Left ventricular systolic dysfunction in outpatients with peripheral atherosclerotic vascular disease: prevalence and association with location of arterial disease
Abstract
Aims
We aimed to determine the prevalence of left ventricular systolic dysfunction (LVSD) in outpatients with peripheral atherosclerotic vascular disease (PAVD). Further, the associations of stenotic internal carotid artery disease (SICAD) and lower extremity artery disease (LEAD) with LVSD were evaluated.
Methods and results
In the Peripheral Artery Disease in Västmanland study, consecutive outpatients with ultrasonographically identified mild to severe stenosis in the internal carotid artery or symptoms of claudication combined with either ankle brachial index of ≤0.90 or ultrasonographic occlusive findings were included (n = 437). Population-based control subjects were matched to the patients (n = 395). LVSD was defined as echocardiographically determined left ventricular ejection fraction (LVEF) <55%, and moderate or greater LVSD was defined as LVEF <45%. The prevalence of LVSD was significantly greater in patients than in controls (13.7% vs. 6.1%, P < 0.001). The prevalence of moderate or greater LVSD in participants not on treatment with a combination of angiotensin-converting enzyme inhibitor and beta-blocker was 2.3% in patients and 1.3% in controls (P = 0.31). When LEAD and SICAD were analysed together, adjusted for potential confounders, SICAD [odds ratio (OR) 2.54, 95% confidence interval (CI) 1.03–6.32], but not LEAD (OR 1.59, 95% CI 0.80–3.18), was independently associated with LVSD.
Conclusions
In outpatients with PAVD, we found a 13.7% prevalence of LVSD. However, the prevalence of at least moderate LVSD in patients not on treatment with angiotensin-converting enzyme inhibitor and a beta-blocker was only 2.3% and not significantly different from controls. Stenotic artery disease in the internal carotid artery, but not in the lower extremities, was independently associated with LVSD.
Introduction
Detection of left ventricular systolic dysfunction (LVSD) in asymptomatic subjects is of paramount importance. Even without symptoms, LVSD is associated with a poor prognosis.1-3 It is well established that with therapeutic interventions, the development of heart failure symptoms, the need for hospitalization, and mortality can all be reduced, and, as a consequence, current guidelines suggest asymptomatic LVSD as a target for a prevention strategy.4-6 However, except for asymptomatic family members of patients with idiopathic cardiomyopathy, current guidelines do not recommend routine screening with echocardiography to detect asymptomatic LVSD.6, 7 This is probably because of concerns about the costs and effectiveness of available screening tools and the lack of evidence showing a benefit of screening programmes. Although asymptomatic mild LVSD has been associated with an adverse prognosis,1 the main target for a screening programme would be moderate or severe LVSD as no beneficial effect of pharmacological treatment has been shown in patients with mild LVSD.6, 7
One important prerequisite for cost effectiveness of a screening programme is a reasonably high prevalence of the targeted condition, and therefore the benefit of general echocardiographic screening for LVSD in the general population is questionable.8 However, screening in high-risk populations might be an alternative. Patients with peripheral atherosclerotic vascular disease, including lower extremity artery disease (LEAD) and carotid artery disease comprise a potentially high-prevalence population.9 Previous studies have reported LVSD in 16–68% of patients with peripheral arterial disease.10-15 Most of these studies have been performed mainly on hospitalized patients and preoperatively, before major vascular surgery. Data are limited on the prevalence of LVSD in outpatients with peripheral atherosclerotic vascular disease.
Although atherosclerosis is a systemic process, there are distinctive differences in risk factor patterns, complications and outcomes depending on the location of the atherosclerotic lesions.16-18 Carotid artery atherosclerosis has been associated with LVSD and incident heart failure, whereas data are scarce concerning the separate relationship between LEAD and LVSD.16, 17 In the present study, we aimed to evaluate the prevalence of LVSD in outpatients with peripheral atherosclerotic vascular disease compared with matched controls from the general population. In addition, we examined the relationship between LVSD and both stenotic carotid artery disease and LEAD.
Methods
Participants
In the Peripheral Artery Disease in Västmanland (PADVa) study, consecutive patients referred to the Vascular Ultrasound Laboratory of the Department of Vascular Surgery, Västmanland County Hospital, Västerås, Sweden between April 2006 and February 2011 were evaluated for inclusion (see the Supporting Information, Figure S1). All patients underwent carotid ultrasonographic duplex screening. Patients with symptoms of claudication (defined as discomfort in the lower limb reproducibly produced during exercise and relieved by rest within 10 minutes) underwent ankle blood pressure measurements and ultrasonographic duplex examination of the arteries in the ipsilateral extremity. At least one of the following criteria were required for inclusion: (i) mild to severe stenosis of the internal carotid artery (ICA); (ii) symptoms of claudication combined with an ankle–brachial index (ABI) ≤0.90; or (iii) symptoms of claudication combined with ultrasonographic evidence of arterial occlusive disease in the ipsilateral extremity. Among 614 patients fulfilling at least one of the inclusion criteria, 452 (73.6%) accepted the invitation to be included.
Control subjects were recruited from the Västmanland Myocardial Infarction Study (VaMIS; ClinicalTrials.gov number, NCT01452178). In the VaMIS, consecutive patients hospitalized from November 2005 to May 2011 for acute myocardial infarction were included (Figure S1). For each included VaMIS patient, a control subject was recruited from the general population. From the Swedish population register, where all Swedish citizens are registered, a person with the closest date of birth, same sex, and same municipality as the VaMIS patient was identified and invited to participate by mail.
Patients in the present study and control subjects from the VaMIS were frequency matched. In a first step, all control subjects fulfilling any of the patient inclusion criteria (i.e., mild or greater ICA stenosis, ABI ≤0.90, or claudication) were excluded. Reports suggest that an ABI ≥1.40 predicts mortality with similar strength to ABI ≤0.90,19 and it has been acknowledged as an important risk factor in recent guidelines.20 Consequently, we also excluded control subjects with an ABI ≥1.40. All patients (n = 452) and the remaining controls (n = 692) were stratified by sex, age (in 5-year intervals), and municipality. Thereafter, control subjects were randomly excluded from strata not proportionally balanced to the patients for matched variables (sex, age, and municipality). Patients and controls with missing values for echocardiographic measurement of LV mass (n = 9), smoking history (n = 1), and serum creatinine (n = 9) were excluded. In total, 437 patients (257 men and 180 women) and 395 control subjects (244 men and 151 women) remained to be analysed. The study was approved by the Ethics Committee of Uppsala University, Sweden (Dnr 2005:382). All participants gave their written informed consent. The study is identified as ClinicalTrials.gov number NCT01452165.
Study protocol
Patients were invited to the Department of Clinical Physiology, Västmanland County Hospital, Västerås, Sweden, for examinations according to the study protocol. Self-reported diagnoses of cardiovascular disease and diabetes mellitus were confirmed from medical records. Hypertension was judged to be present if the participant had been assigned this diagnosis by a physician and had been prescribed antihypertensive medication or had a blood pressure of >140/90 mmHg at two separate occasions. Hyperlipidaemia was defined as treatment with lipid-lowering medication or a serum level of total cholesterol of >5 mmol/L. Ischaemic heart disease was defined as a history of myocardial infarction, angina pectoris (confirmed by positive exercise test, myocardial scintigraphy, or coronary angiography), coronary artery bypass grafting, or percutaneous coronary intervention.
Measurement of arm and ankle blood pressures
Arm and ankle blood pressure were measured in all included participants. One of three experienced nurses measured blood pressure in both arms with the participant in a supine position after at least 5 minutes rest. Ankle systolic blood pressures in the bilateral dorsalis pedis and posterior tibial arteries were obtained using an appropriate-sized leg cuff, an aneroid sphygmomanometer, and a handheld Doppler instrument with a 5 MHz probe. The leg-specific ABI was calculated as the higher of the two pedal artery systolic pressures divided by the highest systolic blood pressure of the two arms. The subject-specific ABI was defined as ≥1.40 if either of the two leg-specific ABIs was ≥1.40. In participants where both leg-specific ABIs were <1.40, the subject-specific ABI was defined as the lower of the two leg-specific ABIs.
Carotid ultrasound
One of three experienced technicians performed the carotid artery duplex examinations utilizing an Acuson Sequoia 512 system (Siemens, Erlangen, Germany) with a 4–9 MHz linear array transducer. The common carotid artery, proximal ICA and proximal external carotid artery were examined bilaterally by B-mode and colour flow mapping. Pulsed spectral Doppler velocities were obtained from all vascular segments. A plaque was defined as a localized protrusion of the vessel wall into the lumen in the common or internal carotid arteries. ICA disease was classified by combining findings in the gray-scale image, colour flow Doppler, and spectral Doppler (Table 1).21
| Grade | Plaque | Colour flow Doppler | Spectral Doppler |
|---|---|---|---|
| Normal artery | Absent | No turbulence | No spectral broadening PSV <1.2 m/s |
| Plaque without flow disturbance | Present | No turbulence | No spectral broadeningPSV <1.2 m/s |
| Mild stenosis | Present | Turbulence | Spectral broadeningPSV 1.2–1.4 m/s |
| Moderate stenosis | Present | Turbulence | Spectral broadeningPSV 1.5–2.5 m/s |
| Severe stenosis | Present | Turbulence | Spectral broadeningPSV > 2.5 m/s |
| Occlusion | Present | No flow | No flow |
- PSV, peak systolic flow velocity.
- a Modified from The Society of Radiologists in Ultrasound.21
Echocardiography
One experienced physician performed all echocardiographic examinations using a GE Vivid 7 harmonic imaging ultrasound machine (General Electric, Horten, Norway). Digital loops and images were recorded with the subjects in the left lateral decubitus position. All measurements were performed offline on a commercial software (EchoPAC PC version 110; General Electric) by a single experienced physician blinded to all clinical data, including the age, sex, and group of the subjects. The LV dimensions and mass were measured and calculated according to the European Association of Echocardiography.22
The LV ejection fraction (LVEF) was assessed by the biplane Simpson's rule.22 LVSD was categorized as absent (LVEF ≥55%), mild (LVEF 45–54%), moderate (LVEF 35–44%), fairly severe (LVEF 25–34%), or severe (LVEF <25%). In subjects for whom it was not possible to obtain the Simpson LVEF (n = 141, 16.7%) a visual estimation of LVEF was made and classified into one of the above categories.
Previous diagnosis of LVSD was extracted from the medical records. Patients with moderate to severe LVSD who were not being treated with an angiotensin converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) in combination with a beta-blocker were identified as the main target group for potential screening for untreated LVSD.
Physical examination and definition of heart failure
All participants underwent a focused interview and physical examination by one of three experienced physicians. In cases where there was a history of distressing dyspnoea when walking on the level, nocturnal dyspnoea, orthopnoea, previous diagnosis of heart failure, or previous medical contact because of dyspnoea, a participant was judged to have possible heart failure and was evaluated according to the Framingham heart failure criteria (see the Supporting Information, Figure S2).23 In addition, each medical record was reviewed to determine whether documented clinical information fulfilled the Framingham heart failure criteria. Subjects fulfilling the Framingham criteria, in the present or from medical records, were defined as having definite heart failure. Participants without possible or definite heart failure but with LVSD at echocardiography were considered to have asymptomatic LVSD.
Biochemistry
After participants fasted overnight, venous blood samples were taken by trained staff and immediately sent to the accredited Laboratory of Clinical Chemistry, Västmanland County Hospital, Västerås, Sweden. Serum levels of creatinine were determined by Synchron LX 20 and UniCel DxC instruments (Beckman Coulter, Fullerton, CA, USA). Plasma levels of N N-terminal pro-brain natriuretic peptide (NT-proBNP) were measured by a commercially available sandwich immunoassay using monoclonal antibodies and separation based on biotin–streptavidin binding (Elecys 1010 and Cobas e411; Roche Diagnostics, Germany). Renal function was quantified as estimated glomerular filtration rate (eGFR) according to the chronic kidney disease epidemiology (CKD-EPI) formula.24
Statistical analyses
Measurement reproducibility is presented in Supporting Information. Data are presented as mean ± SD, count (percentages), or median (25th percentile, 75th percentile) where appropriate. The t-test was used to compare differences between two groups and one-way anova for differences between three or more groups for continuous variables with approximately normal distribution. The Wilcoxon rank-sum test was used to compare two groups, and the Kruskal–Wallis rank test was used for three or more groups for variables with a skewed distribution (NT-proBNP). The Fisher exact test was used to compare differences in categorical variables.
We evaluated the association between LVSD and location of arterial disease. In these analyses, LEAD was defined as either ABI ≤0.90, ABI ≥1.40, claudication in combination with ultrasonic evidence of arterial occlusive disease in the ipsilateral extremity, or previous lower extremity vascular intervention (percutaneous revascularization, bypass, or amputation). Stenotic ICA disease (SICAD) was defined as prevalent stenosis or occlusion in the ICA, or previous carotid endarterectomy. Logistic regression was used to adjust for potentially confounding factors [age, sex, ever smoking, body mass index (BMI), pulse pressure, hypertension, diabetes mellitus, hyperlipidaemia, valvular disease, eGFR, and ischaemic heart disease] in the association between LEAD, SICAD, and LVSD. The number of study participants included was based on a power analysis presented in the Supporting Information. G*Power version 2 (http://www.gpower.hhu.de/en.html) was used for the power analysis and stata version 12.1 (StataCorp LP, College Station, Texas, USA) was used for all the other analyses.
Results
Participant characteristics
Characteristics of the patients and control subjects are presented in Table 2. As a consequence of the study design, there were no significant differences between patients and controls in age or sex, and the control subjects were free from claudication, had normal ABIs and had no ICA stenosis. However, the controls were not free from carotid artery atherosclerosis as 48% had non-stenotic plaques in the common or internal carotid arteries.
| Patients (n = 437) | Controls (n = 395) | P-value | |
|---|---|---|---|
| Women | 180 (41.2%) | 151 (38.2%) | 0.40 |
| Age, years | 69.4 ± 7.3 | 68.8 ± 7.7 | 0.27 |
| Current smoker | 72 (16.5%) | 37 (9.4%) | 0.003 |
| Ever smoker | 331 (75.7%) | 220 (55.7%) | <0.001 |
| Body mass index, kg/m2 | 27.1 ± 4.1 | 26.7 ± 3.9 | 0.13 |
| Atrial fibrillation | 14 (3.2%) | 15 (3.8%) | 0.71 |
| Systolic blood pressure, mmHg | 154 ± 21 | 148 ± 20 | <0.001 |
| Diastolic blood pressure, mmHg | 78 ± 10 | 83 ± 10 | <0.001 |
| Pulse pressure, mmHg | 76 ± 19 | 66 ± 15 | <0.001 |
| Hypertension | 379 (86.7%) | 267 (67.6%) | <0.001 |
| Hyperlipidaemia | 405 (92.7%) | 339 (85.8%) | 0.002 |
| Diabetes mellitus | 106 (24.3%) | 29 (7.3%) | <0.001 |
| Previous TIA/stroke | 43 (9.8%) | 21 (5.3%) | 0.018 |
| Ischemic heart disease | 149 (34.1%) | 35 (8.9%) | <0.001 |
| Myocardial infarction | 81 (18.5%) | 20 (5.1%) | <0.001 |
| Angina pectoris | 114 (26.1%) | 23 (5.8%) | <0.001 |
| Valvular disease | 19 (4.4%) | 6 (1.5%) | 0.024 |
| Possible heart failure | 123 (28.2%) | 54 (13.7%) | <0.001 |
| Definite heart failure | 22 (5.0%) | 2 (0.5%) | <0.001 |
| ABI ≤0.90 | 176 (40.4%) | 0 (0%) | NA |
| ABI ≥1.40 | 10 (2.3%) | 0 (0%) | NA |
| Stenotic carotid plaque | 336 (75.5%) | 0 (0%) | NA |
| Mild stenosis | 202 (45.4%) | 0 (0%) | NA |
| Moderate stenosis | 70 (15.7%) | 0 (0%) | NA |
| Severe stenosis/occlusion | 64 (14.4%) | 0 (0%) | NA |
| Non-stenotic carotid plaque | 86 (19.7%) | 188 (47.6%) | <0.001 |
- TIA, transient ischaemic attack; ABI, ankle–brachial index; NA, not applicable.
- Values are mean ± SD or count (percentages).
Prevalence of LV systolic dysfunction
The prevalence of LVSD was significantly greater among patients than among control subjects (13.7% vs. 6.1%, P < 0.001; Table 3) and was greater in male than in female patients (17.9% vs. 7.8%, P = 0.003). The prevalence of moderate or greater LVSD was 5.5% in the patients and 2.0% in the controls (P = 0.011). Among patients with LVSD, 53.3% (n = 32) were asymptomatic.
| Patients | Controls | |||||
|---|---|---|---|---|---|---|
| Women, n = 180 | Men, n = 257 | All, n = 437 | Women, n = 151 | Men, n = 244 | All, n = 395 | |
| LVSD | 7.8 (4.3–12.7) | 17.9 (13.4–23.1) | 13.7 (10.6–17.3) | 1.3 (0.2–4.7) | 9.0 (5.7–13.3) | 6.1 (3.9–8.9) |
| ALVSD | 3.9 (1.6–7.8) | 9.7 (6.4–14.0) | 7.3 (5.1–10.2) | 1.3 (0.2–4.7) | 6.1 (3.5–9.9) | 4.3 (2.6–6.8) |
| Possible SHF | 1.7 (0.3–4.8) | 5.4 (3.0–9.0) | 3.9 (2.3–6.2) | 0.0 (0.0–2.4) | 2.5 (0.9–5.3) | 1.5 (0.6–3.3) |
| Definite SHF | 2.2 (0.6–5.6) | 2.7 (1.1–5.5) | 2.5 (1.3–4.5) | 0.0 (0.0–2.4) | 0.4 (0.0–2.3) | 0.3 (0.0–1.4) |
- Values are percentages (95% confidence intervals).
Patients without and with LVSD were of similar age (69.2 ± 7.2 years vs. 70.2 ± 7.6 years; P = 0.38), BMI (27.1 ± 4.2 vs. 26.8 ± 3.9; P = 0.60) and ever-smoking history (82% vs. 75%; P = 0.33), and had similar prevalence of hypertension (87% vs. 87%; P = 1.00), diabetes mellitus (32% vs. 23%; P = 0.15) and LEAD (67% vs. 62%; P = 0.57). In contrast, ischaemic heart disease (77% vs. 27%; P < 0.001) and SICAD (87% vs. 74%; P = 0.035) were more common in patients with LVSD than in patients without LVSD. Control subjects with internal carotid artery plaques had LVSD significantly more often (9.0% vs. 3.4%; P = 0.021) than controls without such plaques.
Previous diagnosis of LV systolic dysfunction
From the participants' medical records, it was found that among participants with LVSD, the patients had been previously evaluated with an echocardiographic examination more often than the control subjects (39/60, 65% vs. 7/24, 29%; P = 0.004). In these previous echocardiographic examinations, LVEF was reported to be <55% in 29 (74%) of the patients and in four (57%) of the controls (P = 0.38). Thus, a previously unknown LVSD was diagnosed at the study examination in 31/437 (7.1%) of the patients and in 20/395 (5.1%) of the controls (P = 0.25).
Pharmacological treatment for LV systolic dysfunction
Among the 24 patients with moderate to severe LVSD, 83% (n = 20) were being treated with an ACE-I or an ARB, 67% (n = 16) with a beta-blocker, and 58% (n = 14) with a combination of ACE-I/ARB and beta-blocker. The prevalence of moderate or greater LVSD in participants not on treatment with a combination of ACE-I/ARB and beta-blocker was 2.3% in the patients and 1.3% in the control group (P = 0.31).
Association of LV systolic dysfunction with location of arterial disease
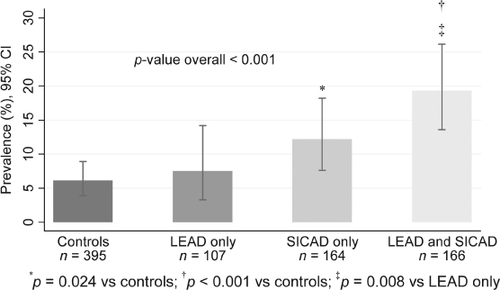
Figure 1 shows the prevalence of LVSD according to occurrence of LEAD and SICAD. Patients with only LEAD did not differ from control subjects in prevalence of LVSD (7.5% vs. 6.1%; P = 0.66). In contrast, patients with SICAD only and patients with a combination of LEAD and SICAD had significantly greater prevalence of LVSD compared with controls. Table 4 shows some characteristics of control subjects and patients according to the presence of LEAD and SICAD. In comparison with patients with LEAD only, patients with SICAD only were less often ever-smokers (66.5% vs. 83.2%; P = 0.003) and had greater prevalence of angina pectoris (27.4% vs. 15.9%; P = 0.028) and LV hypertrophy (45.7% vs. 32.7%; P = 0.043). In addition, there was a trend towards higher NT-proBNP levels (P = 0.059) in patients with SICAD only compared with patients with LEAD only. However, there was no significant difference between patients with SICAD only and those with LEAD only for previous myocardial infarction (P = 0.22).
| Controls | Patients | ||||
|---|---|---|---|---|---|
| No LEAD or SICAD (n = 395) | LEAD only (n = 107) | SICAD only (n = 164) | LEAD and SICAD (n = 166) | P-value overall | |
| Women | 151 (38.2%) | 43 (40.2%) | 74 (45.1%) | 63 (38.0%) | 0.46 |
| Age, years | 68.8 ± 7.7 | 68.6 ± 7.7 | 69.0 ± 7.2 | 70.3 ± 7.1 | 0.14 |
| Current smoker | 37 (9.4%) | 22 (20.6%)b | 23 (14.0%) | 27 (16.3%)a | 0.008 |
| Ever smoker | 220 (55.7%) | 89 (83.2%)c | 109 (66.5%)ae | 133 (80.1%)ch | <0.001 |
| Body mass index, kg/m2 | 26.7 ± 3.9 | 26.2 ± 4.2 | 27.4 ± 4.0ad | 27.3 ± 4.1d | 0.029 |
| Atrial fibrillation | 15 (3.8%) | 3 (2.8%) | 2 (1.2%) | 9 (5.4%) | 0.19 |
| Systolic BP, mmHg | 148 ± 20 | 153 ± 20a | 154 ± 20b | 154 ± 22b | <0.001 |
| Diastolic BP, mmHg | 83 ± 10 | 81 ± 9 | 79 ± 10c | 75 ± 10cfi | <0.001 |
| Pulse pressure, mmHg | 66 ± 15 | 72 ± 18c | 75 ± 17c | 79 ± 20ce | <0.001 |
| Hypertension | 267 (67.6%) | 86 (80.4%)c | 140 (85.4%)c | 153 (92.2%)cfg | <0.001 |
| Hyperlipidaemia | 339 (85.8%) | 95 (88.8%) | 156 (95.1%)b | 154 (92.8%)a | 0.004 |
| Diabetes mellitus | 29 (7.3%) | 19 (17.8%)b | 29 (17.7%)b | 61 (36.8%)cei | <0.001 |
| Previous TIA/stroke | 21 (5.3%) | 6 (5.6%) | 20 (12.2%)b | 17 (10.2%)a | 0.020 |
| Ischaemic heart disease | 35 (8.9%) | 26 (24.3%)c | 54 (32.9%)c | 69 (41.6%)ce | <0.001 |
| Myocardial infarction | 20 (5.1%) | 12 (11.2%)a | 28 (17.1%)c | 41 (24.7%)ce | <0.001 |
| Angina pectoris | 23 (5.8%) | 17 (15.9%)b | 45 (27.4%)cd | 52 (31.3%)ce | <0.001 |
| Valvular disease | 6 (1.5%) | 2 (1.9%) | 4 (2.4%) | 13 (7.8%)cg | 0.002 |
| Possible heart failure | 54 (13.7%) | 28 (26.2%)b | 43 (26.2%)b | 52 (31.3%)c | <0.001 |
| Definite heart failure | 2 (0.5%) | 4 (3.7%)a | 5 (3.0%)a | 13 (7.8%)c | <0.001 |
| Creatinine, µmol/L | 79.5 ± 18.1 | 82.4 ± 17.2 | 81.8 ± 23.3 | 91.4 ± 33.4cdh | <0.001 |
| eGFR, ml/min.1.73 m2 | 79.5 ± 15.0 | 76.2 ± 15.8a | 76.5 ± 16.3a | 71.1 ± 19.4cdh | <0.001 |
| NT-proBNP, ng/L | 94 (52, 187) | 125 (65, 254)a | 156 (90, 304)c | 243 (110, 505)cfh | <0.001 |
| Medication | |||||
| ACE-I/ARB | 91 (23.0%) | 54 (50.5%)c | 83 (50.6%)c | 113 (68.1%)cdh | <0.001 |
| β-Blockers | 97 (24.6%) | 37 (34.6%)a | 73 (44.5%)c | 112 (67.5%)cfi | <0.001 |
| Statins | 83 (21.0%) | 77 (72.0%)c | 138 (84.1%)cd | 141 (84.9%)cd | <0.001 |
| Aspirin | 86 (21.8%) | 86 (80.4%)c | 119 (72.6%)c | 133 (80.1%)c | <0.001 |
| Diuretics | 55 (13.9%) | 19 (17.8%) | 34 (20.7%) | 51 (30.7%)cdg | <0.001 |
| Calcium channel blockers | 48 (12.2%) | 36 (33.6%)c | 58 (35.4%)c | 68 (41.0%)c | <0.001 |
| Echocardiography | |||||
| LVMI, g/m2 | 98 ± 21 | 100 ± 25 | 105 ± 26c | 111 ± 30cb | <0.001 |
| LV hypertrophy | 120 (30.4%) | 35 (32.7%) | 75 (45.7%)bd | 83 (50.0%)ce | <0.001 |
| LVEF <55% | 24 (6.1%) | 8 (7.5%) | 20 (12.2%)a | 32 (19.3%)ce | <0.001 |
| LVEF <45% | 8 (2.0%) | 2 (1.9%) | 8 (4.9%) | 14 (8.4%)bg | 0.004 |
| Simpson LVEF, % | 62.3 ± 7.0(n = 337) | 63.0 ± 7.4(n = 94) | 61.9 ± 9.5(n = 131) | 58.5 ± 10.7cfh(n = 129) | <0.001 |
- ACE-I/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; IHD, ischaemic heart disease; LEAD, lower extremity artery disease; LVMI, left ventricular mass indexed for body mass; NT-proBNP, N-terminal pro-brain natriuretic peptide; SICAD, stenotic internal carotid artery disease.
- Values are mean ± SD, count (percentages), or median (25th percentile, 75th percentile) where appropriate.
- aP < 0.05 vs. controls; bP < 0.01 vs. controls; cP < 0.001 vs. controls; dP < 0.05 vs. LEAD only; eP < 0.01 vs. LEAD only; fP < 0.001 vs. LEAD only; gP < 0.05 vs. SICAD only; hP < 0.01 vs. SICAD only; iP < 0.001 vs. SICAD only.
When analysed unadjusted and separately in a logistic regression model, SICAD, as opposed to LEAD, was significantly associated with LVSD in the patient group (Table 5). After adjustment for potential confounders, except for ischaemic heart disease, the relationship between SICAD and LVSD was strengthened. When introducing ischaemic heart disease into the full model, the influence of SICAD was attenuated but remained significantly associated with LVSD.
| Patients (n = 437) | ||
|---|---|---|
| OR (95% CI) | P-value | |
| Unadjusted | ||
| LEAD | 1.24 (0.70–2.20) | 0.47 |
| SICAD | 2.31 (1.06–5.04) | 0.035 |
| Adjusted for age and sex | ||
| LEAD | 1.62 (0.87–2.99) | 0.13 |
| SICAD | 2.90 (1.27–6.63) | 0.012 |
| Multi-adjusteda | ||
| LEAD | 1.63 (0.85–3.14) | 0.14 |
| SICAD | 3.13 (1.33–7.38) | 0.009 |
| Multi-adjusteda + IHD | ||
| LEAD | 1.59 (0.80–3.18) | 0.19 |
| SICAD | 2.54 (1.03–6.32) | 0.044 |
- IHD, ischemic heart disease; LEAD, lower extremity artery disease; SICAD, stenotic internal carotid artery disease.
- a Multi-adjusted for age, sex, smoking (never/ever), body mass index, pulse pressure, hypertension, diabetes mellitus, hyperlipidaemia, valvular disease, and estimated glomerular filtration rate.
- Logistic regression models were run with forced entry of LEAD, SICAD, and specified covariates except for unadjusted models where LEAD and SICAD were run separately.
Among the 330 patients with SICAD, 6.7% (n = 22) had a moderate to severe LVSD of whom 59% (n = 13) were being treated with a combination of ACE-I/ARB and a beta-blocker. Thus, the prevalence of participants with moderate or severe LVSD not on treatment with a combination of ACE-I/ARB and beta-blocker was 2.7% in the patients with SICAD compared with 1.3% in the control subjects (P = 0.18).
Discussion
In the present study of outpatients with peripheral atherosclerotic vascular disease, LVSD occurred in 13.7%, which was at least twice the prevalence of, and significantly different from, control subjects from the general population matched for age and sex. However, the prevalence of patients with at least moderate LVSD not being treated with ACE-I/ARB and a beta-blocker, the main targets for a potential screening programme, was only 2.3% and not significantly different from that in the controls. We also found that SICAD, as opposed to LEAD, had a significant relationship with LVSD, independent of other potential risk factors.
In our findings, the prevalence of LVSD in patients with peripheral atherosclerotic vascular disease appears to be lower than previously reported. In studies during the last two decades of the twentieth century, LVSD was demonstrated in 16–68% of patients examined preoperatively before elective vascular surgery.11, 12, 25 In 2002, Kelly et al.13 reported a 28% prevalence of moderate or greater LVSD in 255 patients with peripheral vascular disease, compared with 5.5% in our patients. Although their patients were similar to ours regarding age, sex, and prevalence of ischaemic heart disease, they were considerably more often current smokers (78%), had a greater prevalence of atrial fibrillation (11%), and, most importantly, in contrast to our outpatients they were mainly hospitalized. Among 120 outpatients with ABI ≤0.90, Ward et al.14 reported an occurrence of LVSD in 27%, and moderate or greater LVSD in 14%. Their patients differed from ours in being mainly African-American (74%), being more often current smokers (42%), and substantially more often having diabetes mellitus (44%).
In addition to differences in populations, one may speculate whether changes in epidemiology and patient care over time might contribute to the lower prevalence of LVSD in the present study compared with previous data. Coronary artery disease (CAD) and hypertension are considered the most common causes of LVSD. Recent data from Scandinavia and the USA suggest that the incidence of myocardial infarctions is declining.26, 27 In view of the well-known influence of myocardial infarction on LV contractility, a decreasing prevalence of LVSD as a reflection of declining incidence of myocardial infarction might be expected. Another conceivable explanation for lower prevalence of LVSD in the present study compared with previous data might be a temporal change in pharmacological antihypertensive treatment. Although 7% (n = 31) of our patients were diagnosed with a previously unknown LVSD, the majority (68%) of these 31 patients were already receiving treatment with ACE-I or ARB, and 39% were taking a beta-blocker, possibly because of hypertension. Overall, 57% and 51% of our patients were on treatment with ACE-I/ARB and beta-blocker, respectively, at inclusion. The corresponding figures in the patient group reported by Kelly et al.13 in 2002 were 19% and 20%, respectively.
Interestingly, in the present study, SICAD was significantly more strongly associated with LVSD than was LEAD. This may correspond to a stronger association of SICAD than LEAD with CAD. Consistent with this possibility, angina pectoris was more prevalent in the patients with SICAD only than in those with LEAD only. In support of the hypothesis that SICAD is more strongly related to CAD than is LEAD, Sillesen et al.28 reported carotid plaque burden to be the strongest predictor of coronary artery calcium score, compared with ABI and abdominal aortic diameter. Moreover, Lamina et al.29 demonstrated carotid artery disease to be a stronger predictor of nonfatal or fatal myocardial infarction than ABI.
Although SICAD was significantly associated with LVSD, the identification of patients with SICAD appears to be of no substantial benefit in screening for LVSD, as the prevalence of moderate or greater LVSD in patients not under treatment with a combination of ACE-I/ARB and beta-blocker (2.7%) was not significantly different from that in the control group (1.3%).
Limitations
There were limitations to the present study. The PADVa study population comprises Caucasian, middle-aged and older individuals, which may limit the generalizability of our findings. One-quarter of the invited patients declined to participate in the study. Although the dropouts did not differ from the participants in age (P = 0.68) or sex (P = 0.93), they may have been more severely affected by disease, which could have been a source of bias. The planned sample size of the present study was based on previous data13 presenting much higher prevalence of LVSD than we subsequently found. This loss of power has to be taken into account. The subgroup analyses should be regarded as exploratory and needs to be confirmed in future studies.
Conclusions
In outpatients with peripheral atherosclerotic vascular disease, we found a 13.7% prevalence of LVSD, which was significantly different from the 6.1% prevalence in control subjects from the general population. However, the prevalence of at least moderate LVSD in patients not on treatment with ACE-I/ARB and a beta-blocker was only 2.3% and not significantly different from the controls, which casts doubts on the efficiency of a potential screening programme for LVSD in outpatients with peripheral atherosclerotic vascular disease. We also found that SICAD, as opposed to stenotic LEAD, had a significant relationship with LVSD, independently of other potential risk factors. The pathophysiological mechanisms of the effects of carotid vs. femoral atherosclerotic disease on LVSD require further study.
Acknowledgments
The authors thank the participants and the staff of the PADVa study, especially Petra Wahlén, Lena Trollvad, Lolita Backsell, Marja-Leena Ojutkangas, Annika Kärnsund, and Göran Nilsson, for their valuable contribution.
Funding
This study was supported by grants from Sparbanksstiftelsen Nya, the County of Västmanland, Selanders Stiftelse, and the Swedish Medical Association.
Conflict of interest: none declared.




