ICAM-1 isoforms: specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G
Abstract
The extracellular moiety of ICAM-1 consists of five Ig-like domains, the first and third domains mediating adhesion to integrin ligands. The ICAM-1 gene, however, gives rise to the expression of five alternative splice variants containing two, three, or four Ig-like domains. In this work, we have investigated whether the rearrangement of the architecture of ICAM-1 affects its structural properties and function. We showed that, in contrast to the common form, all alternative isoforms of ICAM-1 were susceptible to cleavage by leukocyte elastase and cathepsin G. We found that the length of an isoform did not influence the susceptibility to proteolysis. The molecular diversity provided by the skipping of entire Ig domains and the level of expression on the APC, however, significantly influenced their ability to potentiate the proliferation of T cells. Finally, we found that the expression of minor ICAM-1 isoforms encoding the third Ig-like domains was sufficient to sustain neutrophil infiltration in the liver and confer exon-5-targeted ICAM-1-deficient mice susceptibility to LPS-induced septic shock. These findings not only demonstrate that ICAM-1 isoforms are fully functional, but support the concept that alternative RNA splicing in the Ig superfamily may fulfill distinct roles during the development of the immune response.
Abbreviations:
-
- HLE:
-
Human leukocyte elastase
-
- CF:
-
Cystic fibrosis
-
- MFI:
-
Mean fluorescent intensity
1 Introduction
Intercellular adhesion molecule-1 (ICAM-1) is membrane glycoprotein of the Ig superfamily consisting of five extracellular Ig-like domains, a transmembrane domain, and a short cytoplasmic tail1. ICAM-1 plays an important role in inflammatory processes and immune responses. It functions as a major costimulatory molecule during antigen presentation to T cells, most notably in the context of antigenic peptides complexed to MHC class II molecules 2, 3. Endothelial ICAM-1 promotes firm adhesion of leukocytes to vascular endothelium, allowing their migration to sites of inflammation 4.
The common form of ICAM-1 contains five extracellular Ig-like domains, a hydrophobic domain, and a short cytoplasmic domain 5. The first and third Ig-like domains are responsible for binding to LFA-1 and Mac-1, respectively, both members of the β2 family of leukocyte integrins 6, 7. On the cell surface, ICAM-1 has been shown to exist as a non-covalently-linked dimer and larger multimer 8, 9. Analyses by crystallography 10, electron microscopy 11, 12, and antibody binding 8, 9 suggest that specific structural features of the five Ig-like domains of ICAM-1 (such as a D3-D4 bend, a specific D1 toD2 orientation, which favors a hydrophobic dimerization interface in the first domain opposite of the ligand-binding site, and the implication of D5 and perhaps transmembrane domain 8, 9) result in the formation of a closed ring-like dimeric structure 13. Although ICAM-1 monomers are fully competent to bind LFA-1, it is likely that dimerizationserves to properly orientate ICAM-1 and present it for binding to LFA-1 14.
Generation of ICAM-1-deficient mouse models, targeting either exon 4 or 5 15, 16, which encode the third and fourth Ig-like extracellular domains, respectively, led to the unexpected discovery of the existence of isoforms of ICAM-1 generated by alternative splicing 17. The length of the isoforms varies, the shortest of the isoforms expressing only domains 1 and 5. The impact of such molecular diversity on the ability of ICAM-1 to bind LFA-1 when expressed at the cell surface remains, however, unknown. A similar diversity in structure has been observed in other molecules of the Ig superfamily of adhesion molecules, such as VCAM-1 and CD31 18, 19. These receptors, just like ICAM-1 and its isoforms, have been shown to exist in a soluble form in circulation 20, although the proteases that are responsible for their cleavage remain poorly characterized. How the rearrangement of the architecture of cell adhesion molecules affects their susceptibility to proteolysis or their functions also remains unknown. In the present work, we have characterized the functional properties of ICAM-1 isoforms when expressed at the cell surface, and provide unique information as to the structure-function properties of ICAM-1 isoforms, which suggests that the complex pattern of splicing events observed within members of the family of adhesion molecules may act as a versatile mechanism to modulate intercellular adhesion.
2 Results
2.1 Stable expression of the different isoforms of ICAM-1
We have previously shown that the common form of ICAM-1 is susceptible to cleavage by human leukocyte elastase (HLE) 21. The structure of the five alternative isoforms is shown (Fig. 1A). Here, we have generated a series of stable transfectants expressing each of the isoforms, including the common form of ICAM-1 (Fig. 1B) and tested their susceptibility cleavage by HLE. Our results showed that, although all alternative isoforms were sensitive to HLE, the 2–4 isoform, and to a lesser extent the 2–5 isoform, were the most susceptible to proteolysis by HLE (Fig. 2A). In contrast, the common form of ICAM-1 was undoubtedly more resistant. While most isoforms were cleaved by up to 75% after 2 h, the wild type was not cleaved by more than 15%. A similar pattern of susceptibility was observed when cells were treated with increasing concentrations of HLE for 1 h (Fig. 2B). The susceptibility of cleavage between the common form of ICAM-1 and its isoforms was not due to the turnover/recycling rates of the ICAM-1 molecules, as we found that turnover/recycling rates of the common form was actually slower than that of the 3–6 isoform (Fig. 2C).
To test whether the density of ICAM-1 at cell surface could dictate the susceptibility to HLE, stable transfectants of Hi-7 fibroblasts expressing alternative isoforms of ICAM-1 at levels of expression that differed from those found on RT-4 transfectants were used (Fig. 3A). Our results showed that the level of expression of surface ICAM-1 did not influence the susceptibility of the isoforms to HLE.
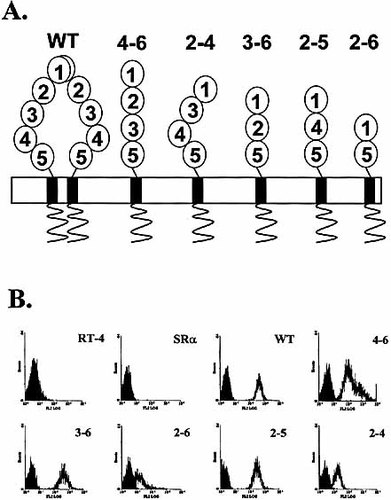
The ICAM-1 isoforms. (A) The structure is based on the nucleotide sequence of mRNA described in the study of King et al. 17. The bend between D3 and D4 and the dimerization is predicted by electron microscopy and by studies at the biochemical and biophysical levels of ICAM-1, as recently discussed in details by Wang and Springer 13. (B) Expression of the ICAM-1 isoforms by RT-4 transfectants. Cell surface external labeling was performed by flow cytometry (shaded area=control). Similar results were obtained using Hi-7 transfectants. The results are representative of at least three independent experiments.
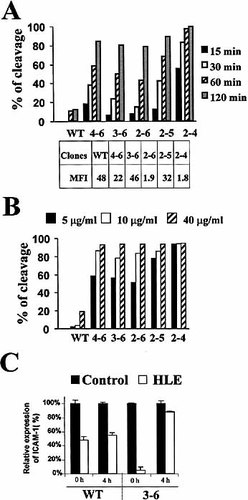
Cleavage of ICAM-1 isoforms by HLE. The RT-4 transfectants were incubated with HLE (5 μg/ml) for various times (A) or at the indicated concentrations of HLE for 60 min (B). Cleavage of isoforms or the common form (WT; wild-type) of ICAM-1 was measured by flow cytometry. Levels of ICAM-1 expression are shown as MFI. MFI on control transfectants (untransfected RT-4 cells or RT-4 cells transfected with the control vector) was less than 0.01. In (C), re-expression of ICAM-1 after cleavage with HLE. RT-4 cells transfected (WT and 3–6) were treated for 2 h with HLE (30 μg/ml). Data are representative of at least three experiments.
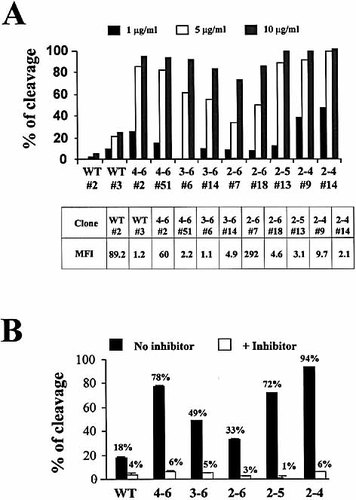
Dose-dependent cleavage of ICAM-1 expressed on Hi-7 stable transfectants and by sputum samples of CF patients. (A) Analysis of cleavage on Hi-7 cells following treatment with the indicated concentrations of HLE for 60 min. MFI on control transfectants (untransfected Hi-7 cells or Hi-7 cells transfected with the control vector) was <0.01. Results are representative of two independent experiments. (B) Cleavage on RT-4 transfectants treated for 2 h with sputum samples (final dilution: 1/10) with (white bars) or without (black bars) HLE-specific inhibitor. Results are representative of at least two independent experiments.
2.2 Cleavage of ICAM-1 isoforms by sputum of cystic fibrosis patients
We have previously demonstrated that sputum samples from cystic fibrosis (CF) patients contained a sufficient concentration of proteases to cleave the common form of ICAM-1 21. We thus tested the pattern of susceptibility of alternative isoforms using sputum of CF patients. We obtained a dose-dependent cleavage of all isoforms and found a similar pattern of susceptibility to that observed using recombinant HLE (Fig. 3B). In all cases, the cleavage obtained using the sputum was mediated mostly by natural HLE, as shown by the ability of HLE-blocking peptides to prevent cleavage.
2.3 Identification of cathepsin G as a novel protease that cleaves ICAM-1
We consistently observed a residual MSAAPVCK-resistant cleavage of ICAM-1 in the sputum; but neither E64 (a cysteine protease inhibitor), nor pepstatin (an aspartate protease inhibitor) could inhibit the cleavage of ICAM-1 by the sputum (data not shown). Since cathepsin G had been identified in the sputum of CF patients 22, 23, we investigated whether this protease could cleave ICAM-1 isoforms. We found that the common form of ICAM-1 was also resistant to cleavage by cathepsin G (Fig. 4). In contrast, all alternative isoforms were sensitive to cathepsin G, although the pattern of susceptibility was somewhat different than that observed with HLE. Indeed, we consistently observed that the 2–4, 2–5 and 2–6 isoforms were more sensitive than the 4–6 and 3–6.
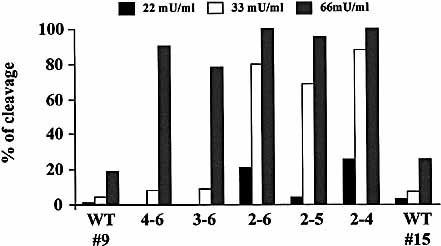
Cleavage of ICAM-1 isoforms by cathepsin G. RT-4 transfectants were treated for 1 h with cathepsin G. Two RT-4 transfectants expressing the common form of ICAM-1 (WT 9: MFI=104, and WT 15: MFI=3.4) were tested. Results are representative of at least two independent experiments.
2.4 Biochemical analysis of ICAM-1 cleavage
HLE and cathepsin G have previously been reported to cleave, with distinctive capacity, other cell surface receptors of the Ig superfamily such as CD2, CD4, and CD8 24. The question arose therefore whether both enzymes cleaved ICAM-1 using the same target site(s). We therefore compared the proteolytic fragments generated after cleavage by both enzymes by Western blotting with a domain 1-specific antibody. We found that, although both enzymes are known to have a distinct repertoire of physiological substrates, they generated a similar pattern of proteolytic fragments, suggesting that they have common cleavage sites on ICAM-1 (Fig. 5).
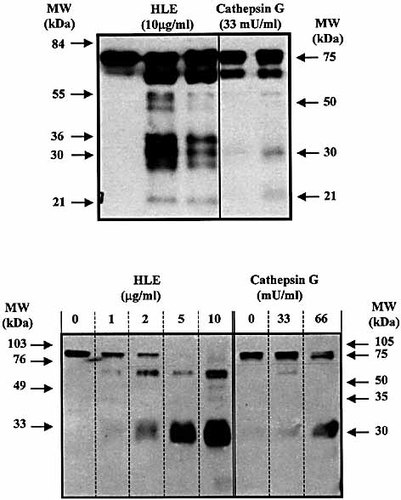
Cleavage of human soluble ICAM-1 by HLE and cathepsin G. Purified human sICAM-1 (300 ng) was incubated for the indicated times (upper gel) or for 1 h (lower gel) with HLE and cathepsin G. Proteolysis of ICAM-1 was determined by gel electrophoresis on SDS-PAGE (12 %) and visualized by Western blot. Results are representative of three independent experiments.
2.5 Do ICAM-1 isoforms act as accessory molecules during antigen presentation?
We used the Hi-7 transfectants expressing the common form of ICAM-1 and its isoforms as well as I-Ek, and tested the ability of isoforms to act as accessory molecules during antigen presentation using Hb(64–76)-specific, I-Ek-restricted, LFA-1-expressing splenic CD4+ T lymphocytes isolated from 2.102tg transgenic mice 25. All clones expressed similar levels of MHC class II type I-Ek (Fig. 6). For each isoform, we chose a clone expressing a similar level of ICAM-1, and another clone expressing a higher level (except for the 2–5 isoform, which has previously shown to be incapable of binding to LFA-1 in its chimeric form) 17. We found a variability in the efficiency of the different ICAM-1 isoforms in presentation of Hb(64–76) peptide to naive CD4+ (Fig. 6). None of the isoforms was more efficient than the common form of ICAM-1 to act as accessory molecules. All, but the 2–5 isoform, could nevertheless increase the ability of CD4-positive T cells to proliferate in response to antigenic peptides. In all cases, the ability of the isoforms to enhance antigen presentation of antigenic peptides was completely inhibited by addition ICAM-1-specific antibody (data not shown). The 2–4 isoform, which is the only one that has an intact D3-D4 domain junction, was the most efficient isoform in its ability to act as an accessory molecule, despite the fact the transfectants used expressed relatively low levels of the isoform at its surface. It is noteworthy that shorter isoforms were functional if they were expressed at very high levels on the cell surface. This was particularly well illustrated in the case of the shortest form of ICAM-1, the 2–6 isoform, which contains only two Ig-like domains.
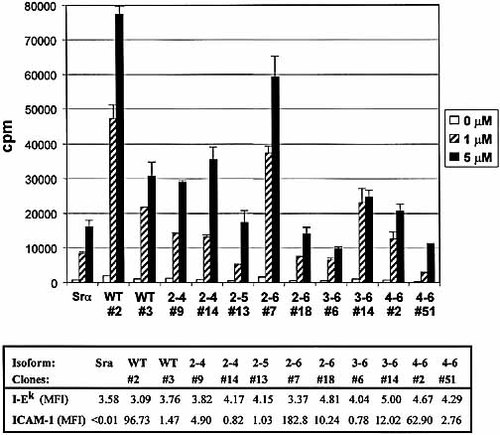
Functional capacity of ICAM-1-isoform in presentation of antigenic peptides. Proliferation of CD4-positive splenic T cells from 2.102 transgenic mice during presentation of Hb(64–76) peptide by Hi-7 transfectants expressing ICAM-1 isoforms versus cells transfected with pSrα vector alone. MFI for MHC class II I-EK and ICAM-1 levels for each clone is indicated below the histogram. Data are representatives of four independent experiments.
2.6 Are ICAM-1 isoforms functional in vivo?
To determine whether isoforms are functional in vivo, we took advantage of the previous observation that exon-4-targeted ICAM-1-deficient mice are resistant to LPS-induced septic shock 15. We also took into consideration that exon 5-targeted ICAM-1-deficient mice express a residual level of a repertoire of putative Mac-1-binding ICAM-1-isoforms 17. Groups of C57BL/6, C57BL/6Tm1Bay and C57BL/6Tm1jcgr mice were injected i.p. with lethal dose of LPS and the mortality rate was compared. Our results showed that the majority of C57BL/6Tm1Bay (10/13) and half of normal C57BL/6 (7/14) mice died of septic shock within 48 h of injection. Interestingly, C57BL/6Tm1jcgr mice survived to the LPS injection (0/12), although they showed symptoms of endotoxin shock such as shivering and lethargy. Immunohistochemical analysis of liver sections showed massive neutrophil invasion in LPS-injected normal and C57BL/6Tm1Bay mice, but not in C57BL/6Tm1jcgr mice (Fig. 7A). Using oligonucleotides specific for murine ICAM-1 domains, we found that C57BL/6Tm1Bay, but not C57BL/6Tm1jcgr mice, expressed ICAM-1 isoforms following LPS injection in the liver, most specifically the 2–6 and the 4–6 isoforms (Fig. 7B). No isoforms were detectable at the mRNA level in the mutant mice in control mice injected with PBS alone (data not shown). These results show that the repertoire of isoforms expressed in the liver of C57BL/6Tm1Bay mice can sustain neutrophil infiltration and is sufficient to confer susceptibility to septic shock.
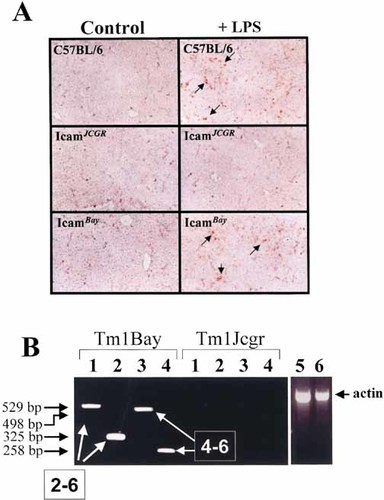
Induction of septic shock in normal and ICAM-1-deficient mice. (A) Analysis of liver for neutrophil infiltration 24 h after induction of septic shock. The presence of infiltrating Ly-6G+ neutrophils, as assessed by immunohistochemistry, appears red on sections. Original magnification, ×100. (B) Expression of ICAM-1-isoforms at the mRNA in the liver of ICAM-1-deficient mice 24 h after injection of LPS. RT-PCR analysis was performed using two sets of sense primers for D1 [lane 1: D1(ATG); lane 2: D2(Xho)], and one for D2 (lane 3), and one for D3 (lane 4), using an D5-specific antisense oligo in all PCR-reactions. This strategy allows to detect specific ICAM-1 isoforms in both TM1Bay and Tm1Jcgr mutant mice 17, 36. β-actin controls for Tm1Bay (lane 5) and Tm1Jcgr (lane 6) are shown. Results are representative of two independent experiments.
3 Discussion
In the present work, we report that alternative spliced forms of ICAM-1 have distinctive functional features. They are more susceptible to proteolytic cleavage by HLE and cathepsin G than the common form; the expression of minor ICAM-1 isoforms encoding the third Ig-like domains can support neutrophil infiltration in the liver and are sufficient to confer susceptibility to LPS-induced septic shock; most of the isoforms have the capacity to act as accessory molecule during presentation of antigenic peptides to T cells, although the molecular structure resulting from alternative splicing and the level of expression significantly influenced their ability to potentiate the proliferation of T cells.
Using transfectants expressing different levels of each of the isoforms, we further established that: (1) cleavage of ICAM-1 molecules was independent of their density at the cell surface and of the number of Ig-like domains, and (2) ICAM-1 molecules show a similar pattern of susceptibility to both HLE and cathepsin G, two major proteases found in neutrophils and sputum samples of CF patients. These results indicate that alternative isoforms of ICAM-1 are physiologically distinct from the common form, and that their high susceptibility to proteolysis may represent a new mechanism to rapidly and efficiently regulate the level of cell surface expression of ICAM-1 and LFA-1-mediated adhesion.
Our findings demonstrate that cleavage of membrane-bound ICAM-1 isoforms is not dependent on the presence of specific Ig-like domains. In contrast to what could have been expected, the length of the molecules does not influence their susceptibility to cleavage. The 2–6 splice variant, which barely protrudes above the glycocalyx, was found to be highly susceptible to cleavage, suggesting that the juxtaposition of the domains rather than the length of a molecule, is a predominant factor in conferring Ig-like molecules susceptibility to proteolysis. In fact, it is the longest of the ICAM-1 molecules, the common form, which is the most resistant to proteolysis. This leads to the hypothesis that the relative susceptibility of the different forms of ICAM-1 to proteolysis is dictated at least in part by their ability to dimerize or to form larger multimeric complexes. Indeed, several observations suggests that formation of a closed ring-like structure of ICAM-1 requires specific structural features, such as a D3-D4 bend and a specific D1 to D2 orientation that might be difficult to achieve by the deletion of D2, D3, and/or D4 13. Thus, the fact that the common form is cleaved by proteases might be explained by the fact that only a fraction of the common form of ICAM-1 is found as a dimer at the cell surface 6, 7. Alternatively, if dimerization requires only the D5, transmembrane and cytoplasmic domains, as recently suggested by Jun et al. 19, the resistance of the common form would be consistent with its unique capacity to form a closed, ring-like structure. Conversion to an "opened", "w-shaped" structure, following interaction with another ICAM-1 dimer, together with some hinge-likemotions between domains 1 and 5, would then allow exposure of putative protease cleavage sites. Questions of the physiological relevance of ICAM-1 dimers have been raised by the recent indications that dimerization of ICAM-1, in contrast to what would have been expected from its capacity to present two LFA-1 binding sites, does not enhance adhesiveness 26. Although alternative roles for dimerization of ICAM-1 most likely include its ability to properly present the binding epitope to LFA-1, we propose that the existence in ICAM-1 of such ring-like dimers may be used to protect ICAM-1-mediated cell-cell interactions from proteolysis during cell trafficking. Alternatively, rapid removal of ICAM-1 isoforms from the surface of antigen-presenting cells (APC) by proteolysiscould be used as a means to modify the type of cytokines produced during T cell responses 27.
Since ICAM-1 molecules contain the first and fifth Ig-like domains, while maintaining the structural integrity of the first domain, it is likely that the cleavage site is located near the membrane proximal domain of the molecules. This view is supported by biochemical characteristics of the soluble forms of ICAM-1 found in serum of humans or animal models, as well as the ability of antibodies to bind to all domains of the soluble form of ICAM-1 27. We have found that soluble forms of the 4–6, 3–6, 2–5 isoforms, and that of the common form, were all susceptible tocleavage and were also all detectable by ELISA testing (data not shown). The dominance of an alternative isoform found in the serum of the mutant mice, or a given pathology, is thus most likely dueto the level of expression of specific splice variants at the membrane level and/or the local release of proteases that are responsible for the cleavage of the isoforms. We found, however, that both HLE and cathepsin G can further cleave ICAM-1 in smaller fragments of approximately 60 and 25 kDa, both of which contain the first Ig-like domain. However, in such conditions as CF, concentrations of proteases in lung fluids might be too high to measure any detectable levels of structurally conserved soluble forms of ICAM-1 21. In the epithelial lining fluid of patients with CF, we and others have reported for instance that concentrations of active elastase found in these fluids vary between 100 and 750 μg/ml, i.e. in sufficient amounts to significantly modulate the cleavage of ICAM-1 from the cell surface of leukocytes 21, 28. One must, thus, exercise care in the use of ELISA for the quantitative measurements of soluble isoforms in biological samples, as this approach is dependent on the proper recognition of specific epitopes. This would be severely compromised here by the facts that: (1) isoforms of ICAM-1 are generated from alternative splicing (thus containing between two and five Ig-like domains), (2) isoforms can theoretically be found in monomeric or multimeric forms in the supernatant; and (3) the Ig-like domains can form distinct inter-domains junctions, again affecting epitope recognition.
It has been well established that secretion of extracellular proteases plays a key role in inflammatory lung disorders by altering the host's immune response against opportunistic infectious agents 29. In CF, for instance, killing of opportunistic pathogens such as Pseudomonas aeruginosa is ineffective because high concentration of neutrophil-derived elastase is released in the extracellular space, thereby reducing phagocytosis of pathogens. We have also recently shown that virus infection of the lungs can lead to a transient increase in proteolyticactivity that favors the establishment of opportunistic infections 30. Indeed, excess production of proteases can modulate the local immune response by cleaving a relatively large repertoire of molecules implicated in the development of the immune response, including ICAM-1 21, 31, 32. Our present work suggests that the common form of ICAM-1 is expressed as a relatively protease-resistant multimeric complex capable of mediating stable cell-cell interaction in a protease-rich environment, while cell-cell interactions implicating minor isoforms would be highly unstable and of short duration in such environment. Furthermore, we have now identified two proteases that can cleave ICAM-1. Both enzymes show very distinct susceptibility to protease inhibitors. While HLE is inhibited by the α-1 protease inhibitor, α2-macroglobulin, as well as smaller inhibitors such as elafin and secretory leukoprotease inhibitor, cathepsin G is mostly inhibited by α1-antichymotrypsin, a major acute phase reactant which is up-regulated during inflammatory episodes to compensate for the excess of enzymes released from activated neutrophils 33. Thus, depending on the physiological situation, cathepsin G could represent a new alternative pathway that modulates the expression of ICAM-1 on the cell surface.
Our results showed that, apart from the common form of ICAM-1, other isoforms were also capable of acting as accessory molecules in antigen presentation. The exception was the 2–5 isoform, consistent with the previous observation that this isoform does not bind LFA-1 17. Among the transfectants, the 2–4 isoform was the most potent if we take into consideration that thelevel of expression required to fulfill its accessory function was the closest to that of the common form. Interestingly, the 2–4 isoform is the only one that has conserved the D3-D4 junction, previously shown to confer the common form with the ability to "bend" and properly orientate its LFA-1 binding site, in contrast with the other isoforms, which have a more rigid conformation and point directly away from the surface of the cell. This leads to the hypothesis that the relative efficiency of the different isoforms in T cells activation might be dictated at least in part by their ability to dimerize or to form larger multimeric complexes. The 4–6 isoform, which is very efficient in binding LFA-1 17 in its chimeric form, showed very limited efficiency in its ability to activate T cells when expressed at the surface of APC. A second useful observation was that the 2–6 isoform could still potentiate antigen presentation despite its short structure. Using a clone expressing very high levels of this short isoform, we were able to establish that a short version of ICAM-1 containing only two Ig-like domain, thus without the capacity to bend or to protrude above the glycocalyx can be a very potent accessory molecule during antigen presentation, provided its expression level was very high. This observation is not only relevant in the context of ICAM-1 isoform, but also in the context of other Ig-like adhesion molecules expressed on APC, such as ICAM-2. ICAM-2 also binds LFA-1 through its first Ig-like domain, is constitutively expressed at high levels on APC, and contains, just like the 2–6 ICAM-1-isoform, only two Ig-like domain.
Our results also demonstrated that, in contrast to exon-4-deficient (Tm1jcgr) mice, exon-5 (Tm1Bay) -deficient mice displayed no resistance to septic shock. The susceptibility of Tm1Bay mice to endotoxin septic shock suggests an implication of ICAM-1 isoforms in the lethal effect of LPS. The remarkable resistance of the Tm1jcgr mice to septic shock induced by LPS has been attributed to the incapacity of neutrophil to bind vascular endothelium and infiltrate liver parenchyma 15. Indeed, our data showing massive infiltration of PMN in liver of Tm1Bay but not Tm1jcgr mice is consistent with the view that recruitment of neutrophils by ICAM-1 isoforms is sufficient to render these mice susceptible to septic shock. The ICAM-1 isoform 2–6 is capable of binding to LFA-1 but not MAC-1 because the third Ig-like domain has been alternatively spliced. The isoform 4–6 contains both the D1 and the D3 domains and can, in contrast to 2–6 isoform, bind both LFA-1 and MAC-1. Isoform 2–6, including the isoforms 3–6 and 2–5, lost their ability to bind to MAC-1 by the deletion of the domain 3 portion of ICAM-1. Theoretically, the 2–6 and the 3–6 isoforms present in the Tm1Bay mice can also be expressed in the septic shock-resistant Tm1jcgr mice, suggesting that susceptibility to septic shock would be conferred by the presence of the 4–6 isoform. Using RT-PCR techniques, we have indeed detected at least two isoforms, 2–6 and 4–6 in the liver of Tm1Bay mice following LPS injection. This possibility, supported by the previous observation that these isoforms are the dominant forms expressed in most tissues of mutant mice 17, firmly establishes its functional capacity to support neutrophil invasion in vivo.
Finally, our results have important implications in the context of tumor immune surveillance. It has been well documented that soluble forms of ICAM-1 are elevated in malignant tumors. Thus, it is possible that tumor cells expressing high levels of a minor isoform could rapidly generate high concentration of sICAM-1 in the peritumoral microenvironment in response to leukocyte invasion, thereby inhibiting LFA-1-mediated conjugate formation between the tumor-infiltrating lymphocytes and the tumor cells and its subsequent MHC-restricted killing 34. Further studies are required to determine the specific tissue distribution of ICAM-1 isoforms in normal tissues, and most importantly, whether tumor cells expressing minor isoforms are more tumorigenic that those expressing the common form.
Together, our findings demonstrate that ICAM-1 isoforms are fully functional and support the concept that alternative splicing in the Ig superfamily may fulfill specific roles related, for example, to their different ability to modulate T cells activation following antigen presentation, or to display a distinct susceptibility to neutrophil proteases that would directly affect their half-life on the cell surface of an APC or on the vascular endothelium, while increasing the release of soluble forms in the blood circulation. Defining more precisely the mechanisms that regulate the expression and tissue distribution of the isoforms of ICAM-1 and other adhesion molecules should help to better understand the role of these splice variants during the development of the immune response.
4 Materials and methods
4.1 Mice
The 2.102 TCR-transgenic mouse (2.102tg) was generated and bred to RAG1-deficient mice as reported elsewhere 35. Colonies of the C57BL/6-ICAM-1-deficient mouse strains (C57BL/6tm1jcgr and C57BL/6tm1Bay) carrying a deletional mutation in the fourth and fifth exons of the ICAM-1 gene, respectively, 15, 16 were bred inour animal facility. These mice do not express the common form of ICAM-1 but do express a limited repertoire of minor isoforms generated by alternative splicing 17. All mice usedin this study were housed in the pathogen-free animal facility in accordance with the guidelines of the Ethical Committee of the INRS-Institut Armand-Frappier.
4.2 Reagents and antibodies
The Hb(64–76) peptide (amino acid sequence: GKKVITAFNEGLK) was synthesized and purified as described 35. HLE (EC 3.4.21.37) and neutrophil cathepsin G (EC 3.4.21.20) were obtained from Calbiochem (La Jolla, CA). Sputum samples of patients with CF containing high levels of HLE were also used as a source of HLE, as described 21. The HLE-specific peptide inhibitor N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (MSAAPVCK) was obtained from Sigma (St. Louis, MO). Purified human sICAM-1 was obtained from R&D Systems (Minneapolis, MN). The biotinylated anti-Ly6G (Gr-1), anti-CD4, and the blocking anti-mouse ICAM-1 3E2 mAb were purchased from BD-PharMingen (San Diego, CA). The mouse G-5 anti-ICAM-1 mAb was obtained from Santa Cruz (Santa Cruz Biotechnology, CA). The human IgG was purchased from Cappel Research Reagents (ICN Biomedicals). The 14-4-4s hybridoma, specific for I-Ek α-chain, was obtained from the American Type Culture Collection (Rockville, MD). The phycoerythrin (PE)- and peroxidase-conjugated streptavidin (SA) complexes were purchased from Immunotech (Westbrook, ME) and Roche (Laval, QC), respectively.
4.3 Cell lines
The RT-4 was obtained from Dr. Ronald N. Germain (National Health Institute, Bethesda). The IKM-9 cell line, a stable transfectant of RT4 expressing the common form of murine ICAM-1, was kindly provided by Dr. Tania H. Watts (University of Toronto). The Hi-7 transfectants expressing the I-Ek class II molecules were obtained from Dr. Paul Allen (University of Washington, St. Louis, MO). Both fibroblastic cell lines were grown in RPMI 1640 (Life Technologies, Burlington, ON) supplemented with 10% (v/v) heat-inactivated FCS (Hyclone), 2 mM glutamine (Life Technologies) and antibiotics.
4.4 Generation of transfectants expressing each ICAM-1 isoform
cDNA encoding the common form of ICAM-1 and the alternative isoforms 16 were subcloned in the Srα/puro vector (kindly provided by Dr. François Denis, INRS-Institut Armand-Frappier), and transfected using lipofectamine (Life Technologies). Integrity of cDNA inserts was confirmed by DNA sequencing. Stable transfectants were selected in complete RPMI with 10% FCS and10 μg/ml puromycin (Sigma), cloned by limiting dilutions, and characterized for their expression of ICAM-1 by flow cytometry using ICAM-1-specific 3E2 mAb. Controls included cells transfected with the empty vector, cells without antibodies or with control antibodies, and cells incubated with SA-PE alone.
4.5 Antigen-presentation assays
Splenic CD4+ T cells from 2.102tg mice were magnetically separated using a ferritin-conjugated anti-CD4 mAb (Miltenyi Biotech, Auburn, CA). Cells isolated in this manner were >9598% CD4+ as determined by flow cytometric analysis. The CD4+ T cells (105 cells/well) were then co-cultured with 1.5×104 irradiated Hi-7 transfectants, which were preloaded for 4 h with the indicated doses of peptide or antibody. After 72 h of incubation, 1 μCi/well of [3H]thymidine (ICN Biomedicals) was added, and after an additional 18 hof culture, the cells were harvested and the incorporated radioactivity was measured.
4.6 Cleavage of ICAM-1 by proteases or sputum samples
Cells (5×105) were seeded in 96-well plates and cultured overnight. Proteases or sputum samples were added, and the cells incubated at 37°C for the indicated times in serum-free RPMI. In experiments with protease inhibitors, sputum samples were incubated for 10 min at 37°C with HLE-specific MSAAPVCK inhibitor (10 μg/ml) before being added to cells. The enzymatic reaction was stopped by adding RPMI medium containing 10% FCS. Cell surface expression of ICAM-1 was measured by flow-cytometric analysis, comparing the mean fluorescent intensity (MFI) of HLE-treated cells with that of untreated cells (MFIuntreated – MFItreated/MFIuntreated)×100.
4.7 Re-expression of ICAM-1 following HLE treatment
RT-4 cells expressing the common form and the 3–6 isoform of ICAM-1 cells were seeded into six-well plates (5×105/well) in serum-free medium and treated with the indicated concentrations of HLE for 2 h at 37°C. The reaction was stopped by adding complete RPMI containing 10% FCS. Cells were then harvested by mild trypsin-treatment and seeded into 24-well dishes. After 4 h ofculture, re-expression of ICAM-1 was measured by flow cytometry.
4.8 Septic shock
Age- and sex-matched mice were injected intraperitoneally with 50 mg/kg LPS (Escherichia coli, serotype 0127:B7; Sigma) and monitored for clinical signs of septic shock twice daily for 7 days. Experiments were terminated on day 7, as required by the guidelines for animal experimentation.
4.9 Immunohistochemistry
Frozen sections were stained with the biotinylated RB6–8C5 antibody, which reacts with Ly-6G, an antigen expressed on the neutrophils in the periphery. The sections were then washed, and stained with SA-peroxidase complexes using an aminoethyl carbazol substrate kit (Zymed Laboratories, San Francisco, CA). The sections were counterstained with hematoxylin.
4.10 RT and PCR conditions for ICAM-1 analysis
Detection of ICAM-1 isoforms at the mRNA level in the liver of normal and ICAM-1-deficient mice was essentially carried out as described 36 using the following primers: Sense D1(ATG) 5′-AGGCCGGAATTCCGGATGGCTTCAACCCGTGCCAAG-3′; sense D1(xho) 5′-CTGAAAGATGAGCTCGAGAGTGGACCCAACTGG-3′; sense D2 5′-CAGGAGCCTCCGGACTTTCG-3′; sense D3 5′GCCGCTGCGCTGCGTTTTG-3′; antisense 5′-GCTATGTACCATTCATCTCC-3′. Actin amplification was used as control using the following primers: sense 5′-CATGGATGACGATATCGCTGCCGC-3′; antisense 5′-GCTGTCGCCACGCTCGGTCAGGATC-3′.
Acknowledgements
This work was supported by a grant from the Canadian Institutes for Health Research (Y.S.P. and E.F.P.). O.R. is supported by a post-doctoral Fellowship from La Fondation Armand-Frappier. Y.S.P is a scholar of the Fonds de la Recherche en Santé du Québec.
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH
- WILEY-VCH




