PP-deficient mice.It has also been shown before that the amount of orally applied OVA which can be detected in peripheral blood is similar in normal and PP-deficient mice 30. Since we have not directly analyzed antigen presentation and T cell activation in PP-deficient mice following feeding of limiting antigen doses we cannot exclude that at lower doses of antigen the transport from the PP to the mLN might contribute to antigen presentation in the mLN. However, it has been shown in PP-deficient mice, that oral tolerance induction with low doses of antigen (<1 mg) was normal 23, 27, 28, arguing against the significant contribution of such a mechanism. Therefore we conclude that the majority of soluble antigens which are presented in mLN and peripheral lymphoid organs are taken up via the large surface of the normal gut epithelium.
4 Materials and methods
4.1 Mice
Mice homozygously transgenic for the DO11.10 α/β-T cell receptor (OVA-TCRtg/tg) 32 on BALB/c background (generous gift of Dennis Y. Loh, Washington University School of Medicine, St. Louis, MO) and BALB/c mice were bred and maintained under specific pathogen-free (SPF) conditions in our animal facility (BgVV, Berlin, Germany). 6–8-week-old females wereused in all experiments.
4.2 Generation of PP-deficient (PP-def.) mice
PP-deficient mice were generated as described 10. Briefly, female and male BALB/c mice were mated overnight and those with a vaginal plug were considered pregnant. Noon of the day when the vaginal plug was identified was calculated as 0.5 d.p.c. Anti-IL7Rα (A7R34 hybridoma was kindly provided by S. Nishikawa, Kyoto) antibody dissolved in PBS (2 mg) was injected i. v. into pregnant mice on day 13.5 and again on day 15.5 of gestation. Absence of PP in littermates was determined by visual inspection of each individual animal.
4.3 Antigens and immunization
A modified OVA-peptide323–339 (iSQAVHAAHAEINEAGRc) was synthesized and purified by J. Schneider-Mergener, Charité, Berlin, Germany. Labeling of the peptide was performed at pH 9.5 with a tenfold molar excess of fluorescein-isothiocyanate (FITC) (Sigma Chemical Co.). Unconjugated FITC was removed from FITC-labeled OVA (FOva) by gel filtration on a Sephadex G10 column (Amersham Pharmacia) using PBS as the eluent. OVA (grade V) was purchased from Sigma.
Antigens were given by oral intubation with a 24-g blunt end gauge (FST, Heidelberg, Germany) or by s. c. injection at the tailbase in complete Freund's adjuvant (CFA) (Sigma)
4.4 Preparation of cells
Lymphoid organs were prepared by dissociating organs into a single-cell suspension. CD62L+ cells were obtained by positive selection with MACS (Miltenyi Biotech, Bergisch Gladbach, Germany). DC were enriched by magnetic sorting for CD11c+ cells with MACS. For analysis of proliferation, cells were labeled before transfer with CFSE (Molecular Probes, Eugene, OR) as described 33. Briefly, cells were resuspended in PBS at 107/ml and incubated with CFSE at a final concentration of 5 μM for 3 min at room temperature, followed by two washes with RPMI 1640 (Life Technologies) containing 10% FCS (Sigma). For transfer, cells were resuspended in sterile PBS and injected i. v. through the tail vein.
4.5 Flow cytometry
The following antibodies were used for flow-cytometric analysis: Mac1 (anti-CD11b)-PE, HL3 (anti-CD11c)-FITC, SA-FITC were purchased from BD PharMingen. N418 (anti-CD11c)-PE, B220 (anti-CD45R)-PE (RA3–6B2), KJ1–26.1 (anti-OVA TCR)-Cy5, anti-CD4 (GK1.5)-PE, anti-CD62L (MEL14)-Biotin, M5–114 (anti-I-A)-PE, anti-FcR (2.4G2) and monoclonal anti-FITC-Dig were conjugated in-house. Rat IgG was purchased from Biotrend, Germany.
Magnetofluorescent liposomes (Cy5) were generated and conjugated to sheep-anti-Dig (Fab fragments; Roche) as described 9. Propidium iodide and scatter gating were used to exclude dead cells. Cytometric analysis was performed on a FACSCalibur using CELLQuest research software (Becton Dickinson).
4.6 Analysis of peptide presentation
FOva was given orally in PBS, controls received only PBS. Mice were killed and cells from spleen, pLN (inguinal, brachial, axillar), mLN and PP were isolated and washed twice with PBS/BSA. Unspecific binding of antibodies was blocked by preincubation with anti-FcR (2.4G2) and rat IgG (both at 50 μg/ml). Peptide presentation was analyzed by staining with anti-FITC-Dig followed by staining with anti-Dig-liposomes as described 9. Specificity of the staining was verified by staining cells of mice that received only PBS. APC subsets were identified by staining forB220 (B cells), CD11c (DC) and CD11b (macrophages), respectively.
4.7 Analysis of protein uptake and T cell activation in vivo
BALB/c mice were adoptively transferred with 2×106 CFSE-labeled CD62L+ OVA-specific CD4+ cells. One day later mice were immunized either orally with OVA (0.5 or20 mg) or s.c. with OVA in CFA (100 μg), controls received only PBS. At the indicated time points after immunization mice were killed and cells from spleen, pLN (inguinal, brachial, axillar), mLN and PP were isolated, stained for CD4 and KJ1–26.1 and analyzed for proliferation of OVA-specific CD4+ cells.
Acknowledgements
We thank Farah Hatam for critical reading of the manuscript. This work was supported by the EC grant BIO4–98–0458.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




