TNF-α impairs peripheral tolerance towards β-cells, and local costimulation by B7.1 enhances the effector function of diabetogenic T cells
Abstract
Maintenance of peripheral tolerance and inactivation of autoreactive T cells is based on a delicate balance between pro-inflammatory and protective cytokines that is poorly understood. We havehere addressed how the local expression of the inflammatory cytokine TNF-α can impair peripheral tolerance and lead to autoreactivity. After transplantation of pancreata that are immunogenic due to β-cell expression of B7.1 and TNF-α, into thymectomized and euthymic syngeneic mice, we found that only euthymic mice rejected the grafts. This result suggests that under normal circumstances autoreactive T cells are functionally inactivated, and initiation of an autoreactive response requires de-novo generation of T cells. By contrast, thymectomized mice expressing TNF-α on the endogenous islets rejected the grafts, showing that expression of TNF-α prevents functional silencing of the autoreactive T cells. Thus, this study provides a mechanism by which TNF-α and possibly chronic inflammatory responses may promote autoimmune diseases. Furthermore, we have investigated whether B7.1 can enhance T cell responses of already activated T cells leading to islet rejection. By transplantation of wild-type and B7.1-expressing islets into overtly diabetic mice we found that only the wild-type islets could restore normoglycemia, suggesting that costimulation by B7.1 is required in the expansion or effector phase of the response.
Abbreviations:
-
- RIP:
-
Rat insulin promoter
-
- STZ:
-
Streptozotocin
-
- wt:
-
Wild-type
1 Introduction
Initiation and development of autoimmune disease is a complex process that may result from failure of self tolerance induction and aberrant activation of autoreactive T cells. Different mechanisms operate to avoid the generation of an anti-self response: negative selection of self-reactive T cells in the thymus, peripheral tolerance, and the activation of regulatory cells that can regulate the activation/differentiation of the self-reactive T cells. The mechanism of peripheral tolerance is not clear. In one model it could be a passive phenomenon where the peripheral tolerance is simply maintained due to lack of costimulatory signals. In this case the self-reactive T cells would persist, and when receiving the proper stimulus may induce autoimmune disease. Alternatively, peripheral tolerance could be an active phenomenon, where the encounter of self-reactive T cells with their cognate self antigen leads to anergy or clonal deletion. In this scenario, even the proper stimulus would not lead to autoreactivity. However, the continuous production of T cells in the thymus would still supply autoreactive T cells to the periphery thus providing a permanent risk of autoimmunity.
Induction of a local inflammatory response due to local production of cytokines and chemokines is a critical parameter in inducing an immune response. Tumor necrosis factor (TNF)-α is a pro-inflammatory cytokine that promotes up-regulation of adhesion molecules and activation of macrophages 1. TNF-α is necessary for the development of diabetes in the NOD mouse 2 and a crucial factor in experimental autoimmune encephalo-myelitis 3. On the other hand, injection of TNF-α in adult NOD mice or β-cell expression of TNF-α in adult NOD mice protects against diabetes 4, 5, showing that TNF-α also has opposing effects, which makes it difficult to precisely predict the effect of TNF-α in any given circumstance.
In this study we used rat insulin promoter (RIP)-TNFα transgenic mice and RIP-B7.1 transgenic mice as models to study aspects of peripheral tolerance and costimulation requirements. In the RIP-TNFα mice the β-cell expression of TNF-α induces up-regulation of the adhesion molecules ICAM-1 and vascular cell adhesion molecule (VCAM)-1 in the islet-associated vascular endothelium and MHC I on the β-cells 6, leading to a non-destructive infiltration of leukocytes in the islets 6, 7. These mice usually remain normoglycemic, but in combination with a second transgene, RIP-B7.1, which by itself is non-pathogenic 8, these mice (termed B7/TNFα mice) develop an immune-mediated β-cell destruction leading to hyperglycemia and diabetes at 5–6 weeks of age 9 with 100% penetrance.
Although it is evident that the combined expression of both B7.1 and TNF-α by the pancreatic β-cells is necessary for diabetes induction 9, it has not been clear at which phases of the immune response (priming, expansion and/or effector) B7.1 and TNF-α are needed in order to induce T cell-mediated β-cell death. In this study we have addressed this question by adoptive transfer of splenocytes from diabetic B7/TNFα mice into irradiated transgenic and wild-type (wt) recipients, and by transplantation of islets or fetal pancreas into diabetic B7/ TNFα mice. Furthermore, we have studied the rejection of fetal pancreata expressing B7.1 and TNF-α after transplantation into thymectomized and euthymic mice as a means to investigate mechanisms of peripheral tolerance.
2 Results
2.1 Spleen cells from both wild-type and diabetic B7/TNFα mice can accelerate diabetes upon transfer into irradiated, prediabetic B7/TNFα mice
The local expression of B7.1 and TNF-α leads to development of diabetes at 5–6 weeks of age in B7/TNFα mice. In order to assess whether this process leads to accumulation of activated, islet-reactive T cells in the spleens of diabetic B7/TNFα mice, we adoptively transferred 2×107 spleen cells from wt or diabetic B7/TNFα mice into 3–4-week-old, irradiated B7/TNFα mice. At this age the infiltration of the islets has just begun. Irradiation of the mice was not able, by itself, to inhibit diabetes but delayed the onset with 3–4 weeks (Fig. 1) thereby providing a window of 3–7 weeks to study the ability of the transferred cells to accelerate diabetes. The transferred cells from diabetic B7/TNFα mice were able to accelerate the onset of diabetes compared to control mice that did not receive cells; but to our surprise cells from wt mice accelerated diabetes onset to a similar extent as cells from diabetic B7/TNFα mice (Fig. 1), suggesting that β-cell destruction was not caused by cells that were activated already at the time of transfer but rather from de novo activated cells.
In line with this experiment we adoptively transferred cells from spleens or mesenteric lymph nodes from diabetic B7/TNFα mice into sublethally irradiated RIP-B7.1, RIP-TNFα and wt recipient mice. However, none of the recipient mice developed hyperglycemia during a 10-week observation period following injection of the cells from the diabetic B7/TNFα donor mice (Table 1, A–E), suggesting that neither the spleen nor the lymph nodes contain a sufficient number of activated islet-reactive T cells to transfer diabetes.
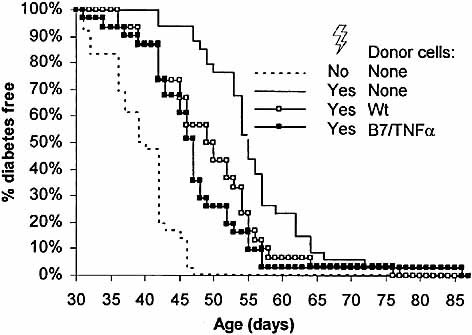
Both splenocytes from wt and diabetic B7/TNFα mice can accelerate diabetes upon transfer into irradiated B7/TNFα mice. Spleen cells (2×107) from wt mice or diabetic B7/TNFα mice were injected i.v. into 3–4-week-old B7/TNFα mice that were irradiated the day before injection. As control one group of mice was irradiated but did not receive any cells and another group was not treated at all. The mice were considered diabetic when the blood glucose level was above 15 mM in two consecutive measurements. For each group n=29–36. p=0.0002 (B7/TNFα transfer vs. no transfer), p=0.007 (wt transfer vs. no transfer) and p=0.34 (B7/TNFα transfer vs. wt transfer) by log rank test.
|
|
Donor mouse |
Tissue/cells from: |
Recipient mousea) |
Diabetes incidence |
Rejection of islet graft |
|---|---|---|---|---|---|
|
A |
B7/TNFα |
Spleen |
Wild-type |
0 of 11 |
Not relevant |
|
B |
B7/TNFα |
Spleen |
RIP-B7 |
0 of 15 |
Not relevant |
|
|
PanLN |
|
0 of 4 |
||
|
C |
B7/TNFα |
Spleen |
RIP-TNFα |
0 of 18 |
Not relevant |
|
|
PanLN |
|
0 of 3 |
||
|
D |
B7/TNFα |
Spleen |
B7/TNFα |
Accelerated (n=12) |
Not relevant |
|
E |
Wt |
Spleen |
B7/TNFα |
Accelerated (n=11) |
Not relevant |
|
F |
Wt |
Isletsb) |
Diabetic B7/TNFα |
0 of 6 |
No |
|
G |
RIP-B7 |
Isletsb) |
Diabetic B7/TNFα |
11 of 11 |
Yes |
|
H |
RIP-B7 |
Isletsb) |
wild-type |
1 of 5 |
No |
|
I |
RIP-B7 |
Isletsb) |
STZ wild-typec) |
0 of 3 |
No |
|
|
After graft removal |
3 of 3 |
|
||
- a) Sublethally irradiated mice were used as recipients for pancreatic lymph node (PanLN) cells or spleen cells.
- b) Typically, 250 islets were engrafted under the left kidney capsule.
- c) Mice were treated with 200 mg/kg STZ the day before engraftment.
2.2 Costimulation by B7.1 is necessary for the expansion/effector phase of T cell-mediated islet destruction
Originally, we assumed that the effect of TNF-α and B7.1 in the islets was to attract, activate and expand a pool of islet-reactive T cells leading to β-cell death. The above results might be explained by the lack of such an activated islet-reactive pool of T cells, suggesting that TNF-α and B7.1 in combination can confer T cell-mediated killing in an antigen-nonspecific manner leading to β-cell death. Alternatively, the majority of the pool of isletreactive T cells might reside in the islet infiltrates rather than the local lymph nodes or the spleen. To distinguish between these possibilities we chose to engraft islets into the diabetic B7/TNFα mice. In such an experimental setup, the islet resident autoreactive T cells may have the chance to leave the islets and gain access to the engrafted tissue. Grafting of islets from wt mice under the kidney capsule of diabetic B7/TNFα mice completely restored normoglycemia in the grafted mice, and the blood sugar level remained stable for the 6–8-week period of analysis (Fig. 2A and Table 1, F).
In contrast, islets from RIP-B7.1 mice could not restore normoglycemia. Rejection was very rapid; in most of the mice we observed only a slight decrease in the blood sugar level from typically >30 mM before grafting to 20–25 mM lasting for 1–2 weeks after grafting (Fig. 2B and Table 1, G). Histology of the grafts, performed 6–8 weeks (wt islets) or 2–3 weeks (RIP-B7.1 islets) after transplantation, showed that the wt islets remained free of infiltration, whereas the RIP-B7.1 islets were heavily infiltrated by mononuclear cells (Fig. 3). These results suggest that indeed diabetes in B7/TNFα mice results from activation of islet-reactive T cells that can destroy engrafted islets provided that the graft also expresses the B7.1 costimulatory molecule.
It is possible that the expression of B7.1 on the β-cells per se renders the β-cells more susceptible to the damage induced in connection to the islet isolation and transplantation procedure, leading to breakdown of some β-cells, which could initiate autoimmune destruction of the remaining β-cells. To exclude this possibility we engrafted the RIP-B7.1 islets into normal or streptozotocin (STZ)-induced diabetic wt mice. The engrafted mice remained normoglycemic in contrast to mice that received STZ but no graft (Fig. 4A and Table 1, H, I). Three weeks after transplantation the grafts were removed by nephrectomy of the engrafted kidney, which led to development of hyperglycemia in the STZ-treated mice. These data show that glucose control was dependent upon the function of the engrafted islets. Furthermore, histology showed that the grafts were free of infiltration and contained a prominent β-cell mass whereas no β-cells were seen in the endogenous islets of the STZ-treated mice (Fig. 4B–E). Thus, diabetes in the B7/ TNFα mice engrafted with the RIP-B7.1 islets is not due to technical failure of the grafts but results from an immune reaction against the graft due to expression of the costimulatory molecule B7.1 by the engrafted islets.
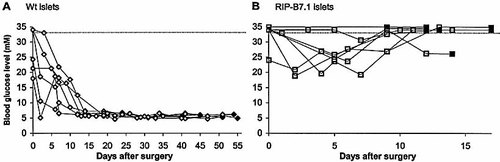
RIP-B7.1 islets but not wt islets are rejected after transplantation into diabetic B7/TNFα mice. Islets were isolated from wt and RIP-B7.1 mice and cultured for 1 week. Diabetic B7/TNFα mice received either 125–300 wt islets (A) or 250 B7.1-expressing islets (B) that were implanted under the left kidney capsule. Each line represents a single mouse. The horizontal dotted line indicates the maximum blood glucose concentration that could be measured by our instrument (33 mM); therefore higher values are arbitrarily shown as higher than 33 mM. The mice were killed after the last measurement (indicated by filled symbols) and the kidney with the graft was fixed in 4% formalin for immunohistochemistry. The data shown have been combined from three independent experiments.
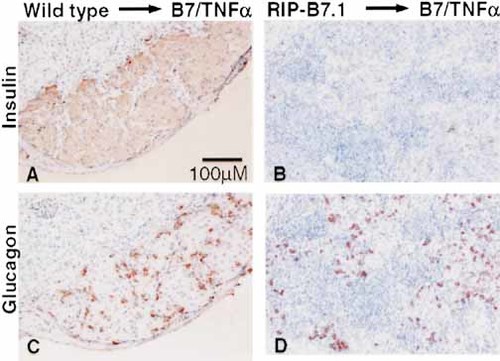
Destruction of B7.1-expressing islets after transplantation. Diabetic B7/TNFα mice were engrafted with wt islets (A, C) or RIP-B7.1 islets (B, D). Grafts were removed at 6–8 weeks (wt islets) or 12–20 days (RIP-B7.1 islets) after transplantation, and paraffin sections of the grafts were stained for insulin (upper panel) or glucagon (lower panel) and counterstained with hematoxylin.
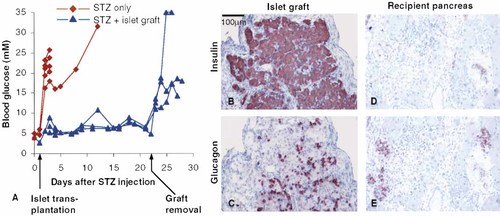
RIP-B7.1 islets can conserve normoglycemia in STZ-induced diabetic mice. Ten mice were injected i.p. with 200 mg/kg body weight of STZ at day 0, and four of the ten mice were engrafted with RIP-B7.1 islets the following day (triangles). After 3 weeks the engrafted kidney was removed and the mice were monitored for another week. Development of hyperglycemia in the remaining six mice (diamonds) was taken to indicate destruction of the endogenous β-cells caused by the STZ treatment. The data have been combined from two independent experiments.
2.3 TNF-α cannot substitute for B7.1 costimulation during the expansion/effector phase of T cell-mediated islet destruction
We further determined whether local inflammation resulting from expression of TNF-α by the islet graft may also lead to rejection of islets engrafted in diabetic B7/TNFα mice. Since it was technically not feasible to isolate intact islets from the RIP-TNFα and B7/TNFα mice due to fragility of the islets, we chose to transplant whole pancreas from day E18 fetus from the transgenic mice. Under these conditions the acinar pancreas degenerates leaving residual engrafted islets. In order to obtain donor pancreata that would only differ in their expression of the transgenes, heterozygous RIP-B7.1 mice were mated with heterozygous RIP-TNFα mice, thus yielding both wt fetuses and heterozygous RIP-B7.1, RIP-TNFα, and B7/TNFα fetuses. In concordance with the results obtained by transplantation of whole islets, the islets derived from wt fetal pancreata remained free of infiltration when fetal pancreata were transplanted into B7/TNFα transgenic mice (Fig. 5A, D). In contrast, 3 weeks after transplantation into B7/TNFα mice, the islets derived from RIP-TNFα fetal pancreata were heavily infiltrated, but retained a significant β-cell mass (Fig. 5B, E). Thus, the islets within the TNF-α-expressing grafts were very similar in appearance to the endogenous islets of RIP-TNFα mice, suggesting that TNF-α cannot efficiently turn on effector functions in T cells infiltrating the islets.
Three weeks post-transplantation of RIP-B7.1 or B7/TNFα pancreas grafts into B7/TNFα mice, the infiltration had already disappeared leaving large empty spaces and few lymphocytes behind. No normal islets were observed, but insulin- and glucagon-positive duct cells and small, islet-like clusters of α- and β-cells close to the ducts were frequently observed, suggesting that endocrine cells were generated de novo from stem cells in the ducts (Fig. 5C, F, G). Thus, islet destruction seems to be strongly accelerated by β-cell expression of B7.1 but not by TNF-α.
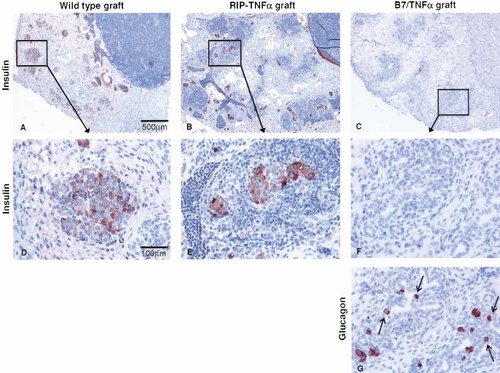
Destruction of fetal islets after transplantation. E18 fetal wt pancreata or pancreata expressing the B7.1 and/or TNF-α molecules were engrafted under the kidney capsule of diabetic B7.1/TNFα mice. The presence of intact β-cells was determined by staining with an anti-insulin antibody (A–F), while remnants of islets were identified with an anti-glucagon antibody (G) that stains all glucagon-producing α-cells. (A) Islets in wt grafts (n=8) remained free of infiltration. (B) In RIP-TNFα grafts (n=8) islets were heavily infiltrated. (C) B7/TNFα grafts (n=10) and RIP-B7.1 grafts (n=7) contained no normal islets and only little infiltration. (D–F) Higher magnification of (A–C), respectively. (G) Section adjacent to (F) stained for glucagon. Small islet-like structures and α-cells seem to be formed de novo from the ducts (indicated by arrows). In some other sections also insulin-positive duct cells were observed (data not shown).
2.4 Autoreactive T cells are functionally inactivated but generated de novo from the thymus
Confirming our previous observation indicating that the spleens of wt mice contain islet-reactive T cells that can, upon transfer into B7/TNFα mice, induce diabetes (Fig. 1), we found that wt mice were able to reject B7/ TNFα pancreas grafts leading to complete β-cell destruction 3 weeks after transplantation (Table 2, group A). We wished to determine whether rejection was mediated by recent thymic emigrants that did not have the time to reach the endogenous pancreas and become tolerant, or whether it reflects the inability of islet cells to silence autoreactive T cells. We therefore thymectomized the recipient mice 1 month before transplantation, thus preventing regeneration of the pool of circulating T cells and leaving sufficient time for tolerance to establish. We then engrafted immunogenic B7/TNFα pancreata into these thymectomized wt mice. When the grafts were examined 5–6 weeks after transplantation, six out of seven adult thymectomized mice failed to reject the B7/TNFα graft, and in four of the non-rejected grafts islets were completely free of infiltration (Table 2, group B). This result shows that thymectomy performed 1 month prior to transplantation leads to acceptance or at least to a profoundly prolonged survival of B7/TNFα grafts that would otherwise have been rejected in euthymic mice.
|
|
Rejectionb) |
|||||
|---|---|---|---|---|---|---|
|
|
Recipientsc) |
ATX |
– |
+ |
++ |
+++ |
|
A) |
–/– |
No |
|
1/7 |
2/7 |
4/7 |
|
B) |
–/– |
Yes |
4/7 |
2/7 |
|
1/7 |
|
C) |
TNFα |
Yes |
1/8 |
|
7/8 |
|
- a) Fetal B7.1/TNFα pancreata, 18 days old, were transplanted under the kidney capsule of wt mice (A, B) or RIP-TNFα mice (C); ATX: thymectomized.
- b) The grafts were read blind. Islets were considered as non-rejected when no lymphocytic infiltration was observed within the graft (scored as –) or when some lymphocytic infiltration was observed but β-cells could clearly be identified by insulin staining (scored as +). The grafts were considered as rejected when only glucagon-producing α-cells but no β-cells could bedetected. Rejected grafts were scored as either 2+ when some lymphocytic infiltration was still observed within the graft, or 3+ when no lymphocytic infiltration was detected. Statistical analysis comparing rejected vs. non-rejected grafts: p=0.03 (A vs. B) and p=0.01 (B vs. C) by Fisher exact test, two-tailed.
- c) Mice from group B and C were thymectomized at 4–5 weeks of age and rested for 1 month before transplantation. Group B and C mice were analyzed 5–6 weeks after transplantation while group A mice were analyzed 3 weeks after transplantation.
2.5 TNF-α inhibits the functional silencing of autoreactive T cells
By a similar approach we determined whether expression of the inflammatory cytokine TNF-α may prevent the silencing of the islet-reactive T cells. As before we transplanted immunogenic B7/TNFα islets into adult thymectomized RIP-TNFα transgenic mice. We found that in seven out of eigth thymectomized RIP-TNFα recipient mice the B7/TNFα pancreas grafts were rejected (Table 2, group C). This result suggests that the expression of TNF-α in the islets sustained the ability of the infiltrating T cells to respond to the engrafted islets and reject the B7/TNFα pancreas grafts.
3 Discussion
Whereas several studies have demonstrated the importance of costimulation by B7.1 and B7.2 for activation of naive T cells, the requirement for costimulation by the B7 molecules in the expansion/effector phase is still obscure. While some studies found that cross-linking of CD28 by APC or antibody could not augment anti-CD3-induced T cell proliferation of activated T cells in vitro 10–13, others have shown that B7 costimulation during the expansion/effector phase is important in vivo, both in a model of experimental autoimmune encephalomyelitis, where disease was dependent on host expression of B7 14, and in rejection of peripheral solid tumors, where tumor control was dependent on tumor expression of B7 15. Here we show that in the B7/TNFα model of autoimmune diabetes, costimulation is important not only during the activation phase but also during the expansion/effector phase of the response, since RIP-B7.1 islets but not wt islets were rejected when transplanted into diabetic B7/TNFα mice. This notion is further supported by the observation that thymectomized RIP-TNFα transgenic mice did not develop diabetes despite rejection of B7.1/TNFα grafts.
The function of B7.1 on the engrafted islets may be to augment proliferation and to protect the T cells against apoptosis, which would lead to expansion of the pool of islet-reactive T cells within the islet grafts. Furthermore, B7.1 might strengthen the binding of cytotoxic T cells to the β-cells, thereby facilitating lysis of the β-cells by CTL. In support of this notion, Flynnet al. 16 showed that B7.1-transfected P815 mastocytoma cells can induce cytolytic activity in T cells isolated from influenza virus-primed mice, even in the absence of antigen on the target cell. Rejection of RIP-B7.1 islets is not the result of minor histo-incompatibilities, as RIP-B7.1 islets are not rejected upon engraftment into wt mice. Furthermore, B7/TNFα mice were always generated by intercrossing homozygous RIP-B7.1 and RIP-TNFα mice; thus the RIP-B7.1 islets are unlikely to express minor histocompatibility genes that are not expressed by the B7/TNFα mice.
The finding that RIP-B7.1 islet grafts are rejected in B7/TNFα mice suggests that TNF-α, in contrast to B7.1, is needed only for the initiation of the response. This is in line with the study by Green et al. 17, which showed that in the B7/TNFα mice β-cell expression of TNF-α is only needed to induce diabetes until 25 days after birth, at which point the islet infiltration has been established. Furthermore, the requirement for B7.1 in the effector phase cannot be replaced by TNF-α, as RIP-TNFα fetal pancreata preserve a significant β-cell mass upon engraftment into B7/TNFα mice. Thus, once an islet-specific response has been primed, the transgenically produced TNF-α is neither required nor sufficient to fuel β-cell destruction in this model.
To our surprise spleen or pancreatic lymph node cells from diabetic B7/TNFα mice were not able to induce diabetes upon transfer into wt or RIP-B7.1 mice. This result could be taken as indication for the lack of antigen specificity in this model, but another possibility is that the expression of chemokines and up-regulation of adhesion molecules induced by islet TNF-α expression might be required even for the activated T cells to home to the islets, thus explaining the inability of spleen or lymph node cells to transfer diabetes to RIP-B7.1 mice. In the RIP-B7.1 islet graftsthe requirement for TNF-α might be circumvented due to inflammation created by the surgical procedure itself.
A third possibility is that activated, islet-reactive T cells do not accumulate in the spleen, implying that the local expression of B7.1 and TNF-α enables the T cells to be activated locally within the pancreas. This notion is supported by the observation that there was no difference in the ability of spleen cells from wt donors compared to spleen cells from diabetic B7/TNFα mice to accelerate diabetes upon transfer into prediabetic, sublethally irradiated B7/TNFα mice. These results further suggest that wt mice that never develop diabetes retain sufficient leukocytes that, when confronted with syngeneic islets expressing the appropriate costimulatory and inflammatory environment, can become activated and cause diabetes.
Despite a large number of studies, the mechanisms that lead to peripheral tolerance have remained controversial. In most cases, transgenic mice with expression of a defined antigen on a tissueare tolerant in vivo. While in some models tolerance is associated with a specific inactivation or deletion of self-reactive T cells 18–20, in other models self-reactive T cells remained functional and could participate in an autoimmune response when appropriately activated 21, 22. It was unclear whether the diversity of the published results reflects the plasticity of the immune system or instead results from artifacts inherent to the transgenic systems used such as leaky gene expression in the thymus, an abnormally high expression of the antigen by the targeted tissue or an abnormally high frequency of self-reactive T cells due to the use of TCR transgenic mice. Taking advantage of the immunogenicity of islets expressing the B7.1 molecule and high level of MHC we could directly analyze the mechanisms leading to tolerance under "normal" conditions, e.g. an unbiased T cell repertoire and naturally expressed peripheral antigens in C57BL/6 mice. Here we report two key findings that might reconcile these previous results.
First, we found that thymectomized, non-transgenic mice do not reject syngeneic pancreatic grafts that express the B7.1 and TNF-α molecules, at least for the 5–6-week time period of observation in our experiments. In contrast, euthymic mice readily reject syngeneic grafts that express the B7.1 and TNF-α molecules, leading to the complete destruction of the islets inside the grafts within 3 weeks. These results suggest that cells capable of graft rejection are constantly exported from the thymus, and that indeed recent thymic emigrants are responsible for islet destruction. When they are eliminated by thymectomy, the remaining islet-reactive T cells, because of a lack of proper activation and costimulation, are functionally inactivated over time and are therefore incapable of rejecting the B7/TNFα-expressing graft. Thus, our results indicate that tolerance is not just maintained due to "ignorance", but additionally results from the active inactivation of autoreactive T cells.
Secondly, we show that thymectomized RIP-TNFα transgenic mice, by contrast to non-transgenic mice, maintained the ability to reject syngeneic islet grafts expressing B7.1 and TNF-α. This observation suggests that in RIP-TNFα transgenic mice T cells with the capability to destroy the β-cells remained functional while in non-transgenic mice these T cells were inactivated. Thus, the expression of TNF-α in islets may prevent tolerance induction by this tissue. This result extends prior observations made in transgenic mice expressing the lymphocytic choriomeningitis virus (LCMV) glyco- or nucleoprotein on the islets of Langerhans 21–23. Indeed, these transgenic mice that appeared tolerant in vivo to the transgenically encoded LCMV protein developed autoimmune diabetes following an infection with the LCMV virus. In both cases self-reactive peripheral T cells are tolerant as long as they do not receive the necessary stimulatory signals as elicited either by TNF-α or by infection with LCMV virus.
The demonstration that the inflammatory cytokine TNF-α can also interfere with tolerance induction by tissue cells provides a novel mechanism by which inflammatory responses could affect immune and autoimmune responses. As suggested by Matzinger 24, these properties of TNF-α in preventing T cell inactivation might reflect the general role of a local inflammation in self/non-self discrimination and thus in the development of a productive immune response. Indeed, TNF-α is a ubiquitous cytokine which is produced early during most immune responses primarily by macrophages but also by some T cells and some tissue cells 25. If it was present at the early stage of an infection (as is likely), TNF-α might not only favor the recruitment of antigen-specific T cells in situ but also prevent the specific inactivation of these T cells by infected tissue and thus increase the frequency of antigen-reactive T cells. Furthermore, TNF-α may cause a decrease in the number of CD4+CD25+ T cells as recently demonstrated by Green et al. in RIP-TNFα mice 26 and by Wu et al. in NOD mice 27, thereby imposing fewer restraints on the proliferation and activation of autoreactive T cells. As exemplified in the RIP-TNFα transgenic mice, this event, by itself, is notsufficient to lead to the activation of autoreactive T cells and thus autoimmunity. In our model, autoreactive T cells are activated only when the engrafted islets are immunogenic owing to the local expression of the B7.1 molecule.
4 Materials and methods
4.1 Mice
RIP-B7.1 transgenic and RIP-TNFα transgenic mice are transgenic for the human B7.1 molecule or the mouse TNF-α cytokine, respectively, driven by RIP II 6, 9. RIP-B7.1 and RIP-TNFα mice were backcrossed at least five and three times, respectively, to C57BL/6. B7/TNFα mice were generated by intercrossing homozygous RIP-B7.1 and RIP-TNFα transgenic mice. In all experiments where wt mice were used, these were C57BL/6 mice.
4.2 Adoptive transfer
The recipient mice received a sublethal dose of γ-irradiation (560–700 rad), and on the following day they were injected i.v. with 2×107 erythrocyte-depleted spleen cells. Wt, RIP-B7.1 and RIP-TNFα recipient mice were 6–11 weeks old at the time of transfer whereas B7/TNFα recipients were 3–4 weeks old. Urinary sugar was measured twice weekly, and when positive, the blood glucose level was determined. The mice were defined as diabetic when the blood glucose level in two consecutive measurements exceeded 15 mM.
4.3 Islet transplantations
Islets were isolated from 6–8-week-old mice by collagenase treatment of the pancreas followed by handpicking of the islets. The islets were cultured for 6 days at 37°C in RPMI supplemented with 10% FBS, 11 mM glucose, 2 mM glutamine, 20 mM Hepes, 100 IU/ml penicillin and 100 μg/ml streptomycin. The day before transplantation, viable islets (as assessed by visual inspection) were recounted and transferred to medium containing 0.5% NU serum instead of calf serum. Diabetic B7/TNFα mice were anesthetized, and 250 islets were placed under the left kidney capsule.
4.4 Streptozotocin injections
STZ was dissolved in 0.1 M citric acid, pH 4.6, to 20 mg/ml, and the mice were immediately injected i.p. with 200 mg STZ/kg body weight. Engraftment of islets was done the following day beforedevelopment of hyperglycemia.
4.5 Grafting of embryonic pancreas
Heterozygous RIP-B7.1 and RIP-TNFα mice were mated to generate all possible combinations of the transgenes, and E18 embryos were collected and sexed, and the fetal liver and pancreas were taken. The pancreata were cultured for 1–2 days prior to engraftment under the kidney capsule. Meanwhile, the embryos were typed by PCR on liver DNA 8.
4.6 Immunohistochemistry
The kidneys with the grafted islets or pancreata were fixed in 4% formalin and embedded in paraffin. Sections (4 μm) were stained for insulin (HUI-18 mouse anti-insulin, Novo Nordisk A/S) or glucagon (Glu-001 mouse anti-glucagon, Novo Nordisk A/S) and counterstained with hematoxylin.
4.7 Scoring of the rejection
The degree of rejection was determined by histological observation as well as immunostaining of insulin-producing β-cells and glucagon-producing α-cells and arbitrarily quantified as indicated in Table 2. Only grafts where some remnant of tissue could be observed were further considered in the study to discard any technical artifacts inherent to the surgery.
4.8 Adult thymectomy
Adult thymectomy was performed on 4–6-week-old mice by suction of the thymic lobes. Mice were allowed to rest for 1 month before grafting.
Acknowledgements
We would like to thank Eva Krøyer and Camilla Stewart for invaluable assistance with animal experiments and D. Butkus for help with the surgery. The Hagedorn Research Institute is an independent research component of Novo Nordisk A/S. R. A. Flavell is an investigator of the Howard Hughes Medical Institute. This work was supported by a research grant toB. K. Michelsen from the Danish Research Agency under the FREJA program, a Ph.D. stipend from the Danish Research Academy to K. Skak, a grant from the NIH to R. A. Flavell, a Cancer Research Institute/Rudolph M. Montgelas Fellowship to S. Guerder, the Yale Diabetes Endocrinology Research Center, and Novo Nordisk A/S.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




