2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure
Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC
Author/Task Force Member affiliations: listed in Author information.
ESC Clinical Practice Guidelines Committee (CPG): listed in the Appendix.
ESC subspecialty communities having participated in the development of this document:
Associations: Association for Acute CardioVascular Care (ACVC), Association of Cardiovascular Nursing & Allied Professions (ACNAP), European Association of Cardiovascular Imaging (EACVI), European Association of Preventive Cardiology (EAPC), European Association of Percutaneous Cardiovascular Interventions (EAPCI), European Heart Rhythm Association (EHRA), Heart Failure Association (HFA).
Councils: Council of Cardio-Oncology, Council on Basic Cardiovascular Science, Council on Valvular Heart Disease.
Working Groups: Adult Congenital Heart Disease, Cardiovascular Pharmacotherapy, Cardiovascular Regenerative and Reparative Medicine, Cardiovascular Surgery, e-Cardiology, Myocardial and Pericardial Diseases, Myocardial Function.
Patient Forum:
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC ([email protected]).
Disclaimer: The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their publication. The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of healthcare or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and, where appropriate and/or necessary, the patient's caregiver. Nor do the ESC Guidelines exempt health professionals from taking into full and careful consideration the relevant official updated recommendations or guidelines issued by the competent public health authorities, in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
These articles are identical except for minor stylistic and spelling differences in keeping with each journal s style. Either citation can be used when citing this article. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC ([email protected]).
Abstract
Document Reviewers: Rudolf A. de Boer (CPG Review Coordinator) (Netherlands), P. Christian Schulze (CPG Review Coordinator) (Germany), Magdy Abdelhamid (Egypt), Victor Aboyans (France), Stamatis Adamopoulos (Greece), Stefan D. Anker (Germany), Elena Arbelo (Spain), Riccardo Asteggiano (Italy), Johann Bauersachs (Germany), Antoni Bayes-Genis (Spain), Michael A. Borger (Germany), Werner Budts (Belgium), Maja Cikes (Croatia), Kevin Damman (Netherlands), Victoria Delgado (Netherlands), Paul Dendale (Belgium), Polychronis Dilaveris (Greece), Heinz Drexel (Austria), Justin Ezekowitz (Canada), Volkmar Falk (Germany), Laurent Fauchier (France), Gerasimos Filippatos (Greece), Alan Fraser (United Kingdom), Norbert Frey (Germany), Chris P. Gale (United Kingdom), Finn Gustafsson (Denmark), Julie Harris (United Kingdom), Bernard Iung (France), Stefan Janssens (Belgium), Mariell Jessup (United States of America), Aleksandra Konradi (Russia), Dipak Kotecha (United Kingdom), Ekaterini Lambrinou (Cyprus), Patrizio Lancellotti (Belgium), Ulf Landmesser (Germany), Christophe Leclercq (France), Basil S. Lewis (Israel), Francisco Leyva (United Kingdom), AleVs Linhart (Czech Republic), Maja-Lisa Løchen (Norway), Lars H. Lund (Sweden), Donna Mancini (United States of America), Josep Masip (Spain), Davor Milicic (Croatia), Christian Mueller (Switzerland), Holger Nef (Germany), Jens-Cosedis Nielsen (Denmark), Lis Neubeck (United Kingdom), Michel Noutsias (Germany), Steffen E. Petersen (United Kingdom), Anna Sonia Petronio (Italy), Piotr Ponikowski (Poland), Eva Prescott (Denmark), Amina Rakisheva (Kazakhstan), Dimitrios J. Richter (Greece), Evgeny Schlyakhto (Russia), Petar Seferovic (Serbia), Michele Senni (Italy), Marta Sitges (Spain), Miguel Sousa-Uva (Portugal), Carlo G. Tocchetti (Italy), Rhian M. Touyz (United Kingdom), Carsten Tschoepe (Germany), Johannes Waltenberger (Germany/Switzerland)
All experts involved in the development of these guidelines have submitted declarations of interest. These have been compiled in a report and published in a supplementary document simultaneously to the guidelines. The report is also available on the ESC website www.escardio.org/guidelines
For the Supplementary Data which include background information and detailed discussion of the data that have provided the basis for the guidelines see European Heart Journal online
Abbreviations and acronyms
-
- 6MWT
-
- 6-minute walk test
-
- 99mTc-PYP
-
- Technetium-labelled pyrophosphate
-
- AATAC
-
- Ablation vs. Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD (trial)
-
- AC
-
- Arrhythmogenic cardiomyopathy
-
- ACE
-
- Angiotensin-converting enzyme
-
- ACE-I
-
- Angiotensin-converting enzyme inhibitor
-
- ACHD
-
- Adult congenital heart disease
-
- ACS
-
- Acute coronary syndrome
-
- ADHF
-
- Acute decompensated heart failure
-
- AF
-
- Atrial fibrillation
-
- AF-CHF
-
- Atrial fibrillation – Congestive Heart Failure (trial)
-
- AFFIRM
-
- Atrial Fibrillation Follow-up Investigation of Rhythm Management (trial)
-
- AFFIRM-AHF
-
- A Randomized, Double-blind Placebo-controlled Trial Comparing the Effect of Intravenous Ferric Carboxymaltose on Hospitalizations and Mortality in Iron-deficient Subjects Admitted for Acute Heart Failure (trial)
-
- AHF
-
- Acute heart failure
-
- AL
-
- Light chain immunoglobulin
-
- AL-CA
-
- Light chain immunoglobulin cardiac amyloidosis
-
- AMICA
-
- Atrial Fibrillation Management in Congestive Heart Failure With Ablation (trial)
-
- ANCA
-
- Antineutrophil cytoplasmic antibody
-
- ARB
-
- Angiotensin-receptor blocker
-
- ARNI
-
- Angiotensin receptor-neprilysin inhibitor
-
- ARVC
-
- Arrhythmogenic right ventricular cardiomyopathy
-
- ATTR
-
- Transthyretin amyloidosis
-
- AV
-
- Atrio-ventricular
-
- b.i.d.
-
- Bis in die (twice daily)
-
- BAG3
-
- Bcl2-associated athanogene 3
-
- BiVAD
-
- Biventricular assist device
-
- BMI
-
- Body mass index
-
- BNP
-
- B-type natriuretic peptide
-
- BP
-
- Blood pressure
-
- b.p.m.
-
- Beats per minute
-
- BTB
-
- Bridge to bridge
-
- BTC
-
- Bridge to candidacy
-
- BTD
-
- Bridge to decision
-
- BTR
-
- Bridge to recovery
-
- BTT
-
- Bridge to transplantation
-
- CA
-
- Cardiac amyloidosis (or amyloid cardiomyopathy)
-
- CABANA
-
- Catheter ABlation vs. ANti-arrhythmic drug therapy for Atrial fibrillation (trial)
-
- CABG
-
- Coronary artery bypass graft
-
- CAD
-
- Coronary artery disease
-
- CANVAS-R
-
- CANagliflozin cardioVascular Assessment Study - Renal
-
- CARE-HF
-
- CArdiac REsynchronization in Heart Failure
-
- CASTLE-AF
-
- Catheter Ablation versus Standard conventional Treatment in patients with LEft ventricular and Atrial Fibrillation (trial)
-
- CCB
-
- Calcium channel blocker
-
- CCS
-
- Chronic coronary syndrome
-
- CHA2DS2-VASc
-
- Congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female) (score)
-
- CHAMPIT
-
- Acute Coronary syndrome/Hypertension emergency/Arrhythmia/acute Mechanical cause/Pulmonary embolism/Infections/Tamponade
-
- CHARM
-
- Candesartan in Heart Failure - Assessment of moRtality and Morbidity (trial)
-
- CHF
-
- Chronic heart failure
-
- CI
-
- Confidence interval
-
- CKD
-
- Chronic kidney disease
-
- CMP
-
- Cardiomyopathy
-
- CMR
-
- Cardiac magnetic resonance
-
- CMV
-
- Cytomegalovirus
-
- COAPT
-
- Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for HF patients with functional mitral regurgitation (trial)
-
- COC
-
- Cardio-Oncology Council (part of the European Society of Cardiology)
-
- COMMANDER-HF
-
- A Study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure (trial)
-
- COMPASS
-
- Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease (trial)
-
- COPD
-
- Chronic obstructive pulmonary disease
-
- CORONA
-
- COntrolled ROsuvastatin multiNAtional (trial)
-
- COVID-19
-
- Coronavirus disease 2019
-
- CR
-
- Controlled release
-
- CREDENCE
-
- Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (trial)
-
- CRT
-
- Cardiac resynchronization therapy
-
- CRT-D
-
- Cardiac resynchronization therapy with defibrillator
-
- CRT-P
-
- Cardiac resynchronization therapy pacemaker
-
- CSA
-
- Central sleep apnoea
-
- CT
-
- Computed tomography
-
- CTCA
-
- Computed tomography coronary angiography
-
- CV
-
- Cardiovascular
-
- DAPA-HF
-
- Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure (trial)
-
- DCM
-
- Dilated cardiomyopathy
-
- DECLARE- TIMI 58
-
- Dapagliflozin Effect on CardiovascuLAR Events (Thrombolysis in Myocardial Infarction) (trial)
-
- DIAMOND
-
- Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi Medications for the Treatment of Heart Failure (trial)
-
- DIG
-
- Digitalis Investigation Group (trial)
-
- DNA
-
- Deoxyribonucleic acid
-
- DOAC
-
- Direct-acting oral anticoagulant
-
- DPD
-
- 3,3-diphosphono-1,2-propanodicarboxylic acid
-
- DPP-4
-
- Dipeptidyl peptidase-4
-
- DSC2
-
- Desmocollin 2
-
- DSG2
-
- Desmoglein 2
-
- DSP
-
- Desmoplakin
-
- DT
-
- Destination therapy
-
- E/e′ (ratio)
-
- E/e′ (ratio) = early filling velocity on transmitral Doppler/early relaxation velocity on tissue Doppler
-
- EACVI
-
- European Association of Cardiovascular Imaging (part of the European Society of Cardiology)
-
- EAST-AFNET 4
-
- Early Treatment of Atrial Fibrillation for Stroke Prevention Trial 4 (trial)
-
- ECG
-
- Electrocardiogram
-
- EchoCRT
-
- Echocardiography Guided Cardiac Resynchronization Therapy (trial)
-
- ECLS
-
- Extracorporeal life support
-
- ECMO
-
- Extracorporeal membrane oxygenation
-
- EF
-
- Ejection fraction
-
- eGFR
-
- Estimated glomerular filtration rate
-
- EHRA
-
- European Heart Rhythm Association
-
- EMA
-
- European Medicines Agency
-
- EMB
-
- Endomyocardial biopsy
-
- EMPA-REG OUTCOME
-
- Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (trial)
-
- EMPEROR- Reduced
-
- EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (trial)
-
- EROA
-
- Effective regurgitant orifice area
-
- ESC
-
- European Society of Cardiology
-
- EU
-
- European Union
-
- EuroSCORE II
-
- European System for Cardiac Operative Risk Evaluation II (score)
-
- FDA
-
- Food and Drug Administration
-
- FDG
-
- Fluorodeoxyglucose
-
- FiO2
-
- Fraction of inspired oxygen
-
- FLN
-
- Filamin
-
- FLNC
-
- Filamin C
-
- GGT
-
- Gamma-glutamyl transferase
-
- GISSI-HF
-
- Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto Miocardico – Heart Failure (trial)
-
- GLP-1
-
- Glucagon-like peptide-1
-
- GUIDE-HF
-
- Hemodynamic-GUIDEd Management of Heart Failure (trial)
-
- h
-
- Hour/hours
-
- H2FPEF
-
- Heavy (BMI >30 kg/m2), Hypertensive (use of ≥2 antihypertensive medications), atrial Fibrillation (paroxysmal or persistent), Pulmonary hypertension (Doppler Echocardiographic estimated Pulmonary Artery Systolic Pressure >35 mmHg), Elderly (age >60 years), Filling pressure (Doppler Echocardiographic E/e′ >9) (score)
-
- HbA1c
-
- Glycated haemoglobin
-
- HCM
-
- Hypertrophic cardiomyopathy
-
- HEART
-
- Heart Failure Revascularisation Trial
-
- HER2
-
- Human epidermal growth factor receptor 2
-
- HF
-
- Heart failure
-
- HFA
-
- Heart Failure Association
-
- HFA-PEFF
-
- Heart Failure Association of ESC diagnostic algorithm, P – Initial Workup (Step 1: Pretest Assessment), E - Diagnostic Workup (Step 2: Echocardiographic and Natriuretic Peptide score), F1 – Advanced Workup (Step 3: Functional testing in Case of Uncertainty), F2 – Aetiological Workup (Step 4: Final Aetiology)
-
- HF-MP
-
- Heart failure management programme
-
- HFmrEF
-
- Heart failure with mildly reduced ejection fraction
-
- HFpEF
-
- Heart failure with preserved ejection fraction
-
- HFrEF
-
- Heart failure with reduced ejection fraction
-
- HHV
-
- Human herpes virus
-
- HIV
-
- Human immunodeficiency virus
-
- HLA-DR
-
- Human leukocyte antigen-DR isotype
-
- HMDP
-
- Hydroxyl-methylene-diphosphonate
-
- HR
-
- Hazard ratio
-
- HT
-
- Heart transplantation
-
- HTM
-
- Home telemonitoring
-
- i.v.
-
- Intravenous
-
- IABP
-
- Intra-aortic balloon pump
-
- ICCU
-
- Intensive coronary care unit
-
- ICD
-
- Implantable cardioverter-defibrillator
-
- ICU
-
- Intensive care unit
-
- IHD
-
- Ischaemic heart disease
-
- INR
-
- International normalized ratio
-
- INTERMACS
-
- Interagency Registry for Mechanically Assisted Circulatory Support
-
- INTrEPID
-
- Investigation of Nontransplant-Eligible Patients Who Are Inotrope Dependent (trial)
-
- IOCM
-
- Iron overload cardiomyopathy
-
- IPD
-
- Individual patient data
-
- I-PRESERVE
-
- Irbesartan in Patients with Heart Failure and PRESERVEd Ejection Fraction (trial)
-
- KCNH2
-
- Potassium voltage-gated channel subfamily H member 2
-
- KCNQ1
-
- Potassium voltage-gated channel subfamily Q member 1
-
- LA
-
- Left atrium/atrial
-
- LAE
-
- Left atrial enlargement
-
- LBBB
-
- Left bundle branch block
-
- LDB3
-
- LIM domain binding 3
-
- LFT
-
- Liver function test
-
- LGE
-
- Late gadolinium enhancement
-
- LMNA
-
- Lamin A/C
-
- LMWH
-
- Low-molecular-weight heparin
-
- LUS
-
- Lung ultrasound
-
- LV
-
- Left ventricular/ventricle
-
- LVAD
-
- Left ventricular assist device
-
- LVEDP
-
- Left ventricular end-diastolic pressure
-
- LVEF
-
- Left ventricular ejection fraction
-
- LVESD
-
- Left ventricular end-systolic diameter
-
- LVH
-
- Left ventricular hypertrophy
-
- LVNC
-
- Left ventricular non-compaction
-
- LVOT
-
- Left ventricular outflow tract
-
- LVOTO
-
- Left ventricular outflow tract obstruction
-
- MADIT-CRT
-
- Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (trial)
-
- MADIT-II
-
- Multicenter Automatic Defibrillator Implantation Trial II (trial)
-
- MADIT-RIT
-
- Multicenter Automatic Defibrillator Implantation Trial – Reduce Inappropriate Therapy (trial)
-
- MAGGIC
-
- Meta-Analysis Global Group in Chronic Heart Failure
-
- MCS
-
- Mechanical circulatory support
-
- MEK
-
- Mitogen-activated protein kinase
-
- MI
-
- Myocardial infarction
-
- MITRA-FR
-
- Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation (trial)
-
- MMR
-
- Mismatch repair
-
- MR
-
- Mitral regurgitation
-
- MRA
-
- Mineralocorticoid receptor antagonist
-
- MRI
-
- Magnetic resonance imaging
-
- mRNA
-
- Messenger ribonucleic acid
-
- MR-proANP
-
- Mid-regional pro-atrial natriuretic peptide
-
- MT
-
- Medical therapy
-
- MV
-
- Mitral valve
-
- mWHO
-
- Modified World Health Organization
-
- MYPC
-
- Myosin-binding protein C
-
- NICM
-
- Non-ischaemic cardiomyopathy
-
- NKX2-5
-
- NK2 transcription factor related, locus 5
-
- NP
-
- Natriuretic peptide
-
- NSAID
-
- Non-steroidal anti-inflammatory drug
-
- NSVT
-
- Non-sustained ventricular tachycardia
-
- NT-proBNP
-
- N-terminal pro-B-type natriuretic peptide
-
- NYHA
-
- New York Heart Association
-
- o.d
-
- Omne in die (once daily)
-
- OMT
-
- Optimal medical therapy
-
- OSA
-
- Obstructive sleep apnoea
-
- PA
-
- Pulmonary artery
-
- PaO2
-
- Partial pressure of oxygen
-
- PARADIGM-HF
-
- Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (trial)
-
- pCO2
-
- Partial pressure of carbon dioxide
-
- PCI
-
- Percutaneous coronary intervention
-
- PCR
-
- Polymerase chain reaction
-
- PCWP
-
- Pulmonary capillary wedge pressure
-
- PEP-CHF
-
- Perindopril in Elderly People with Chronic Heart Failure (trial)
-
- PET
-
- Positron emission tomography
-
- PKP2
-
- Plakophilin 2
-
- PLN
-
- Phospholamban
-
- PPCM
-
- Peripartum cardiomyopathy
-
- PREVEND
-
- Prevention of REnal and Vascular ENd-stage Disease (trial)
-
- PV
-
- Pulmonary vein
-
- PVC
-
- Premature ventricular contraction
-
- PVI
-
- Pulmonary vein isolation
-
- pVO2
-
- Peak exercise oxygen consumption
-
- QI
-
- Quality indicator
-
- QOL
-
- Quality of life
-
- QRS
-
- Q, R, and S waves of an ECG
-
- RAAS
-
- Renin-angiotensin-aldosterone system
-
- RACE II
-
- Rate Control Efficacy in Permanent Atrial Fibrillation: a Comparison between Lenient versus Strict Rate Control II (trial)
-
- RAFT
-
- Resynchronization/Defibrillation for Ambulatory Heart Failure Trial (trial)
-
- RASi
-
- Renin-angiotensin system inhibitor
-
- RATE-AF
-
- Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (trial)
-
- RBM20
-
- Ribonucleic acid binding motif 20
-
- RCT
-
- Randomized controlled trial
-
- REMATCH
-
- Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (trial)
-
- REVERSE
-
- REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (trial)
-
- REVIVED
-
- REVascularization for Ischaemic VEntricular Dysfunction (trial)
-
- RNA
-
- Ribonucleic acid
-
- RRT
-
- Renal replacement therapy
-
- RV
-
- Right ventricular/ventricle
-
- RVAD
-
- Right ventricular assist device
-
- RVEDP
-
- Right ventricular end-diastolic pressure
-
- SARS-CoV-2
-
- Severe acute respiratory syndrome coronavirus 2
-
- SAVR
-
- Surgical aortic valve replacement
-
- SBP
-
- Systolic blood pressure
-
- SCN5a
-
- Sodium channel alpha subunit 5
-
- SCORED
-
- Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (trial)
-
- SENIORS
-
- Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalizations in Seniors with Heart Failure (trial)
-
- SERVE-HF
-
- Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure (trial)
-
- SGLT2
-
- Sodium-glucose co-transporter 2
-
- S-ICD
-
- Subcutaneous implantable cardioverter-defibrillator
-
- SMR
-
- Secondary mitral regurgitation
-
- SPECT
-
- Single-photon emission computed tomography
-
- SpO2
-
- Transcutaneous oxygen saturation
-
- SR
-
- Sinus rhythm
-
- STEMI
-
- ST-elevation myocardial infarction
-
- STICH
-
- Surgical Treatment for Ischemic Heart Failure (trial)
-
- STICHES
-
- Extended follow-up of patients from the STICH trial
-
- STS-PROM
-
- Society of Thoracic Surgeons Predicted Risk of Mortality
-
- SZC
-
- Sodium zirconium cyclosilicate
-
- T2DM
-
- Type 2 diabetes mellitus
-
- TAVI
-
- Transcatheter aortic valve implantation
-
- TFT
-
- Thyroid function test
-
- t.i.d.
-
- Ter in die (three times a day)
-
- TKI
-
- Tyrosine kinase inhibitor
-
- TMEM43
-
- Transmembrane protein 43
-
- TNNT
-
- Troponin-T
-
- TR
-
- Tricuspid regurgitation
-
- TRPM4
-
- Transient receptor potential cation channel subfamily M member 4
-
- TSAT
-
- Transferrin saturation
-
- TSH
-
- Thyroid-stimulating hormone
-
- TTN
-
- Titin
-
- TTR
-
- Transthyretin
-
- UK
-
- United Kingdom
-
- US
-
- United States
-
- VAD
-
- Ventricular assist device
-
- Val-HeFT
-
- Valsartan Heart Failure Trial (trial)
-
- VEGF
-
- Vascular endothelial growth factor
-
- VERTIS-CV
-
- Cardiovascular Outcomes Following Ertugliflozin Treatment in Type 2 Diabetes Mellitus Participants With Vascular Disease (trial)
-
- VEST
-
- Vest Prevention of Early Sudden Death Trial (trial)
-
- VKA
-
- Vitamin K antagonist
-
- VO2
-
- Oxygen consumption
-
- VPB
-
- Ventricular premature beat
-
- vs.
-
- Versus
-
- VV interval
-
- Interventricular delay interval
-
- WARCEF
-
- Warfarin and Aspirin in Reduced Cardiac Ejection Fraction (trial)
-
- wtTTR-CA
-
- Wild-type transthyretin cardiac amyloidosis
-
- XL
-
- Extended release
1 Preamble
Guidelines summarize and evaluate available evidence with the aim of assisting health professionals in proposing the best management strategies for an individual patient with a given condition. Guidelines and their recommendations should facilitate decision making of health professionals in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of guidelines have been issued in recent years by the European Society of Cardiology (ESC), as well as by other societies and organizations. Because of their impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (https://www.escardio.org/Guidelines). The ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
In addition to the publication of Clinical Practice guidelines, the ESC carries out the EURObservational Research Programme of international registries of cardiovascular (CV) diseases and interventions which are essential to assess diagnostic/therapeutic processes, use of resources and adherence to guidelines. These registries aim at providing a better understanding of medical practice in Europe and around the world, based on high-quality data collected during routine clinical practice.
Furthermore, the ESC has developed and embedded in this document a set of quality indicators (QIs), which are tools to evaluate the level of implementation of the guidelines and may be used by the ESC, hospitals, healthcare providers and professionals to measure clinical practice as well as used in educational programmes, alongside the key messages from the guidelines, to improve quality of care and clinical outcomes.
The Members of this Task Force were selected by the ESC, including representation from its relevant ESC sub-specialty groups, in order to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management of a given condition according to ESC Clinical Practice Guidelines (CPG) Committee policy. A critical evaluation of diagnostic and therapeutic procedures was performed, including assessment of the risk-benefit ratio. The level of evidence and the strength of the recommendation of particular management options were weighed and graded according to predefined scales, as outlined below.
The experts of the writing and reviewing panels provided declaration of interest forms for all relationships that might be perceived as real or potential sources of conflicts of interest. Their declarations of interest were reviewed according to the ESC declaration of interest rules and can be found on the ESC website (https://www.escardio.org/guidelines) and have been compiled in a report and published in a supplementary document simultaneously to the guidelines.
This process ensures transparency and prevents potential biases in the development and review processes. Any changes in declarations of interest that arise during the writing period were notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from the healthcare industry.
The ESC CPG supervises and coordinates the preparation of new guidelines. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions the guidelines are signed-off by all the experts involved in the Task Force. The finalized document is signed-off by the CPG for publication in the European Heart Journal. The guidelines were developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
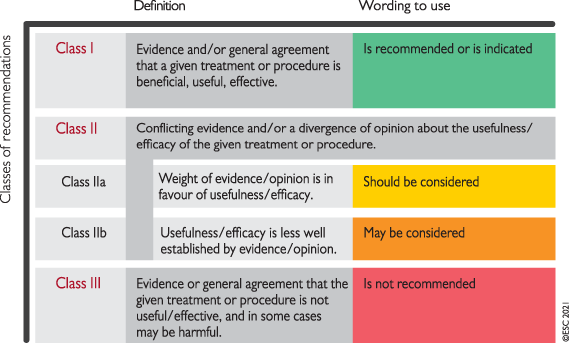 |
The task of developing ESC Guidelines also includes the creation of educational tools and implementation programmes for the recommendations including condensed pocket guideline versions, summary slides, summary cards for non-specialists and an electronic version for digital applications (smartphones, etc.). These versions are abridged and thus, for more detailed information, the user should always access to the full text version of the guidelines, which is freely available via the ESC website and hosted on the European Heart Journal website. The National Cardiac Societies of the ESC are encouraged to endorse, adopt, translate and implement all ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
 |
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic, or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient or the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable in each country to drugs and devices at the time of prescription.
2 Introduction
The aim of this ESC Guideline is to help health professionals manage people with heart failure (HF) according to the best available evidence. Fortunately, we now have a wealth of clinical trials to help us select the best management to improve the outcomes for people with HF; for many, it is now both preventable and treatable. This guideline provides practical, evidence-based recommendations.
We have revised the format of the previous 2016 ESC HF Guidelines1 to make each phenotype of HF stand-alone in terms of its diagnosis and management. The therapy recommendations mention the treatment effect supported by the class and level of evidence and are presented in tables. For HF with reduced ejection fraction (HFrEF), the tabular recommendations focus on mortality and morbidity outcomes. Where there are symptomatic benefits, these are highlighted in the text and/or in the web appendices. Detailed summaries of the trials underpinning the recommendations are available in the web appendices. For diagnostic indications, we have suggested investigations that all patients with HF should receive, and investigations that can be targeted to specific circumstances. As diagnostic tests have rarely been subject to randomized controlled trials (RCTs), most of the evidence would be regarded as level C. However, that does not mean that there has not been appropriate rigorous evaluation of diagnostic tests.
In this guideline, we have decided to focus on the diagnosis and treatment of HF, not on its prevention. Management of CV risk and many CV diseases [especially systemic hypertension, diabetes mellitus, coronary artery disease, myocardial infarction (MI), atrial fibrillation (AF), and asymptomatic left ventricular (LV) systolic dysfunction] will reduce the risk of developing HF, which is addressed by many other ESC Guidelines and in section 9.1 of the current guideline.2-7
This guideline is the result of a collaboration between the Task Force (including two patient representatives), the reviewers, and the ESC CPG Committee. As such, it is a consensus/majority opinion of the experts consulted in its development.
2.1 What is new
In addition to the recommendations listed below, the following table lists some new concepts compared with the 2016 version.
New concepts
| A change of the term ‘heart failure with mid-range ejection fraction’ to ‘heart failure with mildly reduced ejection fraction’ (HFmrEF). |
| A new simplified treatment algorithm for HFrEF. |
| The addition of a treatment algorithm for HFrEF according to phenotypes. |
| Modified classification for acute HF. |
| Updated treatments for most non-cardiovascular comorbidities including diabetes, hyperkalaemia, iron deficiency, and cancer. |
| Updates on cardiomyopathies including the role of genetic testing and new treatments. |
| The addition of key quality indicators. |
- HF = heart failure; HFrEF = heart failure with reduced ejection fraction
New recommendations
| Recommendations | Class |
| Recommendations for the diagnosis of HF | |
| Right heart catheterization should be considered in patients where HF is thought to be due to constrictive pericarditis, restrictive cardiomyopathy, congenital heart disease, and high output states. | IIa |
| Right heart catheterization may be considered in selected patients with HFpEF to confirm the diagnosis. | IIb |
| Recommendations for treatment of chronic HF | |
| HFrEF | |
| Dapagliflozin or empagliflozin are recommended for patients with HFrEF to reduce the risk of HF hospitalization and death. | I |
| Vericiguat may be considered in patients in NYHA class II–IV who have had worsening HF despite treatment with an ACE-I (or ARNI), a beta-blocker and an MRA to reduce the risk of CV mortality or HF hospitalization. | IIb |
| HFmrEF | |
| An ACE-I may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. | IIb |
| An ARB may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. | IIb |
| A beta-blocker may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. | IIb |
| An MRA may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. | IIb |
| Sacubitril/valsartan may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death. | IIb |
| HFpEF | |
| Screening for, and treatment of, aetiologies, and CV and non-CV comorbidities are recommended in patients with HFpEF (see relevant sections of this document). | I |
| Prevention and monitoring | |
| Self-management strategies are recommended to reduce the risk of HF hospitalization and mortality. | I |
| Either home-based and/or clinic-based programmes improve outcomes and are recommended to reduce the risk of HF hospitalization and mortality. | I |
| Influenza and pneumococcal vaccinations should be considered in order to prevent HF hospitalizations. | IIa |
| A supervised, exercise-based, cardiac rehabilitation programme should be considered in patients with more severe disease, frailty, or with comorbidities. | IIa |
| Non-invasive HTM may be considered for patients with HF in order to reduce the risk of recurrent CV and HF hospitalizations and CV death. | IIb |
| Recommendations for management of patients with advanced HF | |
| Patients being considered for long-term MCS must have good compliance, appropriate capacity for device handling and psychosocial support. | I |
| Heart transplantation is recommended for patients with advanced HF, refractory to medical/device therapy and who do not have absolute contraindications. | I |
| Continuous inotropes and/or vasopressors may be considered in patients with low cardiac output and evidence of organ hypoperfusion as bridge to MCS or heart transplantation. | IIb |
| Recommendations for management of patients after HF hospitalization | |
| It is recommended that patients hospitalized for HF be carefully evaluated to exclude persistent signs of congestion before discharge and to optimize oral treatment. | I |
| It is recommended that evidence-based oral medical treatment be administered before discharge. | I |
| An early follow-up visit is recommended at 1–2 weeks after discharge to assess signs of congestion, drug tolerance, and start and/or uptitrate evidence-based therapy. | I |
| Recommendations for management of patients with HF and atrial fibrillation | |
| Long-term treatment with an oral anticoagulant should be considered for stroke prevention in AF patients with a CHA2DS2-VASc score of 1 in men or 2 in women. | IIa |
| Recommendations for management of patients with HF and CCS | |
| CABG should be considered as the first-choice revascularization strategy, in patients suitable for surgery, especially if they have diabetes and for those with multivessel disease. | IIa |
| In LVAD candidates needing coronary revascularization, CABG should be avoided, if possible. | IIa |
| Coronary revascularization may be considered to improve outcomes in patients with HFrEF, CCS, and coronary anatomy suitable for revascularization, after careful evaluation of the individual risk to benefit ratio, including coronary anatomy (i.e. proximal stenosis >90% of large vessels, stenosis of left main or proximal LAD), comorbidities, life expectancy, and patient’s perspectives. | IIb |
| PCI may be considered as alternative to CABG, based on Heart Team evaluation, considering coronary anatomy, comorbidities, and surgical risk. | IIb |
| Recommendations for management of patients with HF and valvular heart disease | |
| Aortic valve intervention, TAVI or SAVR is recommended in patients with HF and severe high-gradient aortic stenosis to reduce mortality and improve symptoms. | I |
| It is recommended that the choice between TAVI and SAVR be made by the Heart Team, according to individual patient preference and features including age, surgical risk, clinical, anatomical and procedural aspects, weighing the risks and benefits of each approach. | I |
| Percutaneous edge-to-edge mitral valve repair should be considered in carefully selected patients with secondary mitral regurgitation, not eligible for surgery and not needing coronary revascularization, who are symptomatic despite OMT and who fulfil criteria to achieve a reduction in HF hospitalizations. | IIa |
| Percutaneous edge-gto-edge mitral valve repair may be considered to improve symptoms in carefully selected patients with secondary mitral regurgitation, not eligible for surgery and not needing coronary revascularization, who are highly symptomatic despite OMT and who do not fulfil criteria for reducing HF hospitalization. | IIb |
| Recommendations for management of patients with HF and diabetes | |
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with T2DM at risk of CV events to reduce hospitalizations for HF, major CV events, end-stage renal dysfunction, and CV death. | I |
| SGLT2 inhibitors (dapagliflozin, empagliflozin, and sotagliflozin) are recommended in patients with T2DM and HFrEF to reduce hospitalizations for HF and CV death. | I |
| The DPP-4 inhibitor saxagliptin is not recommended in patients with HF. | III |
| Recommendations for management of patients with HF and iron deficiency | |
| It is recommended that all patients with HF are periodically screened for anaemia and iron deficiency with a full blood count, serum ferritin concentration, and TSAT. | I |
| Intravenous iron supplementation with ferric carboxymaltose should be considered in symptomatic HF patients recently hospitalized for HF and with LVEF ≤50% and iron deficiency, defined as serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT <20%, to reduce the risk of HF hospitalization. | IIa |
| Treatment of anaemia in HF with erythropoietin stimulating agents is not recommended in the absence of other indications for this therapy. | III |
| Recommendations for management of patients with HF and cancer | |
| It is recommended that cancer patients at increased risk for cardiotoxicity, defined by a history or risk factors of CV disease, previous cardiotoxicity or exposure to cardiotoxic agents, undergo CV evaluation before scheduled anticancer therapy, preferably by a cardiologist with experience/interest in Cardio-Oncology. | I |
| Treatment with an ACE-I and a beta-blocker (preferably carvedilol) should be considered in cancer patients developing LV systolic dysfunction, defined as a 10% or more decrease in LVEF and to a value lower than 50%, during anthracycline chemotherapy. | IIa |
| A baseline CV risk assessment should be considered in all cancer patients scheduled to receive a cancer treatment with the potential to cause HF. | IIa |
| Recommendations for treatment of patients with HF and amyloidosis | |
| Tafamidis is recommended in patients with genetic testing proven hTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality. | I |
| Tafamidis is recommended in patients with wtTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality. | I |
- ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; CABG = coronary artery bypass graft; CCS = chronic coronary syndrome; CHA2DS2-VASc = Congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female) (score); CMP = cardiomyopathy; CV = cardiovascular; DPP-4 = dipeptidyl peptidase-4; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HTM = home telemonitoring; hTTR = hereditary transthyretin; LAD = left anterior descending artery; LV = left ventricular; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; SAVR = surgical aortic valve replacement; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus; TAVI = transcatheter aortic valve implantation; TSAT = transferrin saturation; wtTTR-CA = wild-type transthyretin cardiac amyloidosis.
Changes in recommendations
| 2021 | Class | 2016 | Class |
|---|---|---|---|
| Recommendations for diagnosis of HF | |||
| Invasive coronary angiography may be considered in patients with HFrEF with an intermediate to high pre-test probability of CAD and the presence of ischaemia in non-invasive stress tests. | IIb | Invasive coronary angiography should be considered in patients with HF and intermediate to high pre-test probability of CAD and the presence of ischaemia in non-invasive stress tests (who are considered suitable for potential coronary revascularization) in order to establish the diagnosis of CAD and its severity. | IIa |
| CTCA should be considered in patients with a low to intermediate pre-test probability of CAD or those with equivocal non-invasive stress tests in order to rule out coronary artery stenosis. | IIa | Cardiac CT may be considered in patients with HF and low to intermediate pre-test probability of CAD or those with equivocal non-invasive stress tests in order to rule out coronary artery stenosis. | IIb |
| Recommendations for device therapy in HFrEF | |||
| An ICD should be considered to reduce the risk of sudden death and all-cause mortality in patients with symptomatic HF (NYHA class II–III) of a non-ischaemic aetiology, and an LVEF ≤35% despite ≥3 months of OMT, provided they are expected to survive substantially longer than 1 year with good functional status. | IIa |
Primary prevention An ICD is recommended to reduce the risk of sudden death and all-cause mortality in patients with symptomatic HF (NYHA class II–III), and an LVEF ≤35% despite ≥3 months of OMT, provided they are expected to survive substantially longer than 1 year with good functional status, and they have DCM. |
I |
| CRT should be considered for symptomatic patients with HF in sinus rhythm with a QRS duration of 130–149 ms and LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality. | IIa | CRT is recommended for symptomatic patients with HF in sinus rhythm with a QRS duration of 130–149 ms and LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality. | I |
| Patients with an LVEF ≤35% who have received a conventional pacemaker or an ICD and subsequently develop worsening HF despite OMT and who have a significant proportion of RV pacing should be considered for ‘upgrade’ to CRT. | IIa | Patients with HFrEF who have received a conventional pacemaker or an ICD and subsequently develop worsening HF despite OMT and who have a high proportion of RV pacing may be considered for upgrade to CRT. This does not apply to patients with stable HF. | IIb |
| Recommendations for management of patients with acute HF | |||
| Combination of a loop diuretic with thiazide-type diuretic should be considered in patients with resistant oedema who do not respond to an increase in loop diuretic doses. | IIa | Combination of loop diuretic with either thiazide-type diuretic or spironolactone may be considered in patients with resistant oedema or insufficient symptomatic response. | IIb |
| In patients with AHF and SBP >110 mmHg, i.v. vasodilators may be considered as initial therapy to improve symptoms and reduce congestion. | IIb | In patients with hypertensive AHF, i.v. vasodilators should be considered as initial therapy to improve symptoms and reduce congestion. | IIa |
| Routine use of opiates is not recommended, unless in selected patients with severe/intractable pain or anxiety. | III | Opiates may be considered for cautious use to relieve dyspnoea and anxiety in patients with severe dyspnoea but nausea and hypopnea may occur. | IIb |
| Short-term MCS should be considered in patients with cardiogenic shock as a BTR, BTD, BTB. Further indications include treatment of the cause of cardiogenic shock or long-term MCS or transplantation. | IIa | Short-term MCS may be considered in refractory cardiogenic shock depending on patient age, comorbidities, and neurological function. | IIb |
| Recommendations for management of patients with HF and AF | |||
| DOACs are recommended in preference to VKAs in patients with HF, except in those with moderate or severe mitral stenosis or mechanical prosthetic heart valves. | I | For patients with HF and non-valvular AF eligible for anticoagulation based on a CHA2DS2-VASc score, NOACs rather than warfarin should be considered for anticoagulation as NOACs are associated with a lower risk of stroke, intracranial haemorrhage, and mortality, which outweigh the increased risk of gastrointestinal haemorrhage. | IIa |
| Beta-blockers should be considered for short- and long-term rate control in patients with HF and AF. | IIa | For patients in NYHA class I–III, a beta-blocker, usually given orally, is safe and therefore is recommended as first-line treatment to control ventricular rate, provided the patient is euvolaemic. | I |
| In cases of a clear association between paroxysmal or persistent AF and worsening of HF symptoms, which persist despite medical therapy, catheter ablation should be considered for the prevention or treatment of AF. | IIa | AV node catheter ablation may be considered to control heart rate and relieve symptoms in patients unresponsive or intolerant to intensive pharmacological rate and rhythm control therapy, accepting that these patients will become pacemaker-dependent. | IIb |
| Recommendations for management of patients with HF and CCS | |||
| Coronary revascularization should be considered to relieve persistent symptoms of angina (or an angina-equivalent) in patients with HFrEF, CCS, and coronary anatomy suitable for revascularization, despite OMT including anti-anginal drugs. | IIa | Myocardial revascularization is recommended when angina persists despite treatment with anti-anginal drugs. | I |
| Recommendations for management of patients with HF and diabetes | |||
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with T2DM at risk of CV events to reduce hospitalizations for HF, major CV events, end-stage renal dysfunction, and CV death. | I | Empagliflozin should be considered in patients with T2DM in order to prevent or delay the onset of HF and prolong life. | IIa |
- AF = atrial fibrillation; AHF = acute heart failure; AV = atrio-ventricular; BTB = bridge to bridge; BTD = bridge to decision; BTR = bridge to cardiac recovery; CAD = coronary artery disease; CCS = chronic coronary syndrome; CHA2DS2-VASc = Congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female) (score); CRT = cardiac resynchronization therapy; CT = computed tomography; CTCA = computed tomography coronary angiography; CV = cardiovascular; DCM = dilated cardiomyopathy; DOAC = direct oral anticoagulant; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; NOAC = non-vitamin K antagonist oral anticoagulant; NYHA = New York Heart Association; OMT = optimal medical therapy; QRS = Q, R, and S waves of an ECG; RV = right ventricular/ventricle; SBP = systolic blood pressure; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus; VKA = vitamin K antagonist.
3 Definition, epidemiology and prognosis
3.1 Definition of heart failure
Heart failure is not a single pathological diagnosis, but a clinical syndrome consisting of cardinal symptoms (e.g. breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles, and peripheral oedema). It is due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise.
Identification of the aetiology of the underlying cardiac dysfunction is mandatory in the diagnosis of HF as the specific pathology can determine subsequent treatment. Most commonly, HF is due to myocardial dysfunction: either systolic, diastolic, or both. However, pathology of the valves, pericardium, and endocardium, and abnormalities of heart rhythm and conduction can also cause or contribute to HF.
3.2 Terminology
3.2.1 Heart failure with preserved, mildly reduced, and reduced ejection fraction
-
Reduced LVEF is defined as ≤40%, i.e. those with a significant reduction in LV systolic function. This is designated as HFrEF.
-
Patients with a LVEF between 41% and 49% have mildly reduced LV systolic function, i.e. HFmrEF. Retrospective analyses from RCTs in HFrEF or HF with preserved ejection fraction (HFpEF) that have included patients with ejection fractions in the 40–50% range suggest that they may benefit from similar therapies to those with LVEF ≤40%.8-13 This supports the renaming of HFmrEF from ‘heart failure with mid-range ejection fraction’ to ‘heart failure with mildly reduced ejection fraction’.14
-
Those with symptoms and signs of HF, with evidence of structural and/or functional cardiac abnormalities and/or raised natriuretic peptides (NPs), and with an LVEF ≥50%, have HFpEF.
| Type of HF | HFrEF | HFmrEF | HFpEF | |
|---|---|---|---|---|
| CRITERIA | 1 | Symptoms ± Signsa | Symptoms ± Signsa | Symptoms ± Signsa |
| 2 | LVEF ≤40% | LVEF 41–49%b | LVEF ≥50% | |
| 3 | – | – | Objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/raised LV filling pressures, including raised natriuretic peptidesc | |
- HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LV = left ventricle; LVEF = left ventricular ejection fraction.
- a Signs may not be present in the early stages of HF (especially in HFpEF) and in optimally treated patients.
- b For the diagnosis of HFmrEF, the presence of other evidence of structural heart disease (e.g. increased left atrial size, LV hypertrophy or echocardiographic measures of impaired LV filling) makes the diagnosis more likely.
- c For the diagnosis of HFpEF, the greater the number of abnormalities present, the higher the likelihood of HFpEF.
The diagnosis of HFrEF, HFmrEF, and HFpEF is covered in more detail in their respective sections (sections 5, 7, and 8, respectively). These definitions are consistent with a recent report on the Universal Definition of Heart Failure.15
Patients with non-CV disease, e.g. anaemia, pulmonary, renal, thyroid, or hepatic disease may have symptoms and signs very similar to those of HF, but in the absence of cardiac dysfunction, they do not fulfil the criteria for HF. However, these pathologies can coexist with HF and exacerbate the HF syndrome.
3.2.2 Right ventricular dysfunction
Heart failure can also be a result of right ventricular (RV) dysfunction. RV mechanics and function are altered in the setting of either pressure or volume overload.16 Although the main aetiology of chronic RV failure is LV dysfunction-induced pulmonary hypertension, there are a number of other causes of RV dysfunction [e.g. MI, arrhythmogenic right ventricular cardiomyopathy (ARVC), or valve disease].17 The diagnosis is determined by a quantitative assessment of global RV function, most commonly by echocardiography, using at least one of the following measurements: fractional area change (FAC); tricuspid annular plane systolic excursion (TAPSE); and Doppler tissue imaging-derived systolic S′ velocity of the tricuspid annulus. The diagnosis and management of RV dysfunction is covered comprehensively in a recent Heart Failure Association (HFA) position paper.18
3.2.3 Other common terminology used in heart failure
Heart failure is usually divided into two presentations: chronic heart failure (CHF) and acute heart failure (AHF). CHF describes those who have had an established diagnosis of HF or who have a more gradual onset of symptoms. If CHF deteriorates, either suddenly or slowly, the episode may be described as ‘decompensated’ HF. This can result in a hospital admission or treatment with intravenous (i.v.) diuretic therapy in the outpatient setting. In addition, HF can present more acutely. Both of these are considered in the section on AHF (section 11).
Some individuals with HF may recover completely [e.g. those due to alcohol-induced cardiomyopathy (CMP), viral myocarditis, Takotsubo syndrome, peripartum cardiomyopathy (PPCM), or tachycardiomyopathy]. Other patients with LV systolic dysfunction may show a substantial or even complete recovery of LV systolic function after receiving drug and device therapy.
3.2.4 Terminology related to the symptomatic severity of heart failure
The simplest terminology used to describe the severity of HF is the New York Heart Association (NYHA) functional classification (Table 4). However, this relies solely on symptoms and there are many other better prognostic indicators in HF.19 Importantly, patients with mild symptoms may still have a high risk of hospitalization and death.20 Predicting outcome is particularly important in advanced HF to guide selection of cardiac transplantation and device therapies. This will be covered in detail in the section on advanced HF (section 10).
| Class I | No limitation of physical activity. Ordinary physical activity does not cause undue breathlessness, fatigue, or palpitations. |
| Class II | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in undue breathlessness, fatigue, or palpitations. |
| Class III | Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity results undue breathlessness, fatigue, or palpitations. |
| Class IV | Unable to carry on any physical activity without discomfort. Symptoms at rest can be present. If any physical activity is undertaken, discomfort is increased. |
3.3 Epidemiology and natural history of heart failure
3.3.1 Incidence and prevalence
In developed countries, the age-adjusted incidence of HF may be falling, presumably reflecting better management of CV disease, but due to ageing, the overall incidence is increasing.21-24 Currently, the incidence of HF in Europe is about 3/1000 person-years (all age-groups) or about 5/1000 person-years in adults.25, 26 The prevalence of HF appears to be 1–2% of adults.21,27-31 As studies only usually include recognized/diagnosed HF cases, the true prevalence is likely to be higher.32 The prevalence increases with age: from around 1% for those aged <55 years to >10% in those aged 70 years or over.33-36 It is generally believed that, of those with HF, about 50% have HFrEF and 50% have HFpEF/HFmrEF, mainly based on studies in hospitalized patients.32, 35, 37, 38 The ESC Long-Term Registry, in the outpatient setting, reports that 60% have HFrEF, 24% have HFmrEF, and 16% have HFpEF.39 Somewhat more than 50% of HF patients are female.21, 40, 41
3.3.2 Aetiology of heart failure
The most common causes (as well as some key investigations) of HF are shown in Table 5. The aetiology of HF varies according to geography. In Western-type and developed countries, coronary artery disease (CAD) and hypertension are predominant factors.27
| Cause | Examples of presentations | Specific investigations |
|---|---|---|
| CAD | Myocardial infarction Angina or “angina-equivalent” Arrhythmias | Invasive coronary angiography CT coronary angiography Imaging stress tests (echo, nuclear, CMR) |
| Hypertension | Heart failure with preserved systolic function Malignant hypertension/acute pulmonary oedema | 24 h ambulatory BP Plasma metanephrines, renal artery imaging Renin and aldosterone |
| Valve disease | Primary valve disease e.g., aortic stenosis Secondary valve disease, e.g. functional regurgitation Congenital valve disease | Echo – transoesophageal/stress |
| Arrhythmias | Atrial tachyarrhythmias Ventricular arrhythmias | Ambulatory ECG recording Electrophysiology study, if indicated |
| CMPs | All Dilated Hypertrophic Restrictive ARVC Peripartum Takotsubo syndrome Toxins: alcohol, cocaine, iron, copper | CMR, genetic testing Right and left heart catheterization CMR, angiography Trace elements, toxicology, LFTs, GGT |
| Congenital heart disease | Congenitally corrected/repaired transposition of great arteries Shunt lesions Repaired tetralogy of Fallot Ebstein’s anomaly | CMR |
| Infective | Viral myocarditis Chagas disease HIV Lyme disease | CMR, EMB Serology |
| Drug-induced | Anthracyclines Trastuzumab VEGF inhibitors Immune checkpoint inhibitors Proteasome inhibitors RAF+MEK inhibitors | |
| Infiltrative | Amyloid Sarcoidosis Neoplastic | Serum electrophoresis and serum free light chains, Bence Jones protein, bone scintigraphy, CMR, CT-PET, EMB Serum ACE, CMR, FDG-PET, chest CT, EMB CMR, EMB |
| Storage disorders | Haemochromatosis Fabry disease Glycogen storage diseases | Iron studies, genetics, CMR (T2* imaging), EMB α-galactosidase A, genetics, CMR (T1 mapping) |
| Endomyocardial disease | Radiotherapy Endomyocardial fibrosis/eosinophilia Carcinoid | CMR EMB 24 h urine 5-HIAA |
| Pericardial disease | Calcification Infiltrative | Chest CT, CMR, right and left heart catheterization |
| Metabolic | Endocrine disease Nutritional disease (thiamine, vitamin B1 and selenium deficiencies) Autoimmune disease | TFTs, plasma metanephrines, renin and aldosterone, cortisol Specific plasma nutrients ANA, ANCA, rheumatology review |
| Neuromuscular disease | Friedreich’s ataxia Muscular dystrophy | Nerve conduction studies, electromyogram, genetics CK, electromyogram, genetics |
- 5-HIAA = 5-hydroxyindoleacetic acid; ACE = angiotensin-converting enzyme; ANA = anti-nuclear antibody; ANCA = anti-nuclear cytoplasmic antibody; ARVC = arrhythmogenic right ventricular cardiomyopathy; BP = blood pressure; CAD = coronary artery disease; CMP = cardiomyopathy; CMR = cardiac magnetic resonance; CK = creatinine kinase; CT = computed tomography; ECG = electrocardiogram; Echo = echocardiography; EMB = endomyocardial biopsy; FDG = fluorodeoxyglucose; GGT = gamma-glutamyl transferase; HIV = human immunodeficiency virus; h = hour; LFT = liver function test; LGE = late gadolinium enhancement; MEK = mitogen-activated protein kinase; PET = positron emission tomography; TFT = thyroid function test; VEGF = vascular endothelial growth factor.
With regard to ischaemic aetiology, HFmrEF resembles HFrEF, with a higher frequency of underlying CAD compared to those with HFpEF.38, 42, 43
3.3.3 Natural history and prognosis
The prognosis of patients with HF has improved considerably since the publication of the first treatment trials a few decades ago. However, it remains poor, and quality of life (QOL) is also markedly reduced. The improvement in prognosis has been confined to those with HFrEF.
Mortality rates are higher in observational studies than in clinical trials.44 In the Olmsted County cohort, 1-year and 5-year mortality rates after diagnosis, for all types of HF patients, were 20% and 53%, respectively, between 2000 and 2010.45 A study combining the Framingham Heart Study (FHS) and Cardiovascular Health Study (CHS) cohorts reported a 67% mortality rate within 5 years following diagnosis.46 Despite receiving less evidence-based treatment, women have a better survival than men.47
Overall prognosis is better in HFmrEF compared to HFrEF.39 Of note, transition in ejection fraction over time is common, and patients who progress from HFmrEF to HFrEF have a worse prognosis than those who remain stable or transition to a higher ejection fraction category.48-52
HFpEF is generally considered to confer a better survival than HFrEF, but most observational studies show that this difference is negligible.45, 46 In contrast, the large MAGGIC meta-analysis concluded that the adjusted mortality risk for patients with HFpEF was considerably lower than in patients with HFrEF.53
Studies from several countries have shown that between 1980 and 2000 survival in HF patients has improved markedly.41, 54-57 However, this positive trend may have levelled off since then.45
After the initial diagnosis, HF patients are hospitalized once every year on average.54 From 2000 to 2010, the mean rate of hospitalization in the Olmsted County cohort was 1.3 per person-year. Interestingly, the majority (63%) of hospitalizations were related to non-CV causes.45 Studies from several European countries and the United States (US) have shown that HF hospitalization rates peaked in the 1990s, and then declined.54, 55, 58-60 However, in a recent study of incident HF conducted between 1998 and 2017 in the United Kingdom (UK), age-adjusted rates of first hospitalizations increased by 28% for both all-cause and HF admissions, and by 42% for non-CV admissions.61 These increases were higher in women, perhaps related to higher comorbidity rates. The risk of HF hospitalization is 1.5 times higher in patients with diabetes compared to controls. AF, a higher body mass index (BMI), and higher glycated haemoglobin (HbA1c), as well as a low estimated glomerular filtration rate (eGFR) are strong predictors of HF hospitalizations.29
Due to population growth, ageing, and the increasing prevalence of comorbidities, the absolute number of hospital admissions for HF is expected to increase considerably in the future, perhaps by as much as 50% in the next 25 years.24, 62
4 Chronic heart failure
4.1 Key steps in the diagnosis of chronic heart failure
The diagnosis of CHF requires the presence of symptoms and/or signs of HF and objective evidence of cardiac dysfunction (Figure 1). Typical symptoms include breathlessness, fatigue, and ankle swelling (Table 6). Symptoms and signs lack sufficient accuracy to be used alone to make the diagnosis of HF.63-66
| Symptoms | Signs |
|---|---|
| Typical | More specific |
|
|
| Less typical | Less specific |
|
|
- HF = heart failure.
- a This symptom of advanced HF corresponds to shortness of breath when leaning forward.67
The diagnosis of CHF is made more likely in patients with a history of MI, arterial hypertension, CAD, diabetes mellitus, alcohol misuse, chronic kidney disease (CKD), cardiotoxic chemotherapy, and in those with a family history of CMP or sudden death.
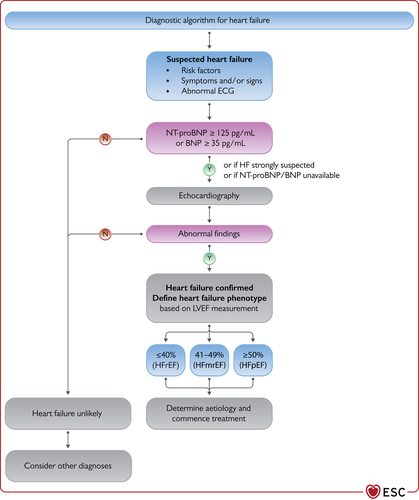
The diagnostic algorithm for heart failure. BNP = B-type natriuretic peptide; ECG = electrocardiogram; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B type natriuretic peptide. The abnormal echocardiographic findings are described in more detail in the respective sections on HFrEF (section 5), HFmrEF (section 7), and HFpEF (section 8).
- (1)
Electrocardiogram (ECG). A normal ECG makes the diagnosis of HF unlikely.63 The ECG may reveal abnormalities such as AF, Q waves, LV hypertrophy (LVH), and a widened QRS complex (Table 7) that increase the likelihood of a diagnosis of HF and also may guide therapy.
- (2)
Measurement of NPs are recommended, if available. A plasma concentration of B-type natriuretic peptide (BNP) <35 pg/mL, N-terminal pro-B-type natriuretic peptide (NT-proBNP) <125 pg/mL, or mid-regional pro-atrial natriuretic peptide (MR-proANP) <40 pmol/L68 make a diagnosis of HF unlikely. These will be discussed in more detail in section 4.2.69, 70
- (3)
Basic investigations such as serum urea and electrolytes, creatinine, full blood count, liver and thyroid function tests are recommended to differentiate HF from other conditions, to provide prognostic information, and to guide potential therapy.
- (4)
Echocardiography is recommended as the key investigation for the assessment of cardiac function. As well as the determination of the LVEF, echocardiography also provides information on other parameters such as chamber size, eccentric or concentric LVH, regional wall motion abnormalities (that may suggest underlying CAD, Takotsubo syndrome, or myocarditis), RV function, pulmonary hypertension, valvular function, and markers of diastolic function.16, 71
- (5)
A chest X-ray is recommended to investigate other potential causes of breathlessness (e.g. pulmonary disease). It may also provide supportive evidence of HF (e.g. pulmonary congestion or cardiomegaly).
Recommended diagnostic tests in all patients with suspected chronic heart failure
| Recommendations | Classa | Levelb |
|---|---|---|
| BNP/NT-proBNPc | I | B |
| 12-lead ECG | I | C |
| Transthoracic echocardiography | I | C |
| Chest radiography (X-ray) | I | C |
| Routine blood tests for comorbidities, including full blood count, urea and electrolytes, thyroid function, fasting glucose and HbA1c, lipids, iron status (TSAT and ferritin) | I | C |
- BNP = B-type natriuretic peptide; ECG = electrocardiogram; HbA1c = glycated haemoglobin; NT-proBNP = N-terminal pro-B-type natriuretic peptide; TSAT = transferrin saturation.
- a Class of recommendation.
- b Level of evidence.
- c References are listed in section 4.2 for this item.
4.2 Natriuretic peptides
Plasma concentrations of NPs are recommended as initial diagnostic tests in patients with symptoms suggestive of HF to rule out the diagnosis. Elevated concentrations support a diagnosis of HF, are useful for prognostication,72 and may guide further cardiac investigation.73 However, it should be noted that there are many causes of an elevated NP—both CV and non-CV—that might reduce their diagnostic accuracy (Table 7). These causes include AF, increasing age, and acute or chronic kidney disease.74 Conversely, NP concentrations may be disproportionately low in obese patients.75
| Cardiac |
|
| Non-cardiac |
|
- ACS = acute coronary syndrome; COPD = chronic obstructive pulmonary disease; ICD = implantable cardioverter-defibrillator.
4.2.1 Use in the non-acute setting
The diagnostic value of NPs, in addition to signs and symptoms and other diagnostic tests, such as an ECG, has been assessed in several studies in the primary care setting.68,76-80 The aim of these studies was to either exclude or establish a diagnosis of HF. The Task Force considered studies of adequate quality that included NP cut-off points in their diagnostic algorithms, below which the probability of having HF was extremely low. The upper limits of normal in the non-acute setting are 35 pg/mL for BNP, and 125 pg/mL for NT-proBNP. In these studies, the negative predictive values of NP concentrations below these thresholds range from 0.94 to 0.98.76-78 Fewer data are available for MR-proANP in CHF than in AHF. A concentration of <40 pmol/L can be used to rule out HF.68
4.3 Investigations to determine the underlying aetiology of chronic heart failure
Recommended tests to determine the underlying aetiology of CHF are summarized in Table 5.
Exercise or pharmacological stress echocardiography may be used for the assessment of inducible ischaemia in those who are considered suitable for coronary revascularization.81 In patients with HFpEF, valve disease, or unexplained dyspnoea, stress echocardiography might help clarify the diagnosis.82
Cardiac magnetic resonance (CMR) imaging with late gadolinium enhancement (LGE), T1 mapping and extracellular volume will identify myocardial fibrosis/scar, which are typically subendocardial for patients with ischaemic heart disease (IHD) in contrast to the mid-wall scar typical of dilated cardiomyopathy (DCM). In addition, CMR allows myocardial characterization in, e.g. myocarditis, amyloidosis, sarcoidosis, Chagas disease, Fabry disease, LV non-compaction CMP, haemochromatosis, and arrhythmogenic cardiomyopathy (AC).83,84
Computed tomography coronary angiography (CTCA) may be considered in patients with a low to intermediate pre-test probability of CAD, or those with equivocal non-invasive stress tests in order to exclude the diagnosis of CAD.5
Single-photon emission CT (SPECT) can also be used to assess myocardial ischaemia and viability, myocardial inflammation or infiltration. Scintigraphy with technetium (Tc)-labelled bisphosphonate has shown high sensitivity and specificity for imaging cardiac transthyretin amyloid.85
Recommendations for specialized diagnostic tests for selected patients with chronic heart failure to detect reversible/treatable causes of heart failure
| Recommendations | Classa | Levelb |
| CMR | ||
| CMR is recommended for the assessment of myocardial structure and function in those with poor echocardiogram acoustic windows. | I | C |
| CMR is recommended for the characterization of myocardial tissue in suspected infiltrative disease, Fabry disease, inflammatory disease (myocarditis), LV non-compaction, amyloid, sarcoidosis, iron overload/haemochromatosis. | I | C |
| CMR with LGE should be considered in DCM to distinguish between ischaemic and non-ischaemic myocardial damage. | IIa | C |
| Invasive coronary angiography (in those who are considered eligible for potential coronary revascularization) | ||
| Invasive coronary angiography is recommended in patients with angina despite pharmacological therapy or symptomatic ventricular arrhythmias.5 | I | B |
| Invasive coronary angiography may be considered in patients with HFrEF with an intermediate to high pre-test probability of CAD and the presence of ischaemia in non-invasive stress tests.89 | IIb | B |
| Non-invasive testing | ||
| CTCA should be considered in patients with a low to intermediate pre-test probability of CAD or those with equivocal non-invasive stress tests in order to rule out coronary artery stenosis. | IIa | C |
| Non-invasive stress imaging (CMR, stress echocardiography, SPECT, PET) may be considered for the assessment of myocardial ischaemia and viability in patients with CAD who are considered suitable for coronary revascularization.90-93 | IIb | B |
| Exercise testing may be considered to detect reversible myocardial ischaemia and investigate the cause of dyspnoea.94-96 | IIb | C |
| Cardiopulmonary exercise testing | ||
| Cardiopulmonary exercise testing is recommended as a part of the evaluation for heart transplantation and/or MCS.94-96 | I | C |
| Cardiopulmonary exercise testing should be considered to optimize prescription of exercise training.94-96 | IIa | C |
| Cardiopulmonary exercise testing should be considered to identify the cause of unexplained dyspnoea and/or exercise intolerance.94-96 | IIa | C |
| Right heart catheterization | ||
| Right heart catheterization is recommended in patients with severe HF being evaluated for heart transplantation or MCS. | I | C |
| Right heart catheterization should be considered in patients where HF is thought to be due to constrictive pericarditis, restrictive cardiomyopathy, congenital heart disease, and high output states. | IIa | C |
| Right heart catheterization should be considered in patients with probable pulmonary hypertension, assessed by echo in order to confirm the diagnosis and assess its reversibility before the correction of valve/structural heart disease. | IIa | C |
| Right heart catheterization may be considered in selected patients with HFpEF to confirm the diagnosis. | IIb | C |
| EMB | ||
| EMB should be considered in patients with rapidly progressive HF despite standard therapy when there is a probability of a specific diagnosis, which can be confirmed only in myocardial samples.97,98 | IIa | C |
- CAD = coronary artery disease; CMR = cardiac magnetic resonance; CTCA = computed tomography coronary angiography; DCM = dilated cardiomyopathy; EMB = endomyocardial biopsy; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LGE = late gadolinium enhancement; LV = left ventricular; MCS = mechanical circulatory support; PET = positron emission tomography; SPECT = single-photon emission computed tomography.
- a Class of recommendation.
- b Level of evidence.
Coronary angiography is recommended in patients with HF, who have angina pectoris or an ‘angina equivalent’ despite pharmacological therapy, in order to establish the diagnosis of CAD and its severity. Coronary angiography may also be considered in patients with HFrEF who have an intermediate to high pre-test probability of CAD and who are considered potentially suitable for coronary revascularization.5
5 Heart failure with reduced ejection fraction
5.1 The diagnosis of heart failure with reduced ejection fraction
The diagnosis of HFrEF requires the presence of symptoms and/or signs of HF and a reduced ejection fraction (LVEF ≤40%). This is most usually obtained by echocardiography. Details about the quality standards that should be adhered to when determining the presence of reduced LV systolic function by echocardiography can be found in the European Association of Cardiovascular Imaging (EACVI) position paper.99 If assessment of EF is not possible by echocardiography, then CMR or rarely, nuclear techniques can be employed.
An algorithm for the diagnosis of HFrEF is depicted in Figure 1. For the investigation of the underlying aetiology, please refer to Table 5.
5.2 Pharmacological treatments for patients with heart failure with reduced ejection fraction
5.2.1 Goals of pharmacotherapy for patients with heart failure with reduced ejection fraction
Pharmacotherapy is the cornerstone of treatment for HFrEF and should be implemented before considering device therapy, and alongside non-pharmacological interventions.
There are three major goals of treatment for patients with HFrEF: (i) reduction in mortality, (ii) prevention of recurrent hospitalizations due to worsening HF, and (iii) improvement in clinical status, functional capacity, and QOL.100-102
The key evidence supporting the recommendations in this section for patients with symptomatic HFrEF is given in Supplementary Table 1.
Figure 2 depicts the algorithm for the treatment strategy, including drugs and devices in patients with HFrEF, for Class I indications for the reduction of mortality (either all-cause or CV). The recommendations for each treatment are summarized below.
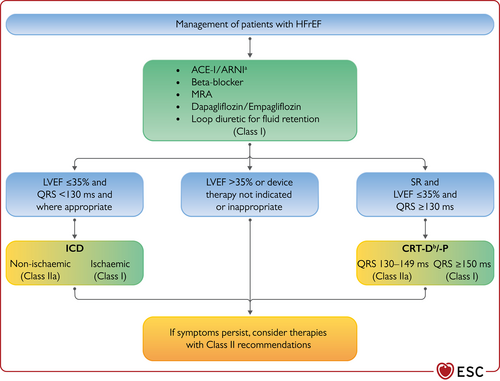
Therapeutic algorithm of Class I Therapy Indications for a patient with heart failure with reduced ejection fraction. ACE-I = angiotensin-converting enzyme inhibitor; ARNI = angiotensin receptor-neprilysin inhibitor; CRT-D =cardiac resynchronization therapy with defibrillator; CRT-P = cardiac resynchronization therapy pacemaker; ICD = implantable cardioverter-defibrillator; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; QRS = Q, R, and S waves of an ECG; SR = sinus rhythm. aAs a replacement for ACE-I. bWhere appropriate. Class I = green. Class IIa = Yellow.
5.2.2 General principles of pharmacotherapy for heart failure with reduced ejection fraction
Modulation of the renin-angiotensin-aldosterone (RAAS) and sympathetic nervous systems with angiotensin-converting enzyme inhibitors (ACE-I) or an angiotensin receptor-neprilysin inhibitor (ARNI), beta-blockers, and mineralocorticoid receptor antagonists (MRA) has been shown to improve survival, reduce the risk of HF hospitalizations, and reduce symptoms in patients with HFrEF. These drugs serve as the foundations of pharmacotherapy for patients with HFrEF. The triad of an ACE-I/ARNI, a beta-blocker, and an MRA is recommended as cornerstone therapies for these patients, unless the drugs are contraindicated or not tolerated.103-105 They should be uptitrated to the doses used in the clinical trials (or to maximally tolerated doses if that is not possible). This guideline still recommends the use of ARNI as a replacement for ACE-I in suitable patients who remain symptomatic on ACE-I, beta-blocker, and MRA therapies; however, an ARNI may be considered as a first-line therapy instead of an ACE-I.106, 107 The recommended doses of these drugs are given in Table 8. Angiotensin-receptor blockers (ARBs) still have a role in those who are intolerant to ACE-I or ARNI.
| Starting dose | Target dose | |
|---|---|---|
| ACE-I | ||
| Captoprila | 6.25 mg t.i.d. | 50 mg t.i.d. |
| Enalapril | 2.5 mg b.i.d. | 10–20 mg b.i.d. |
| Lisinoprilb | 2.5–5 mg o.d. | 20–35 mg o.d. |
| Ramipril | 2.5 mg b.i.d. | 5 mg b.i.d. |
| Trandolaprila | 0.5 mg o.d. | 4 mg o.d. |
| ARNI | ||
| Sacubitril/valsartan | 49/51 mg b.i.d.c | 97/103 mg b.i.d. |
| Beta-blockers | ||
| Bisoprolol | 1.25 mg o.d. | 10 mg o.d. |
| Carvedilol | 3.125 mg b.i.d. | 25 mg b.i.d.e |
| Metoprolol succinate (CR/XL) | 12.5–25 mg o.d. | 200 mg o.d. |
| Nebivolold | 1.25 mg o.d. | 10 mg o.d. |
| MRA | ||
| Eplerenone | 25 mg o.d. | 50 mg o.d. |
| Spironolactone | 25 mg o.d.f | 50 mg o.d. |
| SGLT2 inhibitor | ||
| Dapagliflozin | 10 mg o.d. | 10 mg o.d. |
| Empagliflozin | 10 mg o.d. | 10 mg o.d. |
| Other agents | ||
| Candesartan | 4 mg o.d. | 32 mg o.d. |
| Losartan | 50 mg o.d. | 150 mg o.d. |
| Valsartan | 40 mg b.i.d. | 160 mg b.i.d. |
| Ivabradine | 5 mg b.i.d. | 7.5 mg b.i.d. |
| Vericiguat | 2.5 mg o.d. | 10 mg o.d. |
| Digoxin | 62.5 µg o.d. | 250 µg o.d. |
| Hydralazine/ Isosorbide dinitrate | 37.5 mg t.i.d./20 mg t.i.d. | 75 mg t.i.d./40 mg t.i.d. |
- ACE-I = angiotensin-converting enzyme inhibitor; ARNI = angiotensin receptor-neprilysin inhibitor; b.i.d. = bis in die (twice daily); CR = controlled release; CV = cardiovascular; MRA = mineralocorticoid receptor antagonist; o.d. = omne in die (once daily); SGLT2 = sodium-glucose co-transporter 2; t.i.d. = ter in die (three times a day); XL = extended release.
- a Indicates an ACE-I where the dosing target is derived from post-myocardial infarction trials.
- b Indicates drugs where a higher dose has been shown to reduce morbidity/mortality compared with a lower dose of the same drug, but there is no substantive randomized, placebo-controlled trial and the optimum dose is uncertain.
- c Sacubitril/valsartan may have an optional lower starting dose of 24/26 mg b.i.d. for those with a history of symptomatic hypotension.
- d Indicates a treatment not shown to reduce CV or all-cause mortality in patients with heart failure (or shown to be non-inferior to a treatment that does).
- e A maximum dose of 50 mg twice daily can be administered to patients weighing over 85 kg.
- f Spironolactone has an optional starting dose of 12.5 mg in patients where renal status or hyperkalaemia warrant caution.
The sodium-glucose co-transporter 2 (SGLT2) inhibitors dapagliflozin and empagliflozin added to therapy with ACE-I/ARNI/beta-blocker/MRA reduced the risk of CV death and worsening HF in patients with HFrEF.108, 109 Unless contraindicated or not tolerated, dapagliflozin or empagliflozin are recommended for all patients with HFrEF already treated with an ACE-I/ARNI, a beta-blocker, and an MRA, regardless of whether they have diabetes or not.
Other drugs may be used for selected patients with HFrEF. These are discussed in section 5.4.
5.3 Drugs recommended in all patients with heart failure with reduced ejection fraction
Pharmacological treatments indicated in patients with (NYHA class II–IV) heart failure with reduced ejection fraction (LVEF ≤40%)
| Recommendations | Classa | Levelb |
| An ACE-I is recommended for patients with HFrEF to reduce the risk of HF hospitalization and death.110-113 | I | A |
| A beta-blocker is recommended for patients with stable HFrEF to reduce the risk of HF hospitalization and death.114-120 | I | A |
| An MRA is recommended for patients with HFrEF to reduce the risk of HF hospitalization and death.121,122 | I | A |
| Dapagliflozin or empagliflozin are recommended for patients with HFrEF to reduce the risk of HF hospitalization and death.108,109 | I | A |
| Sacubitril/valsartan is recommended as a replacement for an ACE-I in patients with HFrEF to reduce the risk of HF hospitalization and death.105 | I | B |
- ACE-I = angiotensin-converting enzyme inhibitor; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association.
- a Class of recommendation.
- b Level of evidence.
5.3.1 Angiotensin-converting enzyme inhibitors
ACE-Is were the first class of drugs shown to reduce mortality and morbidity in patients with HFrEF.110-113 They have also been shown to improve symptoms.111 They are recommended in all patients unless contraindicated or not tolerated. They should be uptitrated to the maximum tolerated recommended doses.
Practical guidance on how to use ACE-Is is given in Supplementary Table 2.
5.3.2 Beta-blockers
Beta-blockers have been shown to reduce mortality and morbidity in patients with HFrEF, in addition to treatment with an ACE-I and diuretic.114-120 They also improve symptoms.123 There is consensus that ACE-I and beta-blockers can be commenced together as soon as the diagnosis of symptomatic HFrEF is established. There is no evidence favouring the initiation of a beta-blocker before an ACE-I and vice versa.124 Beta-blockers should be initiated in clinically stable, euvolaemic, patients at a low dose and gradually uptitrated to the maximum tolerated dose. In patients admitted with AHF, beta-blockers should be cautiously initiated in hospital, once the patient is haemodynamically stabilized.
An individual patient data (IPD) meta-analysis of all major beta-blocker trials in HFrEF has shown no benefit on hospital admissions and mortality in the subgroup of patients with HFrEF with AF.125 However, since this is a retrospective subgroup analysis, and because beta-blockers did not increase risk, the guideline committee decided not to make a separate recommendation according to heart rhythm.
Practical guidance on how to use beta-blockers is given in Supplementary Table 3.
5.3.3 Mineralocorticoid receptor antagonists
MRAs (spironolactone or eplerenone) are recommended, in addition to an ACE-I and a beta-blocker, in all patients with HFrEF to reduce mortality and the risk of HF hospitalization.121,122 They also improve symptoms.121 MRAs block receptors that bind aldosterone and, with different degrees of affinity, other steroid hormones (e.g. corticosteroid and androgen) receptors. Eplerenone is more specific for aldosterone blockade and, therefore, causes less gynaecomastia.
Caution should be exercised when MRAs are used in patients with impaired renal function and in those with serum potassium concentrations >5.0 mmol/L.
Practical guidance on how to use MRAs is given in Supplementary Table 4.
5.3.4 Angiotensin receptor-neprilysin inhibitor
In the PARADIGM-HF trial, sacubitril/valsartan, an ARNI, was shown to be superior to enalapril in reducing hospitalizations for worsening HF, CV mortality, and all-cause mortality in patients with ambulatory HFrEF with LVEF ≤40% (changed to ≤35% during the study). Patients in the trial had elevated plasma NP concentrations, an eGFR ≥30 mL/min/1.73 m2 and were able to tolerate enalapril and then sacubitril/valsartan during the run-in period.105 Additional benefits of sacubitril/valsartan included an improvement in symptoms and QOL,105 a reduction in the incidence of diabetes requiring insulin treatment,126 and a reduction in the decline in eGFR,127 as well as a reduced rate of hyperkalaemia.128 Additionally, the use of sacubitril/valsartan may allow a reduction in loop diuretic requirement.129 Symptomatic hypotension was reported more commonly in patients treated with sacubitril/valsartan as compared to enalapril, but despite developing hypotension, these patients also gained clinical benefits from sacubitril/valsartan therapy.128, 130
Therefore, it is recommended that an ACE-I or ARB is replaced by sacubitril/valsartan in ambulatory patients with HFrEF, who remain symptomatic despite optimal treatment outlined above. Two studies have examined the use of ARNI in hospitalized patients, some of whom had not been previously treated with ACE-I. Initiation in this setting appears safe and reduces subsequent CV death or HF hospitalizations by 42% compared to enalapril.106, 107, 131 As such, initiation of sacubitril/valsartan in ACE-I naive (i.e. de novo) patients with HFrEF may be considered (class of recommendation IIb, level of evidence B). Patients being commenced on sacubitril/valsartan should have an adequate blood pressure (BP), and an eGFR ≥30 mL/min/1.73 m2. A washout period of at least 36 h after ACE-I therapy is required in order to minimize the risk of angioedema.
Practical guidance on how to use ARNI is given in Supplementary Table 5.
5.3.5 Sodium-glucose co-transporter 2 inhibitors
The DAPA-HF trial investigated the long-term effects of dapagliflozin (SGLT2 inhibitor) compared to placebo in addition to optimal medical therapy (OMT), on morbidity and mortality in patients with ambulatory HFrEF.108 Patients participated in the trial if they were in NYHA class II–IV, and had an LVEF ≤40% despite OMT. Patients were also required to have an elevated plasma NT-proBNP and an eGFR ≥30 mL/min/1.73 m2.108
Therapy with dapagliflozin resulted in a 26% reduction in the primary endpoint: a composite of worsening HF (hospitalization or an urgent visit resulting in i.v. therapy for HF) or CV death. Both of these components were significantly reduced. Moreover, dapagliflozin reduced all-cause mortality,108 alleviated HF symptoms, improved physical function and QOL in patients with symptomatic HFrEF.132 Benefits were seen early after the initiation of dapagliflozin, and the absolute risk reduction was large. Survival benefits were seen to the same extent in patients with HFrEF with and without diabetes, and across the whole spectrum of HbA1c values.108
Subsequently, the EMPEROR-Reduced trial found that empagliflozin reduced the combined primary endpoint of CV death or HF hospitalization by 25% in patients with NYHA class II–IV symptoms, and an LVEF ≤40% despite OMT.109 This trial included patients with an eGFR >20 mL/min/1.73 m2 and there was also a reduction in the decline in eGFR in individuals receiving empagliflozin. It was also associated with an improvement in QOL.133 Although there was not a significant reduction in CV mortality in the EMPEROR-Reduced trial, a recent meta-analysis of the DAPA-HF and EMPEROR-Reduced trials found no heterogeneity in CV mortality.134
Therefore, dapagliflozin or empagliflozin are recommended, in addition to OMT with an ACE-I/ARNI, a beta-blocker and an MRA, for patients with HFrEF regardless of diabetes status. The diuretic/natriuretic properties of SGLT2 inhibitors may offer additional benefits in reducing congestion and may allow a reduction in loop diuretic requirement.135
The combined SGLT-1 and 2 inhibitor, sotagliflozin, has also been studied in patients with diabetes who were hospitalized with HF. The drug reduced CV death and hospitalization for HF.136 It is discussed further in the AHF and comorbidity sections.
Therapy with SGLT2 inhibitors may increase the risk of recurrent genital fungal infections. A small reduction in eGFR following initiation is expected and is reversible and should not lead to premature discontinuation of the drug.
Practical guidance on how to use the SGLT2 inhibitors dapagliflozin and empagliflozin are given in Supplementary Table 6.
5.4 Other drugs recommended or to be considered in selected patients with heart failure with reduced ejection fraction
Other pharmacological treatments indicated in selected patients with NYHA class II–IV heart failure with reduced ejection fraction (LVEF ≤40%)
| Recommendations | Classa | Levelb |
| Loop diuretics | ||
| Diuretics are recommended in patients with HFrEF with signs and/or symptoms of congestion to alleviate HF symptoms, improve exercise capacity, and reduce HF hospitalizations.137 | I | C |
| ARB | ||
| An ARBc is recommended to reduce the risk of HF hospitalization and CV death in symptomatic patients unable to tolerate an ACE-I or ARNI (patients should also receive a beta-blocker and an MRA).138 | I | B |
| If-channel inhibitor | ||
| Ivabradine should be considered in symptomatic patients with LVEF ≤35%, in SR and a resting heart rate ≥70 b.p.m. despite treatment with an evidence-based dose of beta-blocker (or maximum tolerated dose below that), ACE-I/(or ARNI), and an MRA, to reduce the risk of HF hospitalization and CV death.139 | IIa | B |
| Ivabradine should be considered in symptomatic patients with LVEF ≤35%, in SR and a resting heart rate ≥70 b.p.m. who are unable to tolerate or have contraindications for a beta-blocker to reduce the risk of HF hospitalization and CV death. Patients should also receive an ACE-I (or ARNI) and an MRA.140 | IIa | C |
| Soluble guanylate cyclase stimulator | ||
| Vericiguat may be considered in patients in NYHA class II–IV who have had worsening HF despite treatment with an ACE-I (or ARNI), a beta-blocker and an MRA to reduce the risk of CV mortality or HF hospitalization.141 | IIb | B |
| Hydralazine and isosorbide dinitrate | ||
| Hydralazine and isosorbide dinitrate should be considered in self-identified black patients with LVEF ≤35% or with an LVEF <45% combined with a dilated left ventricle in NYHA class III–IV despite treatment with an ACE-I (or ARNI), a beta-blocker and an MRA to reduce the risk of HF hospitalization and death.142 | IIa | B |
| Hydralazine and isosorbide dinitrate may be considered in patients with symptomatic HFrEF who cannot tolerate any of an ACE-I, an ARB, or ARNI (or they are contraindicated) to reduce the risk of death.143 | IIb | B |
| Digoxin | ||
| Digoxin may be considered in patients with symptomatic HFrEF in sinus rhythm despite treatment with an ACE-I (or ARNI), a beta-blocker and an MRA, to reduce the risk of hospitalization (both all-cause and HF hospitalizations).144 | IIb | B |
- ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; b.p.m. = beats per minute; CV = cardiovascular; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association; SR = sinus rhythm.
- a Class of recommendation.
- b Level of evidence.
- c The ARBs with evidence in HFrEF are candesartan, losartan, and valsartan.
5.4.1 Diuretics
Loop diuretics are recommended to reduce the signs and/or symptoms of congestion in patients with HFrEF. The quality of the evidence regarding diuretics is poor and their effects on morbidity and mortality have not been studied in RCTs. However, it should also be remembered that the major disease-modifying treatment trials for HFrEF were conducted with a high background use of loop diuretic therapy. One meta-analysis has shown that in patients with HFrEF, loop and thiazide diuretics appear to reduce the risk of death and worsening HF compared with a placebo, and compared with an active control, diuretics improve exercise capacity.137
Loop diuretics produce a more intense and shorter diuresis than thiazides, although they act synergistically (sequential nephron blockade) and the combination may be used to treat diuretic resistance. However, adverse effects are more likely, and these combinations should only be used with care. Of note, ARNI, MRAs, and SGLT2 inhibitors may also possess diuretic properties.129, 145
The aim of diuretic therapy is to achieve and maintain euvolaemia with the lowest diuretic dose. In some euvolaemic/hypovolaemic patients, the use of a diuretic drug might be reduced or discontinued.146 Patients should be trained to self-adjust their diuretic dose based on monitoring of symptoms/signs of congestion and daily weight measurements.
Practical guidance on how to use diuretics is given in Supplementary Table 7.
5.4.2 Angiotensin II type 1 receptor blockers
The place of ARBs in the management of HFrEF has changed over the last few years. They are now recommended for patients who cannot tolerate ACE-I or ARNI because of serious side effects. Candesartan in the CHARM-Alternative study reduced CV deaths and HF hospitalizations in patients who were not receiving an ACE-I due to previous intolerance.138 Valsartan, in addition to usual therapy, including ACE-I, reduced HF hospitalizations in the Val-HeFT trial.147 However, no ARB has reduced all-cause mortality in any trial.
5.4.3 If-channel inhibitor
Ivabradine slows heart rate by inhibition of the If channel in the sinus node and is therefore only effective in patients in sinus rhythm (SR). Ivabradine reduced the combined endpoint of CV mortality and HF hospitalization in patients with symptomatic HFrEF with an LVEF ≤35%, with HF hospitalization in recent 12 months, in SR and with a heart rate ≥70 b.p.m. who were on evidence-based therapy including an ACE-I (or ARB), a beta-blocker, and an MRA.139,140 Our recommendation is based on the heart rate of ≥70 b.p.m. used in the SHIFT trial. However, the European Medicines Agency (EMA) approved ivabradine for use in Europe in patients with HFrEF with LVEF ≤35% and in SR with a resting heart rate ≥75 b.p.m., because in this group ivabradine conferred a survival benefit148 based on a retrospective subgroup analysis. Every effort should be made to commence and uptitrate beta-blocker therapy to guideline recommended/maximally tolerated doses prior to considering ivabradine.
Practical guidance on how to use ivabradine is given in Supplementary Table 8.
5.4.4 Combination of hydralazine and isosorbide dinitrate
There is no clear evidence to suggest the use of this fixed-dose combination therapy in all patients with HFrEF. A small RCT conducted in self-identified black patients showed that an addition of the combination of hydralazine and isosorbide dinitrate to conventional therapy (an ACE-I, a beta-blocker, and an MRA) reduced mortality and HF hospitalizations in patients with HFrEF and NYHA classes III–IV.142 These results are difficult to translate to patients of other racial or ethnic origins.
Additionally, a combination of hydralazine and isosorbide dinitrate may be considered in symptomatic patients with HFrEF who cannot tolerate any of an ACE-I, ARNI, or an ARB (or if they are contraindicated) to reduce mortality. However, this recommendation is based on the results of the relatively small Veterans Administration Cooperative Study, which included only male patients with symptomatic HFrEF who were treated with digoxin and diuretics.143
5.4.5 Digoxin
Digoxin may be considered in patients with HFrEF in SR to reduce the risk of hospitalization,144 although its effect on those routinely treated with beta-blockers has not been tested. In the DIG trial, the overall effect on mortality with digoxin was neutral.
The effects of digoxin in patients with HFrEF and AF have not been studied in RCTs. Some studies have suggested a potentially higher risk of events in patients with AF receiving digoxin,149, 150 whereas another meta-analysis concluded, on the basis of non-RCTs, that digoxin has no deleterious effect on mortality in patients with AF and HF, most of whom had HFrEF.151 Therefore, in patients with symptomatic HF and AF, digoxin may be useful for the treatment of patients with HFrEF and AF with rapid ventricular rate, when other therapeutic options cannot be pursued.150, 152-155
Digoxin has a narrow therapeutic window and so levels should be checked aiming for a serum digoxin concentration <1.2 ng/mL.156, 157 Caution should also be exercised when using it in females, the elderly, frail, hypokalaemic, and malnourished subjects. In patients with reduced renal function, digitoxin could be considered. Digitoxin use in HF and SR is currently being investigated.158
5.4.6 Recently reported advances from trials in heart failure with reduced ejection fraction
Soluble guanylate cyclase stimulator
The VICTORIA study assessed the efficacy and safety of the oral soluble guanylate cyclase stimulator, vericiguat, in patients with a reduced EF and recently decompensated CHF. The incidence of the primary endpoint of death from CV causes or hospitalization for HF was lower among those who received vericiguat than among those who received placebo.141 There was no reduction in either all-cause or CV mortality. Thus, vericiguat may be considered, in addition to standard therapy for HFrEF, to reduce the risk of CV mortality and hospitalizations for HF.
Cardiac myosin activator
The GALACTIC-HF study assessed the efficacy and safety of the cardiac myosin activator, omecamtiv mecarbil, in HFrEF patients, enrolling patients in both the inpatient and outpatient settings. The primary endpoint of a first HF event or CV death was reduced by 8%. There was no significant reduction in CV mortality. Currently, this drug is not licensed for use in HF. However, in the future it may be able to be considered, in addition to standard therapy for HFrEF to reduce the risk of CV mortality and hospitalization for HF.159
5.5 Strategic phenotypic overview of the management of heart failure with reduced ejection fraction
In addition to the general therapies considered in section 5, other therapies are appropriate to consider in selected patients. These are covered in detail in later sections. Some of the main ones (i.e. those with Class I and IIa Mortality/Hospitalization indications) are depicted in Figure 3. The effect of some interventions on symptoms/QOL are outlined in Supplementary Table 9.
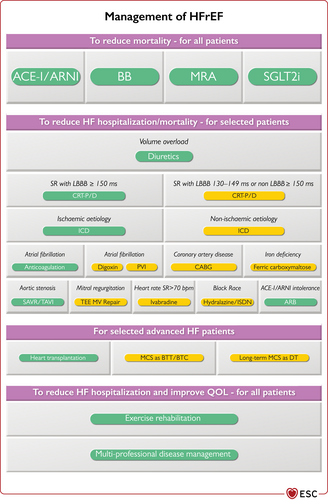
Central illustration. Strategic phenotypic overview of the management of heart failure with reduced ejection fraction. ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta-blocker; b.p.m. = beats per minute; BTC = bridge to candidacy; BTT = bridge to transplantation; CABG = coronary artery bypass graft; CRT-D = cardiac resynchronization therapy defibrillator; CRT-P = cardiac resynchronization therapy pacemaker; DT = destination therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; ISDN = isosorbide dinitrate; LBBB = left bundle branch block; MCS = mechanical circulatory support; MRA = mineralocorticoid receptor antagonist; MV = mitral valve; PVI = pulmonary vein isolation; QOL = quality of life; SAVR = surgical aortic valve replacement; SGLT2i = sodium-glucose co-transporter 2 inhibitor; SR = sinus rhythm; TAVI = transcatheter aortic valve implantation; TEE = transcatheter edge to edge. Colour code for classes of recommendation: Green for Class of recommendation I; Yellow for Class of recommendation IIa (see Table 1, 2 for further details on classes of recommendation). The Figure shows management options with Class I and IIa recommendations. See the specific Tables for those with Class IIb recommendations.
6 Cardiac rhythm management for heart failure with reduced ejection fraction
This section provides recommendations on the use of implantable cardioverter-defibrillators (ICD) and cardiac resynchronization therapy (CRT). Other implantable devices will be discussed at the end of this section.
6.1 Implantable cardioverter-defibrillator
A high proportion of deaths among patients with HF, especially in those with milder symptoms, occur suddenly and unexpectedly. Many of these may be due to electrical disturbances, including ventricular arrhythmias, bradycardia, and asystole, although some are due to other acute vascular events. Treatments that improve or delay the progression of CV disease have been shown to reduce the annual rate of sudden death,105, 160 but they do not treat arrhythmic events when they occur. ICDs are effective at correcting potentially lethal ventricular arrhythmias, and in the case of transvenous systems, also prevent bradycardia. Some antiarrhythmic drugs might reduce the rate of tachyarrhythmias and sudden death, but they do not reduce overall mortality,161 and may increase it.
Recommendations for an implantable cardioverter-defibrillator in patients with heart failure
| Recommendations | Classa | Levelb |
| Secondary prevention | ||
| An ICD is recommended to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status, in the absence of reversible causes or unless the ventricular arrhythmia has occurred <48 h after a MI.162-164 | I | A |
| Primary prevention | ||
| An ICD is recommended to reduce the risk of sudden death and all-cause mortality in patients with symptomatic HF (NYHA class II–III) of an ischaemic aetiology (unless they have had a MI in the prior 40 days—see below), and an LVEF ≤35% despite ≥3 months of OMT, provided they are expected to survive substantially longer than 1 year with good functional status.161,165 | I | A |
| An ICD should be considered to reduce the risk of sudden death and all-cause mortality in patients with symptomatic HF (NYHA class II–III) of a non-ischaemic aetiology, and an LVEF ≤35% despite ≥3 months of OMT, provided they are expected to survive substantially longer than 1 year with good functional status.161,166,167 | IIa | A |
| Patients should be carefully evaluated by an experienced cardiologist before generator replacement, because management goals, the patient’s needs and clinical status may have changed.168-172 | IIa | B |
| A wearable ICD may be considered for patients with HF who are at risk of sudden cardiac death for a limited period or as a bridge to an implanted device.173-176 | IIb | B |
| ICD implantation is not recommended within 40 days of a MI as implantation at this time does not improve prognosis.177,178 | III | A |
| ICD therapy is not recommended in patients in NYHA class IV with severe symptoms refractory to pharmacological therapy unless they are candidates for CRT, a VAD, or cardiac transplantation.179-183 | III | C |
- CRT = cardiac resynchronization therapy; HF = heart failure; ICD = implantable cardioverter-defibrillator; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NYHA = New York Heart Association; OMT = optimal medical therapy; VAD = ventricular assist device.
- a Class of recommendation.
- b Level of evidence.
6.1.1 Secondary prevention of sudden cardiac death
Compared with amiodarone treatment, ICDs reduce mortality in survivors of cardiac arrest and in patients who have experienced sustained symptomatic ventricular arrhythmias. An ICD is recommended in such patients when the intent is to increase survival; the decision to implant should take into account the patient’s view and their QOL, the LVEF (survival benefit is uncertain when LVEF >35%) and the absence of other diseases likely to cause death within the following year.162-164,184
6.1.2 Primary prevention of sudden cardiac death
In an analysis of over 40 000 patients from 12 pivotal HF trials, rates of sudden cardiac death decreased by 44% over the 20-year period (from the mid-1990s to 2015).160 This is almost certainly due to advances in HF treatment, as many key guideline-recommended therapies, including beta-blockers, MRAs, sacubitril/valsartan, and CRT pacemakers (CRT-P), reduce the risk of sudden death. While the afore-mentioned HF therapies have been shown to reduce mortality in patients with HFrEF, amiodarone has not.161 However, if it is to be used, it should be with caution due to its significant side-effect profile. Conversely, dronedarone185 and the class I antiarrhythmic agents disopyramide, encainide, and flecainide186 should not be used for prevention of arrhythmias due to the increase in mortality seen in clinical studies.
Although an ICD reduces the rate of sudden arrhythmic death in patients with HFrEF,187 it would be expected that, in well-managed patients, the additional benefit afforded by an ICD would be lower. In the DANISH trial, rates of sudden death were low in patients with non-ischaemic cardiomyopathy (NICM); only 70 patients out of 1116 followed up over 5 years had sudden death.166 Whilst there was a modest absolute reduction in sudden death with a defibrillator-containing device, this did not significantly improve the overall risk of mortality. However, subgroup analysis suggested there was a benefit in those ≤70 years.188 In a recent meta-analysis of studies that examined the effect of ICDs in NICM a survival benefit was still seen, although the effect was significantly weakened by the inclusion of the DANISH trial.167
On average, patients with IHD are at greater risk of sudden death than patients with NICM and therefore, although the relative benefits are similar, the absolute benefit is greater in patients with IHD.187 Two RCTs showed no benefit in patients who had an ICD implanted within 40 days after a MI.177, 178 Although sudden arrhythmic deaths were reduced, this was balanced by an increase in non-arrhythmic deaths. Accordingly, an ICD for primary prevention is contraindicated in this time period. Furthermore, ICD implantation is recommended only if a minimum of 3 months of OMT has failed to increase the LVEF to >35%. OMT ideally includes the use of Class I recommended drugs for HFrEF. However, the ICD trials we cite predate the use of ARNI and SGLT2 inhibitors. Whether implantation of ICDs reduces mortality in those with an LVEF >35% is unknown. There is an ongoing trial of ICD therapy in such patients with the presence of scar on CMR imaging.189
6.1.3 Patient selection for implantable cardioverter-defibrillator therapy
Patients with HFrEF and a QRS duration ≥130 ms can be considered for CRT with a defibrillator (CRT-D) rather than ICD. See the section on CRT for further details (section 6.2).
In patients with moderate or severe HF, a reduction in sudden death may be partially or wholly offset by an increase in death due to worsening HF.161 As such, ICD therapy is not recommended in patients in NYHA class IV, with severe symptoms refractory to pharmacological therapy, who are not candidates for a ventricular assist device (VAD) or cardiac transplantation. Such patients have a very limited life expectancy and are likely to die from pump failure. Similarly, patients with serious comorbidities who are unlikely to survive substantially more than 1 year with good QOL are unlikely to obtain substantial benefit from an ICD.179-183
Although the DANISH trial did not show a significant benefit from ICD therapy in patients with NICM, it should be remembered that NICM is a heterogeneous condition, and certain subgroups (e.g. laminopathies, sarcoidosis) are at higher risk of sudden death and therefore merit careful consideration of ICD implantation. Tools to help risk stratification (e.g. scar burden on magnetic resonance imaging) can be helpful in that regard.190-192
Patients should be counselled as to the purpose of an ICD and involved in the decision-making process. They should also be aware of the potential complications related to implantation, any additional implications for driving, and the risk of inappropriate shocks. Furthermore, patients should be informed about the circumstances where the defibrillator (or defibrillator component of a CRT-D) might be deactivated (e.g. terminal disease) or explanted (e.g. infection or recovery of LV function).193 Subsequent timely conversations regarding defibrillator deactivation should be held with the patient and caregiver(s).
When an ICD generator reaches its end of life or requires explantation, it should not be replaced automatically. Rather, shared decision making should be undertaken.168-172 Patients should be carefully evaluated by an experienced cardiologist as treatment goals may have changed since implantation (the risk of fatal arrhythmia may be lower, or the risk of non-arrhythmic death may be higher). It is a matter of some controversy whether patients whose LVEF has greatly improved and who have not required device therapy during the lifetime of the ICD should have another device implanted.168-172
6.1.4 Implantable cardioverter-defibrillator programming
Routine defibrillation threshold testing is no longer performed following implantation of an ICD or CRT-D as it does not improve shock efficacy or reduce arrhythmic death.194 Conservative programming with long delays195 between detection and the ICD delivering therapy dramatically reduces the risk of both inappropriate and appropriate but unnecessary shocks.194, 196, 197 Generally, for primary prevention, defibrillators are programmed to minimize pacing (e.g. ventricular demand pacing VVI at 40/min), and with a tachycardia treatment zone >200/min.194, 198 Ultimately—and particularly for secondary prevention—programming should be adapted according to the patient’s specific needs.
6.1.5 Subcutaneous and wearable implantable cardioverter-defibrillators
Subcutaneous ICDs (S-ICDs) appear to be as effective as conventional transvenous ICDs with a similar complication rate. Although the risk of inappropriate shocks appeared to be higher initially, improved patient selection has shown S-ICDs are non-inferior to transvenous ICDs in this regard.199-202 They may be the preferred option for patients with difficult venous access or those who require ICD explantation due to infection. Patients must be carefully selected, as S-ICDs cannot treat bradyarrhythmia (except post-shock pacing) and cannot deliver either anti-tachycardia pacing or CRT. Substantial RCTs with these devices and more long-term data on safety and efficacy are awaited.
A wearable cardioverter-defibrillator that is able to recognize and treat ventricular arrhythmias may be considered for a limited period of time in selected patients with HF who are at high risk for sudden death but otherwise are not suitable for ICD implantation.162, 175, 176, 203 However, the large VEST trial failed to show that the wearable cardioverter-defibrillator reduced arrhythmic death in patients with an LVEF ≤35% following a recent acute MI.204
For more detailed recommendations on the use/indications of ICD we refer the reader to the ESC/European Heart Rhythm Association (EHRA) Guidelines on ventricular tachyarrhythmias and sudden cardiac death.201
6.2 Cardiac resynchronization therapy
Recommendations for cardiac resynchronization therapy implantation in patients with heart failure
| Recommendations | Classa | Levelb |
| CRT is recommended for symptomatic patients with HF in SR with a QRS duration ≥150 ms and LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality.205-215 | I | A |
| CRT rather than RV pacing is recommended for patients with HFrEF regardless of NYHA class or QRS width who have an indication for ventricular pacing for high degree AV block in order to reduce morbidity. This includes patients with AF.216-219 | I | A |
| CRT should be considered for symptomatic patients with HF in SR with a QRS duration ≥150 ms and non-LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality.205-215 | IIa | B |
| CRT should be considered for symptomatic patients with HF in SR with a QRS duration of 130–149 ms and LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality.211,220 | IIa | B |
| Patients with an LVEF ≤35% who have received a conventional pacemaker or an ICD and subsequently develop worsening HF despite OMT and who have a significant proportion of RV pacing should be considered for ‘upgrade’ to CRT.221 | IIa | B |
| CRT may be considered for symptomatic patients with HF in SR with a QRS duration of 130–149 ms and non-LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality.208,213 | IIb | B |
| CRT is not recommended in patients with a QRS duration <130 ms who do not have an indication for pacing due to high degree AV block.222-224 | III | A |
- AF = atrial fibrillation; AV = atrio-ventricular; CRT = cardiac resynchronization therapy; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; OMT = optimal medical therapy (class I recommended medical therapies for at least 3 months); QRS = Q, R, and S waves of an ECG; RV = right ventricular; SR = sinus rhythm.
- a Class of recommendation.
- b Level of evidence.
In appropriately selected individuals, CRT reduces morbidity and mortality.211 Furthermore, CRT improves cardiac function, and enhances QOL.209, 225
Whilst the CARE-HF206, 208 and COMPANION210 trials compared the effect of CRT with medical therapy (MT), the majority of CRT studies have compared CRT-D with ICD, and a few have compared CRT-P with backup pacing. The prevention of fatal bradycardia might be an important mechanism of benefit shared by all pacing devices. In CARE-HF, at baseline, 25% of patients had a resting heart rate of ≤60 b.p.m.206, 208, 209 If prevention of bradycardia is important, the effect of CRT will appear greater in trials where there is no device in the control group. However, in MADIT-II, 35% of those who died with an ICD did so suddenly even though they were protected from both brady- and tachyarrhythmia.226
Most studies of CRT have specified that the LVEF should be ≤35%, but RAFT212 and MADIT-CRT213, 214 specified an LVEF ≤30%, while REVERSE207,215,227 specified ≤40% and BLOCK-HF216 ≤50%. Relatively few patients with an LVEF of 35–40% have been randomized, but an IPD meta-analysis suggests no diminution of the effect of CRT in this group.211
Assessing the ‘response’ to CRT is challenging. Indeed, many who do not appear to ‘respond’ favourably in terms of their symptoms or LV function may well have experienced the mortality benefit. Several characteristics predict improvement in morbidity and mortality. The extent of reverse remodelling is one of the most important mechanisms of action of CRT. Patients with HFrEF of an ischaemic aetiology have less improvement in LV function due to myocardial scar tissue, which is less likely to undergo favourable remodelling.228 Conversely, women may be more likely to respond than men, possibly due to smaller body and heart size.220,224,229 QRS width predicts CRT response and was the inclusion criterion in all randomized trials,211 but QRS morphology has also been related to a beneficial response to CRT. Several studies have shown that patients with left bundle branch block (LBBB) morphology are more likely to respond favourably to CRT, whereas there is less certainty about patients with non-LBBB morphology.230 This latter group is also underrepresented in the large CRT trials.206,210,213 However, patients with LBBB morphology often have wider QRS durations, and there is a current debate about whether QRS durations or QRS morphology is the main predictor of a beneficial response to CRT. Evidence from two IPD meta-analyses indicates that after accounting for QRS duration, there is little evidence to suggest that QRS morphology or aetiology of disease influence the effect of CRT on morbidity or mortality.211,220 In addition, none of the landmark trials selected patients for inclusion according to QRS morphology, sex, or ischaemic aetiology, nor were they powered for subgroup analyses.
The Echo-CRT trial222,223 and an IPD meta-analysis213 suggest possible harm from CRT when QRS duration is <130 ms, thus implantation of CRT is not recommended if QRS duration is <130 ms.
If a patient is scheduled to receive an ICD and is in SR, with a LBBB, CRT-D should be considered if the QRS is between 130 and 149 ms and is recommended if QRS is ≥150 ms. However, clinical practice varies widely among countries and if the primary reason for implanting CRT is for the relief of symptoms, then the clinician should choose CRT-P or CRT-D, whichever they consider appropriate. The only randomized trial to compare CRT-P and CRT-D210 did not demonstrate a difference in morbidity or mortality between these technologies (although the trial was not powered to show such a difference). Furthermore, in the DANISH study in patients with NICM where 58% of patients received CRT there was no suggestion from subgroup analysis that CRT-P was inferior to CRT-D.166,167
When LVEF is reduced, RV pacing may exacerbate cardiac dyssynchrony. This can be prevented by CRT, which might improve patient outcomes.216-218,231 However, a difference in outcome was not observed between CRT and RV pacing in a subgroup analysis of RAFT.212 On balance, CRT rather than RV pacing is recommended for patients with HFrEF regardless of NYHA class who have an indication for ventricular pacing in order to reduce morbidity, although no clear effect on mortality was observed. Patients with HFrEF who have received a conventional pacemaker or an ICD and subsequently develop worsening HF with a high proportion of RV pacing, despite OMT, should be considered for ‘upgrading’ to CRT.
Only two small trials have compared pharmacological therapy alone vs. CRT in patients with AF, with conflicting results. Several studies have indicated that CRT is superior to RV pacing in patients undergoing atrio-ventricular (AV) node ablation.217,218,231 However, AF is not an indication to carry out AV node ablation in patients with CRT except in a few cases when ventricular rate remains persistently high despite attempts at pharmacological rate control. A subgroup analysis of patients with AF from the RAFT study found no benefit from CRT-D compared with ICD, although less than half of patients had >90% biventricular capture.219 In view of the paucity of evidence for the efficacy of CRT in patients with AF, it may be an option in selected patients—particularly those with a QRS ≥150 ms—ensuring a proportion of biventricular pacing as high as possible.
Observational studies report that when biventricular capture is <98%, the prognosis of patients with CRT declines.218,232 Whether this association reflects a loss of resynchronization (which might be remedied by device programming), poor placement of the LV lead, or greater difficulty in pacing severely diseased myocardium is uncertain. This observation has not been confirmed in any randomized trial.
Early studies suggested that imaging tests for dyssynchrony were not of value in selecting patients for CRT.233 However, a recent study has suggested that two novel markers of dyssynchrony (apical rocking and septal flash) are associated with a response to CRT, but these have not been tested as selection criteria or as prespecified subgroups in a randomized trial.234 Patients with extensive myocardial scar will have less improvement in LV function with CRT, but this is true of any treatment for HFrEF and does not reliably predict less clinical benefit. Pacing thresholds are higher in scarred myocardium and, if possible, lead placement should avoid such regions.235,236 Although patients with extensive scarring have an intrinsically worse prognosis, there is little evidence that they obtain less prognostic benefit from CRT.211
The value of trying to optimize AV intervals or interventricular delay intervals (VV intervals) after implantation using echo- or electrocardiographic criteria or BP response is uncertain but may be considered for patients who have had a disappointing response to CRT.237,238 Other options to consider to optimize response to CRT are covered in a recently published practical article.239
Following CRT implantation, a review of diuretic therapy is advised as a reduction in dose or its discontinuation may be required. In addition, CRT implantation may afford an opportunity to further optimize MT for HFrEF.240
The reader is directed to guidelines on pacing and CRT for recommendations on device implantation procedures.241
6.3 Devices under evaluation
Cardiac contractility modulation (CCM) has been evaluated in patients with NYHA class III–IV HF, with an LVEF ≥25% to ≤45% and QRS duration <130 ms, and was associated with a small improvement in exercise tolerance and QOL.242,243
Technologies that involve modification of the activity of the autonomic nervous system, e.g. baroreflex activation therapy,244,245 have also been shown to offer a modest improvement in effort capacity and QOL. However, currently, the evidence is considered insufficient to support specific guideline recommendations for a reduction in mortality or hospitalization for these and a variety of other implantable electrical therapeutic technologies (see also Gaps in Evidence in section 16).
7 Heart failure with mildly reduced ejection fraction
7.1 The diagnosis of heart failure with mildly reduced ejection fraction
The diagnosis of HFmrEF requires the presence of symptoms and/or signs of HF, and a mildly reduced EF (41–49%) The presence of elevated NPs (BNP ≥35 pg/mL or NT-proBNP ≥125 pg/mL) and other evidence of structural heart disease [e.g. increased left atrial (LA) size, LVH or echocardiographic measures of LV filling] make the diagnosis more likely but are not mandatory for diagnosis if there is certainty regarding the measurement of LVEF.
An algorithm for the diagnosis of HFmrEF is depicted in Figure 1. For the investigation of the underlying aetiology, please refer to Table 5 (which refers to investigations regardless of LVEF).
7.2 Clinical characteristics of patients with heart failure with mildly reduced ejection fraction
There is a substantial overlap of clinical characteristics, risk factors, patterns of cardiac remodelling, and outcomes among the LVEF categories in HF. Patients with HFmrEF have, on average, features that are more similar to HFrEF than HFpEF, in that they are more commonly men, younger, and are more likely to have CAD (50–60%),38,42,43 and less likely to have AF and non-cardiac comorbidities (Supplementary Table 10). However, ambulatory patients with HFmrEF have a lower mortality than those with HFrEF, more akin to those with HFpEF.
Patients with HFmrEF may include patients whose LVEF has improved from ≤40% or declined from ≥50%.50
7.3 Treatments for patients with heart failure with mildly reduced ejection fraction
As in other forms of HF, diuretics should be used to control congestion. No substantial prospective RCT has been performed exclusively in patients with HFmrEF (Supplementary Table 11). Some data can be gleaned from subgroup analysis of trials in HFpEF, none of which have met their primary endpoint. Although strong recommendations cannot be made about specific therapies at this point in time, we have included a Table of Recommendations to help guide the management of patients in this category.
Pharmacological treatments to be considered in patients with (NYHA class II–IV) heart failure with mildly reduced ejection fraction
| Recommendations | Classa | Levelb |
| Diuretics are recommended in patients with congestion and HFmrEF in order to alleviate symptoms and signs.137 | I | C |
| An ACE-I may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death.11 | IIb | C |
| An ARB may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death.246 | IIb | C |
| A beta-blocker may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death.12,119 | IIb | C |
| An MRA may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death.247 | IIb | C |
| Sacubitril/valsartan may be considered for patients with HFmrEF to reduce the risk of HF hospitalization and death.13,248 | IIb | C |
- ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; NYHA = New York Heart Association.
- a Class of recommendation.
- b Level of evidence.
7.3.1 Angiotensin-converting enzyme inhibitors
There are no specific trials of ACE-I in patients with HFmrEF. Although, the PEP-CHF trial was conducted in patients with HFpEF and included patients with an LVEF >40%, it did not report outcomes according to LVEF.11
However, in patients with HFmrEF, many will also have CAD, hypertension, or post-MI LV systolic dysfunction and will, therefore, already be treated with ACE-I.
Therefore, ACE-I use may be considered in patients with HFmrEF.
7.3.2 Angiotensin receptor II type 1 receptor blockers
There are no specific trials of ARBs in HFmrEF. The CHARM-Preserved trial missed its primary endpoint of CV death or HF hospitalizations.246 However, a retrospective analysis showed that candesartan reduced the number of patients hospitalized for HF among those with HFmrEF (with similar trends for CV and all-cause mortality).8 Moreover, a recurrent-event analysis suggested a reduction in hospitalizations for HF among the entire CHARM-Preserved cohort, including those with HFmrEF.249
As for ACE-I, many with HFmrEF will already be on an ARB for other CV indications. Therefore, treatment with ARBs may be considered in patients with HFmrEF.
7.3.3 Beta-blockers
There is no specific trial of beta-blockade in HFmrEF. An IPD meta-analysis of landmark trials of beta-blockers suggested similar reductions in CV and all-cause mortality (of 50%) for patients in SR with HFrEF and HFmrEF.12 This IPD meta-analysis included the SENIORS trial where nebivolol reduced the composite primary endpoint of all-cause mortality or CV hospital admissions in the overall population. No interaction between LVEF (35% of patients had an LVEF of 35–50%) and the effect of nebivolol on the primary outcome was observed.119,250 Many patients with HFmrEF may have another CV indication, such as AF or angina, for a beta-blocker. Therefore, treatment with beta-blockers may be considered in patients with HFmrEF.
7.3.4 Mineralocorticoid receptor antagonists
There is no specific trial of MRAs in HFmrEF. In a retrospective analysis of the TOPCAT trial in patients with an LVEF ≥45%,9 spironolactone reduced hospitalizations for HF in those with an LVEF <55%. There was a similar trend for CV but not all-cause mortality.
Treatment with an MRA may be considered in patients with HFmrEF.
7.3.5 Angiotensin receptor-neprilysin inhibitor
There is no specific trial of ARNI in HFmrEF. In the PARAGON-HF trial, which included patients with EF ≥45%, although the trial missed its primary endpoint overall, a significant EF-by-treatment interaction was observed. Sacubitril/valsartan, compared with valsartan, reduced the likelihood of the primary composite outcome of CV death and total HF hospitalizations by 22% in those with an EF below or equal to the median of 57%.13 Further data are available from a combined analysis of the PARADIGM-HF and PARAGON-HF trials showing that sacubitril/valsartan, compared to other forms of RAAS blockade, has a beneficial effect, especially on hospitalizations for HF in those with HFmrEF.248
Treatment with an ARNI may be considered in patients with HFmrEF.
7.3.6 Other drugs
In the DIG trial,10 for those with HFmrEF in SR, there was a trend to fewer hospitalizations for HF in those assigned to digoxin, but no reduction in mortality and a trend to an excess of CV deaths. Therefore, there are insufficient data to recommend its use.
There are also insufficient data on ivabradine in HFmrEF to draw any conclusions.
7.3.7 Devices
While post hoc analyses of landmark CRT trials suggest that CRT may benefit patients with LVEF >35%, trials of CRT for HFmrEF were abandoned due to poor recruitment.251 There are no substantial trials of ICDs for primary prevention of ventricular arrhythmias for HFmrEF; trials conducted more than 20 years ago suggested no benefit from ICD implantation for secondary prevention of ventricular arrhythmias for HFmrEF.
Therefore, there is insufficient evidence to advise CRT or ICD therapy in patients with HFmrEF.
In HF patients with an LVEF ≥40%, the implantation of an interatrial shunt device was found to be safe, and this device is subject to investigation in a larger study before any recommendation on their use in HFpEF or HFmrEF can be given.252
8 Heart failure with preserved ejection fraction
8.1 The background to heart failure with preserved ejection fraction
This guideline acknowledges the historical changes in nomenclature and the lack of consensus on the optimal LVEF cut-off to define the group of patients with HF without overtly reduced EF. The term ‘preserved’ was originally proposed in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Programme to refer to patients with an EF (>40%) that was not clearly ‘reduced’ or completely ‘normal’.253 While the current guidelines have designated patients with an LVEF 41–49% as HFmrEF, we recognize that there will be debate about what constitutes ‘mildly reduced’ EF, what these EF cut-offs should be, and whether they should be different for men and women.14,254 The EACVI defines systolic dysfunction as being <52% for males and <54% for females.16
Whether patients with higher EFs and HF should be named HF with ‘normal’ EF has also been considered.14,255 However, given the known variability of echocardiographic measurements of LVEF, the difficulties in interpreting LVEF measured using different imaging modalities, and remaining controversies regarding the precise LVEF cut-off to define ‘normal’ , which may vary not only with sex but also with other factors such as age and ethnicity,256 this guideline has kept the nomenclature of HFpEF using an EF cut-off of 50%. Importantly, clinicians should be aware that LVEF is a continuous variable with a normal distribution in the general population, and the EF cut-offs used in definitions are therefore arbitrary. Moreover, while the LVEF cut-off to define ‘normal’ will likely be higher than 50%, the presence of a very high EF (e.g. above 65–70%) should also prompt a search for pathology, such as cardiac amyloidosis (CA) or hypertrophic cardiomyopathy (HCM), where a ‘supra-normal’ EF may result from shrinkage of the LV end-diastolic volume (denominator of EF).257,258
8.2 Clinical characteristics of patients with heart failure with preserved ejection fraction
HFpEF differs from HFrEF and HFmrEF in that HFpEF patients are older and more often female. AF, CKD, and non-CV comorbidities are more common in patients with HFpEF than in those with HFrEF.259
There are numerous potential causes of HFpEF (Table 5). The pathophysiology of various HFpEF syndromes differs, and thus they require distinct therapies. Red flags for the potential presence of CA include low normal BP in patients with a history of hypertension, intolerance to beta-blockers or ACE-I, history of bilateral carpal tunnel syndrome, low voltage on ECG and echocardiographic features such as thickening of the septum, posterior wall, or RV wall, enlarged atria, a small pericardial effusion, or valve thickening [for more details see the section on CMP (section 14.2)]. Furthermore, it is important to exclude other conditions that might mimic the HFpEF syndrome (e.g. lung disease, anaemia, obesity, and deconditioning). For a more comprehensive overview on HFpEF, see the ESC/HFA position statement.260
8.3 The diagnosis of heart failure with preserved ejection fraction
The diagnosis of HFpEF remains challenging. Several diagnostic criteria have been proposed by societies and in clinical trials.261 These criteria vary widely in their sensitivities and specificities for diagnosing HFpEF. More recently, two score-based algorithms (H2FPEF and HFA-PEFF) have been proposed to aid the diagnosis.260,262 While the generalizability of the scores has been tested in various trial and observational cohorts, their diagnostic performance has varied.263-270
Both scores assign a substantial proportion of suspected HFpEF patients as intermediate likelihood, wherein additional diagnostics are proposed. Thus, depending on which score is used, different patients will be referred for additional testing or allocated as having HFpEF. Furthermore, physicians may not have access to all the specialized tests recommended by the specific diagnostic algorithms. This limits the broad clinical applicability of the scores and demonstrates the ongoing diagnostic uncertainty in HFpEF.268
To facilitate broad clinical application, this guideline recommends a simplified pragmatic approach that distils the common major elements in prior diagnostic criteria and emphasizes the most frequently used variables widely available to clinicians. Some of these variables, in particular, LA size (LA volume index >32 mL/m2), mitral E velocity >90 cm/s, septal e′ velocity <9 cm/s, E/e′ ratio >9 have been shown to be pivot points beyond which the risk of CV mortality is increased, underscoring their value.271 This recommendation is therefore consistent with the consensus document of the HFA, and does not represent a new algorithm or diagnostic score but rather a simplified approach. Physicians with access to expertise may refer to the full diagnostic approach recommended by the HFA.260
- (1)
Symptoms and signs of HF.
- (2)
An LVEF ≥50%.*
- (3)
Objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of LV diastolic dysfunction/raised LV filling pressures, including raised NPs (Table 9).
*Of note, patients with a history of overtly reduced LVEF (≤40%), who later present with LVEF ≥50%, should be considered to have recovered HFrEF or ‘HF with improved LVEF’ (rather than HFpEF). Continued treatment for HFrEF is recommended in these patients.272 It is not known whether starting HF therapy in patients with recovered LVEF is beneficial. Patients with HFpEF tend to have stable trajectory of LVEF over time.273 However, in those who develop a clinical indication for a repeat echo during follow-up, around one third have a decline in LVEF.274
| Parametera | Threshold | Comments |
|---|---|---|
| LV mass index Relative wall thickness | ≥95 g/m2 (Female), ≥115 g/m2 (Male) >0.42 | Although the presence of concentric LV remodelling or hypertrophy is supportive, the absence of LV hypertrophy does not exclude the diagnosis of HFpEF |
| LA volume indexa | >34 mL/m2 (SR) | In the absence of AF or valve disease, LA enlargement reflects chronically elevated LV filling pressure (in the presence of AF, the threshold is >40 mL/m2) |
| E/e’ ratio at resta | >9 | Sensitivity 78%, specificity 59% for the presence of HFpEF by invasive exercise testing, although reported accuracy has varied. A higher cut-off of 13 had lower sensitivity (46%) but higher specificity (86%).71,260,275 |
| NT-proBNP BNP | >125 (SR) or >365 (AF) pg/mL >35 (SR) or >105 (AF) pg/mL |
Up to 20% of patients with invasively proven HFpEF have NPs below diagnostic thresholds, particularly in the presence of obesity |
| PA systolic pressure TR velocity at resta | >35 mmHg >2.8 m/s |
Sensitivity 54%, specificity 85% for the presence of HFpEF by invasive exercise testing260,262 |
- AF = atrial fibrillation; BNP = B-type natriuretic peptide; E/e’ratio = early filling velocity on transmitral Doppler/early relaxation velocity on tissue Doppler; HFpEF = heart failure with preserved ejection fraction; LA = left atrial; LV = left ventricular; NP = natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PA = pulmonary artery; SR = sinus rhythm; TR = tricuspid regurgitation.
- Note: The greater the number of abnormalities present, the higher the likelihood of HFpEF.
- a Only commonly used indices are listed in the table; for less commonly used indices refer to the consensus document of the ESC/HFA.260
In the presence of AF, the threshold for LA volume index is >40 mL/m2. Exercise stress thresholds include E/e′ ratio at peak stress ≥15 or tricuspid regurgitation (TR) velocity at peak stress >3.4 m/s.276 LV global longitudinal strain <16% has a sensitivity of 62% and a specificity of 56% for the diagnosis of HFpEF by invasive testing.262
The approach to the diagnosis should involve additional confirmatory tests in cases of diagnostic uncertainty, such as cardiopulmonary exercise testing (to confirm a reduction in exercise capacity and to help differentiate the cause of dyspnoea), exercise stress testing, and invasive haemodynamic testing.260
If resting echocardiographic and laboratory markers are equivocal, a diastolic stress test is recommended.260,275 The confirmatory test for the diagnosis of HFpEF is invasive haemodynamic exercise testing. An invasively measured pulmonary capillary wedge pressure (PCWP) of ≥15 mmHg (at rest) or ≥25 mmHg (with exercise) or LV end-diastolic pressure ≥16 mmHg (at rest) is generally considered diagnostic.267 However, instead of an exercise PCWP cut-off, some have used an index of PCWP to cardiac output for the invasive diagnosis of HFpEF261,277. Recognizing that invasive haemodynamic exercise testing is not available in many centres worldwide, and is associated with risks, its main use is limited to the research setting. In the absence of any disease-modifying treatments, the current guidelines do not mandate gold standard testing in every patient to make the diagnosis, but emphasize that the greater the number of objective non-invasive markers of raised LV filling pressures (Table 9), the higher the probability of a diagnosis of HFpEF.
8.4 Treatment of heart failure with preserved ejection fraction
To date, no treatment has been shown to convincingly reduce mortality and morbidity in patients with HFpEF, although improvements have been seen for some specific phenotypes of patients within the overall HFpEF umbrella. However, none of the large RCTs conducted in HFpEF have achieved their primary endpoints. These include PEP-CHF (perindopril),278 CHARM-Preserved (candesartan),246 I-PRESERVE (irbesartan),279 TOPCAT (spironolactone),247 DIG-Preserved (digoxin),280 and PARAGON-HF (sacubitril/valsartan)13 (see Supplementary Table 12 for the details about these and additional trials). Hospitalizations for HF were reduced by candesartan and spironolactone and there was a trend towards reduction with sacubitril/valsartan, although as these trials were neutral for their primary endpoints, these are hypothesis-generating findings only. Although nebivolol significantly reduced the combined primary endpoint of all-cause mortality or CV hospital admission in the SENIORS trial, this trial included only 15% with an LVEF >50%.119,250 Trials targeting the nitric oxide-cyclic guanosine monophosphate pathway have also failed to improve exercise capacity or QOL in HFpEF, e.g. NEAT-HFpEF,281 INDIE-HFpEF,282 VITALITY-HFpEF,283 and CAPACITY-HFpEF (praliciguat).284
Despite the lack of evidence for specific disease-modifying therapies in HFpEF, as the vast majority of HFpEF patients have underlying hypertension and/or CAD, many are already treated with ACE-I/ARB, beta-blockers, or MRAs. In the PARAGON-HF study at baseline, more than 86% of patients were on ACE-I/ARBs, 80% were on beta-blockers, and more than 24% were on MRAs.13
The Task Force acknowledge that the treatment options for HFpEF are being revised as this guideline is being published. We note that the Food and Drug Administration (FDA) has endorsed the use of sacubitril/valsartan and spironolactone in those with an LVEF ‘less than normal’. These statements relate to patients within both the HFmrEF and HFpEF categories. For sacubitril/valsartan, this decision was based on the subgroup analysis from the PARAGON-HF study, which showed a reduction in HF hospitalizations in those with an LVEF <57%, and a meta-analysis of the PARADIGM-HF and PARAGON-HF studies, showing a reduction in CV death and HF hospitalization in those with an LVEF below the normal range.248 Regarding spironolactone, the subgroup of individuals in the TOPCAT study recruited in the Americas had a significant reduction in the primary endpoint of CV death and HF hospitalization, and a subsequent post hoc analysis by EF showed a significant reduction in outcomes for those with an LVEF <55%.9,248 There are also ongoing trials with SGLT2 inhibitors. These developments may well accelerate a redefinition of HFpEF in the future and have therapeutic implications.
In the absence of recommendations regarding disease-modifying therapies, treatment should be aimed at reducing symptoms of congestion with diuretics. Loop diuretics are preferred, although thiazide diuretics may be useful for managing hypertension. Reducing body weight in obese patients and increasing exercise may further improve symptoms and exercise capacity and should therefore be considered in appropriate patients.285,286
It is important to identify and treat the underlying risk factors, aetiology, and coexisting comorbidities in HFpEF (e.g. hypertension in section 12.4, CAD in section 12.2, amyloidosis in section 14.6, AF in section 12.1.1, and valvular heart disease in section 12.3). Undoubtably, treatment of some of the underlying phenotypes of the the HFpEF syndrome leads to improved outcomes.
Recommendations for the treatment of patients with heart failure with preserved ejection fraction
| Recommendations | Classa | Levelb |
| Screening for, and treatment of, aetiologies, and cardiovascular and non-cardiovascular comorbidities is recommended in patients with HFpEF (see relevant sections of this document). | I | C |
| Diuretics are recommended in congested patients with HFpEF in order to alleviate symptoms and signs.137 | I | C |
- HFpEF = heart failure with preserved ejection fraction.
- a Class of recommendation.
- b Level of evidence.
9 Multidisciplinary team management for the prevention and treatment of chronic heart failure
9.1 Prevention of heart failure
General advice about risk factors for the development of HF (see Supplementary Figure 1) and strategies to prevent HF early in the CV continuum are summarized in Table 10.
| Risk factors for heart failure | Preventive strategies |
|---|---|
| Sedentary habit | Regular physical activity |
| Cigarette smoking | Cigarette smoking cessation |
| Obesity | Physical activity and healthy diet |
| Excessive alcohol intake287 | General population: no/light alcohol intake is beneficial Patients with alcohol-induced CMP should abstain from alcohol |
| Influenza | Influenza vaccination |
| Microbes (e.g. Trypanosoma cruzi, Streptococci) | Early diagnosis, specific antimicrobial therapy for either prevention and/or treatment |
| Cardiotoxic drugs (e.g., anthracyclines) | Cardiac function and side effect monitoring, dose adaptation, change of chemotherapy |
| Chest radiation | Cardiac function and side effect monitoring, dose adaptation |
| Hypertension | Lifestyle changes, antihypertensive therapy |
| Dyslipidaemia | Healthy diet, statins |
| Diabetes mellitus | Physical activity and healthy diet, SGLT2 inhibitors |
| CAD | Lifestyle changes, statin therapy |
- CAD = coronary artery disease; CMP = cardiomyopathy; SGLT2 = sodium-glucose co-transporter 2.
Recommendations for the primary prevention of heart failure in patients with risk factors for its development
| Recommendations | Classa | Levelb |
| Treatment of hypertension is recommended to prevent or delay the onset of HF, and to prevent HF hospitalizations.288-291 | I | A |
| Treatment with statins is recommended in patients at high risk of CV disease or with CV disease in order to prevent or delay the onset of HF, and to prevent HF hospitalizations.292,293 | I | A |
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with diabetes at high risk of CV disease or with CV disease in order to prevent HF hospitalizations.294-298 | I | A |
| Counselling against sedentary habit, obesity, cigarette smoking, and alcohol abuse is recommended to prevent or delay the onset of HF.299-303 | I | C |
- CV = cardiovascular; HF = heart failure; SGLT2 = sodium-glucose co-transporter 2.
- a Class of recommendation.
- b Level of evidence.
It is widely recognized that, in addition to optimizing medical and device therapies for HF, attention should also be given to how HF care is delivered. The HFA of the ESC has issued several position papers that cover non-pharmacological management, discharge planning, and standards for delivering HF care.304-306 It has also underscored the need for specialist HF cardiologists and specialist HF nurses to help provide care. Detailed curricula, to aid training of these, are available to be adapted for national implementation.307,308 This section focuses on areas where recommendations with an evidence level can be given: multidisciplinary team management, lifestyle advice, exercise training, follow-up, and monitoring.
9.2 Multidisciplinary management of chronic heart failure
9.2.1 Models of care
In order to reduce hospitalizations and mortality, earlier guidelines1 recommended the use of multidisciplinary HF management programmes (HF-MPs), which enable patients to have the correct investigations, an accurate diagnosis, appropriate evidence-based therapy, education, and suitable follow-up. The optimal implementation of a HF-MP requires a multidisciplinary team that is active along the whole HF trajectory; from onset, through critical events, periods of apparent stability, and its terminal stages.304 Since the 2016 guidelines, new studies have been published that underscore the need for HF-MPs and reveal more insights into how care can be delivered.
A network meta-analysis including 53 randomized trials published in 2017, concluded that both disease-management clinics and home visits by nurses reduced all-cause mortality compared to usual care; home visits being most effective.309 An IPD meta-analysis of 20 studies, including 5624 patients, concluded that self-management interventions in HF patients improve outcomes despite heterogeneity in the intensity, content, and the personnel who deliver the interventions.310
HF-MPs vary in their components and can apply different service models, such as clinic-based approaches (in primary, secondary, or tertiary care), home-based programmes, case management, or hybrids of these. Components used in the services vary, e.g. some HF-MPs use telemonitoring that may be applied at a local, regional, or national level. No service model has been shown to be consistently superior to others.311 While home visits and HF clinics do reduce all-cause admissions and mortality, educational programmes, used alone, do not.309,310 HF-MPs should be patient-centred and take a holistic approach to the patient rather than focussing solely on HF; management of comorbid conditions, such as arrhythmias, hypertension, diabetes, renal dysfunction, and depression, improve patient well-being and self-management, leading to better outcomes.310,312 The organization of a HF-MP should be adapted to the healthcare system, available resources (infrastructure, facilities, staff, and finances), administrative policies, and tailored to the patient’s needs.
Many patients with HF would derive benefit from the early integration of a palliative and supportive approach within the care provided by all members of the HF multidisciplinary team.313,314 Palliative and supportive care should be thought about for all patients with HF, regardless of stage of their illness. Patients in the advanced stages and those considered for mechanical circulatory support (MCS) or heart transplantation should receive a palliative care consultation before such interventions as a matter of protocol (see section 10.2.4).
9.2.2 Characteristics and components of a heart failure management programme
Clinical trials have included complex, bundled interventions, making it difficult to determine the efficiency and effectiveness of each specific component. Table 11 presents an overview of characteristics and components that are important to consider in a HF-MP.
Multidisciplinary interventions recommended for the management of chronic heart failure
| Recommendations | Classa | Levelb |
| It is recommended that HF patients are enrolled in a multidisciplinary HF management programme to reduce the risk of HF hospitalization and mortality.310,315,316,317 | I | A |
| Self-management strategies are recommended to reduce the risk of HF hospitalization and mortality.310 | I | A |
| Either home-based and/or clinic-based programmes improve outcomes and are recommended to reduce the risk of HF hospitalization and mortality.311,318 | I | A |
| Influenza and pneumococcal vaccinations should be considered in order to prevent HF hospitalizations.316,317 | IIa | B |
- HF = heart failure.
- a Class of recommendation.
- b Level of evidence.
Characteristics
|
Components
|
- HF = heart failure; QOL = quality of life.
9.3 Patient education, self-care and lifestyle advice
Adequate patient self-care is essential in the effective management of HF and allows patients to understand what is beneficial, and to agree to self-monitoring and management plans.320 HF patients who report more effective self-care have a better QOL, lower readmission rates, and reduced mortality.310
Misunderstandings, misconceptions, and lack of knowledge all contribute to insufficient self-care and therefore patient education is vital. Improving patients’ knowledge of their condition is fundamental for the development of self-care skills.305
Education to improve self-care should be tailored to the individual patient and based on, where available, scientific evidence or expert opinion. There is little evidence that specific lifestyle advice improves QOL or prognosis; however, providing this information has become a key component of education for self-care.
-
Providing information in a variety of formats that take into account educational grade and health literacy. Consider approaches with active roles for patients and caregivers such as ‘ask-tell-ask’, ‘teach back’, or motivational interviewing. Reinforce messages at regular time intervals.
-
Recognizing barriers to communication (language, social skills, cognition, anxiety/depression, hearing or visual challenges).
-
Recommending ‘HFmatters.org’. Offer help and guidance to use it and offer discussion of questions arising.
-
Inviting patients to be accompanied by a family member or friend.
Key topics to include are recommended in Table 12.
| Education topic | Goal for the patient and caregiver | Professional behaviour and educational tools |
|---|---|---|
| Explanation about HF | To understand the cause of their HF, symptoms and treatment choice. | Provide tailored information. |
| The HF trajectory |
|
Sensitively communicate information on prognosis at time of diagnosis, during decision making about treatment options, when there is a change in the clinical condition and whenever the patient requests. |
| Medical treatment | ||
| Medication |
|
|
| Implanted devices |
|
|
| Self-care aspects | ||
| Activity and exercise |
|
|
| Sleep and breathing |
|
|
| Fluids |
|
|
| Healthy diet | To be able to prevent malnutrition and know how to eat healthily, avoiding excessive salt intake (>5 g/day) and maintaining a healthy body weight. |
|
| Alcohol |
|
|
| Immunization | To be aware of the need for immunization for influenza and pneumococcal disease. |
|
| Smoking and recreational drugs |
|
|
| Travel, leisure, driving |
|
|
| Sexual activity |
|
|
| Symptom monitoring and symptom self-management |
|
|
| Living with HF | ||
| Psychological issues |
|
|
| Family and informal caregivers | To be able to ask for support. |
|
- CMP = cardiomyopathy; CV = cardiovascular; HF = heart failure.
- a 1 unit is 10 mL of pure alcohol (e.g., 1 glass of wine, ½ pint of beer, 1 measure of spirit).
9.4 Exercise rehabilitation
There is consistent evidence that physical conditioning by exercise training improves exercise tolerance, and health-related QOL in patients with HF. Clinical trials and meta-analyses in people with HFrEF show that exercise rehabilitation improves exercise capacity and QOL. Several meta-analyses also show that it reduces all-cause and HF hospitalizations, although uncertainty persists about its effects on mortality.323-329 The effect on hospitalization is seen in those who are highly adherent to the exercise programme.329 High-intensity interval training, in patients who are able and willing, may improve peak oxygen consumption (VO2).330,331 Supervised exercise-based rehabilitation should be considered in those who are frail, who have more severe disease or comorbidities.95
Recommendations for exercise rehabilitation in patients with chronic heart failure
| Recommendations | Classa | Levelb |
| Exercise is recommended for all patients who are able in order to improve exercise capacity, QOL, and reduce HF hospitalization.c 325-329,336-338 | I | A |
| A supervised, exercise-based, cardiac rehabilitation programme should be considered in patients with more severe disease, frailty, or with comorbidities.95,325-328,339 | IIa | C |
- HF = heart failure; QOL = quality of life.
- a Class of recommendation.
- b Level of evidence.
- c In those who are able to adhere to the exercise programme.
Physical conditioning also improves exercise capacity and QOL.333-336 No data on HFmrEF are available, but benefits observed in the other groups of HF should also apply to this group.
9.5 Follow-up of chronic heart failure
9.5.1 General follow-up
This is a relatively understudied area. Patients with HF, even if symptoms are well controlled and stable, require follow-up to ensure continued optimization of therapy, to detect asymptomatic progression of HF or its comorbidities and to discuss any new advances in care. These guidelines recommend follow-up at intervals no longer than 6 months to check symptoms, heart rate and rhythm, BP, full blood count, electrolytes, and renal function. For patients recently discharged from hospital, or in those undergoing uptitration of medication, follow-up intervals should be more frequent. Whether such stable patients need to be followed-up by cardiologists is uncertain. Some studies suggest that follow-up in primary care may be appropriate.304,340 However, uptake of evidence-based interventions is poor in many settings341,104 and several studies suggest that care and follow-up provided by HF specialists, and use of quality improvement registries can lead to higher rates of optimal therapy and improved outcomes.342-344
An ECG should be done annually to detect QRS prolongation345 as such patients may become candidates for CRT. Furthermore, it may identify conduction disturbances and AF.
Serial echocardiography is generally not necessary, although an echocardiogram should be repeated if there has been a deterioration in clinical status. An echocardiogram is also advised 3–6 months after optimization of standard therapies for HFrEF to determine the need for addition of newer pharmacological agents and implanted devices.
9.5.2 Monitoring with biomarkers
Trials investigating the use of biomarkers (particularly BNP and/or NT-proBNP) to guide pharmacotherapy for HFrEF have produced conflicting results.346-353 They are undoubtedly good prognostic markers.72,354,355 Conceptually, it is not clear what a biomarker-supported strategy might offer in addition to assiduous application of guideline-recommended therapy. Current evidence, therefore, does not support the routine measurement of BNP or NT-proBNP to guide titration of therapy.
9.6 Telemonitoring
Telemonitoring enables patients to provide, remotely, digital health information to support and optimize their care. Data such as symptoms, weight, heart rate, and BP, can be collected frequently, stored in an electronic health record and used to guide patients (directly or through a healthcare professional), to adjust therapy or to seek further advice. Home telemonitoring (HTM) can help maintain quality of care, facilitate rapid access to care when needed, reduce patient travel costs, and minimize the frequency of clinic visits.356 Enforced cessation of face-to-face consultations in many countries during the recent COVID-19 pandemic have highlighted some of the potential advantages of HTM.357
Trials of HTM are diverse. Patients are usually required to make measurements and, as for many other aspects of HF management, adherence may be incomplete. HTM may be provided as a local, regional, or national service. Systems that focus on optimizing management rather than detecting and managing medical emergencies need only to be staffed during standard working hours. Some systems are designed also to offer support at any time requested by the patient. The comparative effectiveness and cost effectiveness of each strategy is uncertain. Systems that focus on continuous optimization of care (a health maintenance approach) rather than trying to anticipate and manage episodes of worsening (a strategy that is plagued by a large number of false-positive alerts), appear more successful.358 HTM is an efficient method for providing patient education and motivation and aiding delivery of care, but it should be adapted to work in synergy with existing healthcare provision.359
A Cochrane systematic review conducted in 2017 identified 39 relevant trials of HTM, largely based on assessments of symptoms, weight, heart rate and rhythm, and BP and found that HTM was associated with a reduction in all-cause mortality of 20% and HF hospitalization of 37%.360 Since then, several neutral trials and at least one positive trial have been published.358,361-365 These are unlikely to change the positive results of the systematic review. Importantly, if social distancing and the ‘green’ agenda are important, HTM only needs to show that it is not inferior to contemporary methods of delivering care to be an appropriate means of supporting care.357
Whether wearable technologies for monitoring heart rate and rhythm or lung congestion (bio-impedance or lung radar) offer additional benefits to conventional HTM described above is uncertain.366–368
Many implanted therapeutic devices can provide, wirelessly and remotely, information either on the device itself (generator and lead function), arrhythmias, or on patient physiology (heart rate, activity, heart sounds, bio-impedance). There is strong evidence that monitoring can detect device malfunction earlier than by conventional monitoring and that it may be useful for detecting arrhythmias such as AF. However, there is little evidence that device monitoring reduces admissions for HF or mortality.369-371,372
Devices that only provide a monitoring function are also available. Implantable loop-recorders can be injected subcutaneously and used to monitor heart rate and rhythm, activity, and bio-impedance. Monitoring devices can also be placed in the pulmonary artery to monitor pressure wirelessly, although the external reader required to detect the device signal is rather bulky and requires patient co-operation. A rise in diastolic pulmonary artery pressure may be one of the earliest signs of congestion. A preliminary, but fairly substantial, trial showed a reduction in the risk of recurrent HF hospitalization.373 A much larger trial has completed recruitment (GUIDE-HF).374
Recommendations for telemonitoring
| Recommendations | Classa | Levelb |
| Non-invasive HTM may be considered for patients with HF in order to reduce the risk of recurrent CV and HF hospitalizations and CV death.375 | IIb | B |
| Monitoring of pulmonary artery pressure using a wireless haemodynamic monitoring system may be considered in symptomatic patients with HF in order to improve clinical outcomes.373 | IIb | B |
- CV = cardiovascular; HF = heart failure; HTM = home telemonitoring; LVEF = left ventricular ejection fraction.
- a Class of recommendation.
- b Level of evidence.
Thus, non-invasive HTM may be considered for patients with HF in order to reduce the risk of recurrent CV and HF hospitalizations and CV death; further evidence on management guided by implanted systems is awaited.375
10 Advanced heart failure
10.1 Epidemiology, diagnosis, and prognosis
Many patients with HF progress into a phase of advanced HF, characterized by persistent symptoms despite maximal therapy.376-378 The prevalence of advanced HF is increasing due to the growing number of patients with HF, ageing of the population, and better treatment and survival of HF. Prognosis remains poor, with a 1-year mortality ranging from 25% to 75%.379–381
The updated HFA-ESC 2018 criteria for the definition of advanced HF are reported in Table 13.377 A severely reduced LVEF is common but not required for a diagnosis of advanced HF as it may develop in patients with HFpEF as well. In addition to the reported criteria, extra-cardiac organ dysfunction due to HF (e.g. cardiac cachexia, liver or kidney dysfunction) or type II pulmonary hypertension may be present, but are not required for the definition of advanced HF.377
| All the following criteria must be present despite optimal medical treatment: |
| 1. Severe and persistent symptoms of heart failure [NYHA class III (advanced) or IV]. |
2. Severe cardiac dysfunction defined by at least one of the following:
|
| 3. Episodes of pulmonary or systemic congestion requiring high-dose i.v. diuretics (or diuretic combinations) or episodes of low output requiring inotropes or vasoactive drugs or malignant arrhythmias causing >1 unplanned visit or hospitalization in the last 12 months. |
| 4. Severe impairment of exercise capacity with inability to exercise or low 6MWT distance (<300 m) or pVO2 <12 mL/kg/min or <50% predicted value, estimated to be of cardiac origin. |
- 6MWT = 6-minute walk test; ARVC = arrhythmogenic right ventricular cardiomyopathy; BNP = B-type natriuretic peptide; HFpEF = heart failure with preserved ejection fraction; i.v. = intravenous; LV = left ventricular; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; pVO2 = peak oxygen consumption; RV = right ventricular. Modified from 377.
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profiles, developed to classify patients with a potential indication for durable MCS devices, describes clinical parameters and characteristics consistent with a need for advanced therapies (Table 14).382 This classification has also been shown to be useful in estimating the prognosis of patients undergoing urgent heart transplantation383 or LV assist device (LVAD) implantation,384 and for risk assessment in ambulatory advanced HF patients.385
| Profile | Time frame for intervention |
|---|---|
| Profile 1. Critical cardiogenic shock Patient with life-threatening hypotension despite rapidly escalating inotropic support, critical organ hypoperfusion, often confirmed by worsening acidosis and/or lactate levels. “Crash and burn.” | Definitive intervention needed within hours. |
| Profile 2. Progressive decline Patient with declining function despite i.v. inotropic support, may be manifest by worsening renal function, nutritional depletion, inability to restore volume balance. “Sliding on inotropes.” Also describes declining status in patients unable to tolerate inotropic therapy. | Definitive intervention needed within few days. |
| Profile 3. Stable on inotrope or inotrope-dependent Patient with stable blood pressure, organ function, nutrition, and symptoms on continuous i.v. inotropic support (or a temporary circulatory support device or both) but demonstrating repeated failure to wean from support due to recurrent symptomatic hypotension or renal dysfunction. “Dependent stability.” | Definitive intervention elective over a period of weeks to few months. |
| Profile 4. Frequent Flyer Patient can be stabilized close to normal volume status but experiences daily symptoms of congestion at rest or during activities of daily living. Doses of diuretics generally fluctuate at very high levels. More intensive management and surveillance strategies should be considered, which may in some cases reveal poor compliance that would compromise outcomes with any therapy. Some patients may shuttle between 4 and 5. | Definitive intervention elective over a period of weeks to few months. |
| Profile 5. Housebound Comfortable at rest and with activities of daily living but unable to engage in any other activity, living predominantly within the house. Patients are comfortable at rest without congestive symptoms, but may have underlying refractory elevated volume status, often with renal dysfunction. If underlying nutritional status and organ function are marginal, patients may be more at risk than INTERMACS 4, and require definitive intervention. | Variable urgency, depends upon maintenance of nutrition, organ function, and activity. |
| Profile 6. Exertion limited Patient without evidence of fluid overload, comfortable at rest and with activities of daily living and minor activities outside the home but fatigues after the first few minutes of any meaningful activity. Attribution to cardiac limitation requires careful measurement of peak oxygen consumption, in some cases with haemodynamic monitoring, to confirm severity of cardiac impairment. “Walking wounded.” | Variable, depends upon maintenance of nutrition, organ function, and activity level. |
| Profile 7. Advanced NYHA class III symptoms Patient without current or recent episodes of unstable fluid balance, living comfortably with meaningful activity limited to mild physical exertion. | Heart transplantation or MCS may not be currently indicated. |
| Modifiers for profiles | Possible profiles that can be modified |
| Temporary MCS can modify profile only in hospitalized patients. They include IABP, ECMO, TandemHeart, LVAD, Impella. | 1, 2, 3 |
| Arrhythmia can modify any profile. They include recurrent ventricular tachyarrhythmias that have recently contributed substantially to clinical compromise, frequent ICD shocks or requirement for external defibrillation, usually more than twice weekly. | 1–7 |
| Frequent episodes of HF decompensation characterize patients requiring frequent emergency visits or hospitalizations for diuretics, ultrafiltration, or temporary i.v. vasoactive therapy. Frequent episodes may be considered as at least two emergency visits/admissions in the past 3 months or three in the past 6 months. | 3 if at home, 4, 5, 6. Rarely for profile 7. |
- ECMO = extracorporeal membrane oxygenation; HF = heart failure; IABP = intra-aortic balloon pump; ICD = implantable cardioverter-defibrillator; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; i.v. = intravenous; LVAD = left ventricular assist device; MCS = mechanical circulatory support; NYHA = New York Heart Association. Modified from 382.
Prognostic stratification is important to identify the ideal time for referral to an appropriate centre (i.e. one capable of providing advanced HF therapies), to properly convey expectations to patients and families, and to plan treatment and follow-up strategies (Figure 4).377 Patients with contraindications to MCS or heart transplantation should be considered for palliative care (see section 10.2.4).
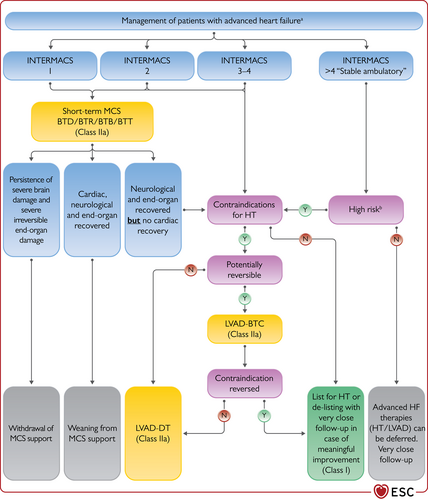
Algorithm for the treatment of patients with advanced heart failure. BTB = bridge to bridge; BTC = bridge to candidacy; BTD = bridge to decision; BTR = bridge to recovery; BTT = bridge to transplantation; CA = cardiac amyloidosis; DT = destination therapy; ESC = European Society of Cardiology; HCM = hypertrophic cardiomyopathy; HF = heart failure; HFA = Heart Failure Association; HT = heart transplantation; INTERMACS = Interagency Registry for Mechanically Assisted Circulatory Support; LVAD = left ventricular assist device; LVAD-BTC = left ventricular assist device – bridge to candidacy; LVAD-DT = left ventricular assist device – destination therapy; MCS = mechanical circulatory support. aThis algorithm can be applied to all patients with advanced HF defined according to the ESC/HFA criteria,377 with exception of HCM, CA, arrhythmic storm, adult congenital heart disease, refractory angina. bRecurrent hospitalization, progressive end-organ failure, refractory congestion, inability to perform cardiopulmonary exercise test or peak oxygen consumption <12 mL/min/kg or <50% of expected value.386 Colour code for classes of recommendation: Green for Class of recommendation I and Yellow for Class of recommendation IIa (see Table 1 for further details on classes of recommendation).
Despite many prognostic parameters (Supplementary Table 13), predicting outcomes remains difficult and patients are often referred to advanced HF centres too late. Identifying warning signs in patients with non-advanced symptoms may allow early referral so that MCS and heart transplantation may be offered before the development of end-organ failure (Figure 5; Supplementary Table 14).377,387 An organizational model between centres with different levels of care complexity, based on a ‘Hub and Spoke’ network is the key to good patient management.377
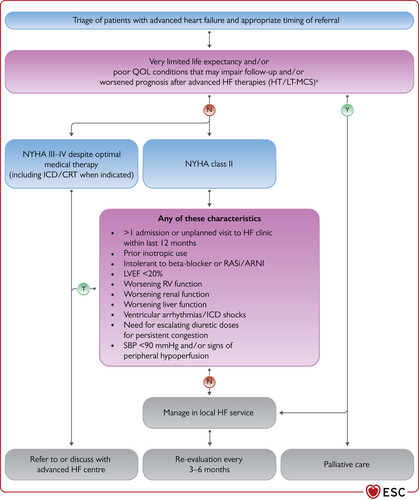
Triage of patients with advanced heart failure and appropriate timing of referral.377 ARNI = angiotensin receptor-neprilysin inhibitor; CRT = cardiac resynchronization therapy; HF = heart failure; HT = heart transplantation; ICD = implantable cardioverter-defibrillator; LT-MCS = long-term mechanical circulatory support; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RASi = renin-angiotensin system inhibitor; RV = right ventricular; SBP = systolic blood pressure; QOL = quality of life. aLimited life expectancy may be due by major comorbidities such as cancer, dementia, end-stage organ dysfunction; other conditions that may impair follow-up or worsen post-treatment prognosis include frailty, irreversible cognitive dysfunction, psychiatric disorder, or psychosocial issues.
10.2 Management
In patients with advanced HF, pharmacological therapy and short-term MCS may be needed until the implantation of long-term MCS or heart transplantation becomes available.
10.2.1 Pharmacological therapy and renal replacement
Inotropes may improve haemodynamic parameters, reducing congestion, augmenting cardiac output, and aiding peripheral perfusion. Although not proven, this may help to prevent worsening end-organ function. Conversely, traditional inotropes may favor myocardial ischaemia and/or tachyarrhythmias and worsen the clinical course.388,389 They can be used as palliative therapy for the relief of symptoms in patients without other treatment options. Intermittent long-term use of inotropes may be considered in outpatients to improve functional class and QOL.390,391
Kidney dysfunction and loop diuretic resistance often characterize the clinical course of patients with advanced HF. Doubling of the loop diuretic dose is proposed, in the first instance, followed by concomitant administration of thiazides or metolazone (see section 11.3.3).145 In patients who fail to respond to diuretic-based strategies, renal replacement therapies should be considered. Ultrafiltration is one of the most common approaches. It may be considered in those with diuretic resistance even if data about its effects on outcomes are unsettled.392,393
10.2.2 Mechanical circulatory support
MCS can improve survival and symptoms of patients with advanced HF.377,394 The use of MCS should be considered for the different scenarios listed in Table 15. Indications for short- and long-term MCS should be based on the INTERMACS profiles (Table 14, Figure 4).
| Bridge to decision (BTD)/ Bridge to bridge (BTB) | Use of short-term MCS (ECMO or Impella) in patients with cardiogenic shock until haemodynamics and end-organ perfusion are stabilized, contraindications for long-term MCS are excluded (brain damage after resuscitation) and additional therapeutic options including long-term VAD therapy or heart transplant can be evaluated. |
| Bridge to candidacy (BTC) | Use of MCS (usually LVAD) to improve end-organ function and/or to make an ineligible patient eligible for heart transplantation. |
| Bridge to transplantation (BTT) | Use of MCS (LVAD, BiVAD or TAH) to keep a patient alive who is otherwise at high risk of death before transplantation until a donor organ becomes available. |
| Bridge to recovery (BTR) | Use of MCS (short-term or long-term) to keep a patient alive until cardiac function recovers sufficiently to remove MCS. |
| Destination therapy (DT) | Long-term use of MCS (LVAD) as an alternative to transplantation in patients with end-stage HF ineligible for transplantation. |
- BiVAD = biventricular assist device; ECMO = extracorporeal membrane oxygenation; HF = heart failure; LVAD = left ventricular assist device; MCS = mechanical circulatory support; TAH = total artificial heart; VAD = ventricular assist device.
Short-term mechanical circulatory support
Short-term MCS devices are indicated to reverse critical end-organ hypoperfusion and hypoxia in the setting of cardiogenic shock. They can be used for a short, limited, period of time, from a few days up to several weeks. The aim is to support the central nervous system and organ perfusion, to reverse acidosis and multi-organ failure until the patient’s outcome becomes clearer be that of cardiac recovery, transition to durable MCS or heart transplantation, or, in some cases, towards a more palliative approach. The care of patients on short-term MCS is complex and requires dedicated expertise including having specific plans for stopping support when neither cardiac nor brain injury recovers. Short-term MCS should be used in patients with INTERMACS profiles 1 or 2 as a bridge to decision (BTD), bridge to recovery (BTR), bridge to bridge (BTB) for either long-term MCS or urgent heart transplantation (Figure 4).395 Further details about short-term MCS are reported in the Supplementary text 11.4.
Long-term mechanical circulatory support
Long-term MCS is indicated in selected patients when MT is insufficient or when short-term MCS has not led to cardiac recovery or clinical improvement, to prolong life and improve QOL, or to keep the patient alive until transplantation (bridge to transplantation, BTT) or to reverse contraindications to heart transplantation (bridge to candidacy, BTC), or as destination therapy (DT) (Table 15).
Long-term MCS should be considered in patients with INTERMACS profiles 2 to 4 and also in patients with INTERMACS profile 5–6, when they have high-risk characteristics. Patients with no irreversible end-organ failure other than cardiac, recovering from INTERMACS level 1 while on short-term MCS, may also qualify for long-term MCS (Figure 4).377,379,384,396-403 The characteristics of patients potentially eligible for implantation of an LVAD are reported in Table 16.
| Patients with persistence of severe symptoms despite optimal medical and device therapy, without severe right ventricular dysfunction and/or severe TR, with a stable psychosocial background and absence of major contraindications*, and who have at least one of the following: |
| • LVEF <25% and unable to exercise for HF or, if able to perform cardiopulmonary exercise testing, with peak VO2 <12 mL/kg/min and/or <50% predicted value. |
| • ≥3 HF hospitalizations in previous 12 months without an obvious precipitating cause. |
| • Dependence on i.v. inotropic therapy or temporary MCS. |
| • Progressive end-organ dysfunction (worsening renal and/or hepatic function, type II pulmonary hypertension, cardiac cachexia) due to reduced perfusion and not to inadequately low ventricular filling pressure (PCWP ≥20 mmHg and SBP ≤90 mmHg or cardiac index ≤2 L/min/m2). |
- HF = heart failure; i.v. = intravenous; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; PCWP = pulmonary capillary wedge pressure; SBP = systolic blood pressure; TR = tricuspid regurgitation; VO2 = oxygen consumption.
- * Stable psychosocial background includes demonstrated understanding of the technology and patient living in the same household with a caregiver that will help the patient (i.e. living alone and poor psychosocial background is LVAD contraindication). Major contraindications include contraindication to long-term oral anticoagulation, infection, severe renal dysfunction, ventricular arrhythmias.
The details of the devices and studies on long-term MCS are summarized in Supplementary Table 15.
Current 2-year survival rates in patients receiving the latest continuous-flow LVADs are comparable to those after heart transplantation, although adverse events negatively affect QOL. Among patients with continuous flow LVADs, actuarial survival was reported of 80% at 1 year and 70% at 2 years.404,405 Two-year survival was 84.5% and survival free of disabling stroke or need of reoperation for LVAD malfunction was 76.9% with a centrifugal-flow LVAD in MOMENTUM 3.406 The fully magnetically levitated centrifugal-flow LVAD has significantly reduced pump thrombosis. In MOMENTUM 3, the need for reoperation to replace a malfunctioning device was 2.3% per 24 months, with only 0.6% per 24 months risk of pump replacement because of pump thrombosis. Stroke (namely, disabling stroke), major bleeding, and gastrointestinal haemorrhage were also lower in the centrifugal-flow pump group than in the axial-flow pump group. However, the incidence of all bleeding events, thromboembolism and driveline infection remained similar to that with older devices.403
Data on fully magnetically centrifugal-flow LVAD use in real-world studies with the 2-year outcomes from the ELEVATE registry showed an overall survival of 74.5%, with gastrointestinal bleeding in 9.7%, stroke in 10.2%, and pump thrombosis in 1.5% of patients.407 According to the IMACS Registry, a new composite endpoint including QOL and adverse events beyond survival was proposed to help in guiding decision making. In this sense ‘living well at one year’ defined as freedom from death, stroke, bleeding requiring operation, RV assist device, pump replacement, or device-related infection within the first year, was 56.8% after isolated, centrifugal flow-LVAD.384
Although now outdated, REMATCH was the only RCT comparing an LVAD as DT with OMT in patients with advanced HF, NYHA class IV and a contraindication to transplantation. REMATCH showed lower all-cause mortality with LVAD therapy when compared with medical treatment (primary endpoint). However, there were high mortality rates at 2 years in both arms.379 Other studies were not randomized (INTrEPID, ROADMAP)397,408,409 or compared different devices (ADVANCE, ENDURANCE, MOMENTUM 3).400,403,410 The two strategies of early LVAD implantation vs. medical treatment with LVAD implantation only after serious deterioration of the patient's condition are currently being compared in a prospective trial, Early-VAD (ClinicalTrials.gov Identifier: NCT02387112). Also, the Swedish evaluation of LVAD (SweVAD) study is comparing the survival of patients with advanced HF ineligible for heart transplantation prospectively randomized to LVAD as DT vs. MT (ClinicalTrials.gov Identifier: NCT02592499).411
10.2.3 Heart transplantation
Heart transplantation remains the gold standard for the treatment of advanced HF in the absence of contraindications. Post-transplant 1-year survival is around 90% with a median survival of 12.5 years.386,412,413 Transplantation significantly improves QOL and functional status, although, for unclear reasons, the percentage of patients returning to work is lower than expected.413 Apart from primary graft dysfunction, the main challenges after heart transplantation relate to either the efficacy or side effects of immunosuppression (e.g. rejection, infection, cardiac allograft vasculopathy, late graft dysfunction, malignancy, renal failure, hypertension, diabetes mellitus).
Organ donor shortage remains the main limitation to heart transplantation. Thus, the donor heart criteria have now been extended to allow an increased upper limit of the donor age, particularly in Europe. Moreover, careful recipient selection is needed, based on pre-transplant and post-transplant life expectancy (both are influenced by pre-operative status and comorbidities).
The main indications and contraindications for heart transplantation are listed in Table 17.377,386
| Indications |
| Advanced HF377 |
| No other therapeutic option, except for LVAD as BTT |
| Contraindications |
| Active infectiona |
| Severe peripheral arterial or cerebrovascular disease |
| Pharmacologic irreversible pulmonary hypertension (LVAD should be considered to reverse elevated pulmonary vascular resistance with subsequent re-evaluation to establish candidacy) |
| Malignancy with poor prognosis (a collaboration with oncology specialists should occur to stratify each patient as regards their risk of tumour progression or recurrence which increases with the use of immunosuppression) |
| Irreversible liver dysfunction (cirrhosis) or irreversible renal dysfunction (e.g. creatinine clearance <30 mL/min/1.73 m²). Combined heart-liver or heart-kidney transplant may be considered |
| Systemic disease with multiorgan involvement |
| Other serious comorbidity with poor prognosis |
| Pre-transplant BMI >35 kg/m² (weight loss is recommended to achieve a BMI <35 kg/m²) |
| Current alcohol or drug abuse |
| Psychological instability that jeopardizes proper follow-up and intensive therapeutic regime after heart transplantation |
| Insufficient social supports to achieve compliant care in the outpatient setting |
- BMI = body mass index; BTT = bridge to transplantation; HF = heart failure; LVAD = left ventricular assist device.
- a Active infection is a relative contraindication to transplant although in some cases of infected LVADs it may actually be an indication.
- Adapted from Crespo-Leiro et al.377
Active infection is a relative contraindication to transplant but in some cases of infected LVADs it may actually be an indication. Elderly age is not an absolute contraindication. Although patients aged <65 years might be more appropriate candidates due to their overall life expectancy, most programmes accept patients up to 70 years of age, and biological age as well as chronological age must be taken into account. Surgical complexity [previous sternotomies, mediastinal radiation, adult congenital heart disease (ACHD)] should also be considered.
The decision pathway to transplantation or LVAD is never straightforward and is unique to each patient. Eligibility for each option may change according to the particular conditions of each patient, which may also change over time. Other factors, not related to the patient, such as time on the heart transplant waiting list, the centre’s surgical experience, and resources, can also influence decision making.414
Recommendations for the treatment of patients with advanced heart failure
| Recommendations | Classa | Levelb |
| Patients being considered for long-term MCS must have good compliance, appropriate capacity for device handling and psychosocial support.415-417 | I | C |
| Heart transplantation is recommended for patients with advanced HF, refractory to medical/device therapy and who do not have absolute contraindications. | I | C |
| Long-term MCS should be considered in patients with advanced HFrEF despite optimal medical and device therapy, not eligible for heart transplantation or other surgical options, and without severe right ventricular dysfunction, to reduce the risk of death and improve symptoms.379,397,398,402,403,405,418 | IIa | A |
| Long-term MCS should be considered in patients with advanced HFrEF refractory to optimal medical and device therapy as a bridge to cardiac transplantation in order to improve symptoms, reduce the risk of HF hospitalization and the risk of premature death.399-401,403,405 | IIa | B |
| Renal replacement therapy should be considered in patients with refractory volume overload and end-stage kidney failure. | IIa | C |
| Continuous inotropes and/or vasopressors may be considered in patients with low cardiac output and evidence of organ hypoperfusion as bridge to MCS or heart transplantation.390,391 | IIb | C |
| Ultrafiltration may be considered in refractory volume overload unresponsive to diuretic treatment.392,393 | IIb | C |
- HF = heart failure; HFrEF = heart failure with reduced ejection fraction; MCS = mechanical circulatory support.
- a Class of recommendation.
- b Level of evidence.
10.2.4 Symptom control and end-of-life care
While the disease trajectory of each patient with HF is unique, there is a generalizable pattern of gradual decline, punctuated by episodes of acute deterioration leading either to sudden death or death due to progressive HF. Communication about the disease trajectory and anticipatory planning should start when a patient is diagnosed with advanced HF. Indications for and key components of a palliative care service are reported in Tables 18 and 19.313,419
| Progressive functional decline (physical and mental) and dependence in most activities of daily living. |
| Severe heart failure symptoms with poor QOL despite optimal pharmacological and non-pharmacological therapies. |
| Frequent admissions to hospital or other serious episodes of decompensation despite optimal treatment. |
| Heart transplantation and MCS ruled out. |
| Cardiac cachexia. |
| Clinically judged to be close to end of life. |
- MCS = mechanical circulatory support; QOL = quality of life.
| Focus on improving or maintaining the QOL of a patient and his/her family as well as possible until he/she dies. |
| Frequent assessment of symptoms (including dyspnoea and pain) resulting from advanced heart failure and other comorbidities and focus on symptom relief. |
| Access for the patient and his/her family to psychological support and spiritual care according to need. |
| Advanced care planning, taking into account preferences for place of death and resuscitation (which may include deactivating devices, such as ICD or long-term MCS that may require a multidisciplinary team decision). |
- ICD = implantable cardioverter-defibrillator; MCS = mechanical circulatory support; QOL = quality of life.
A team-based approach to palliative and end-of-life care for patients with HF has been proposed.420 Specific models of palliative care for patients with advanced HF have been also reported. They reduce hospitalizations, without a clear effect on survival, and have some effects on QOL and symptom burden.421,422
Symptom assessment should be performed on a regular basis. In addition to clinical assessment, symptoms can be assessed using the Numeric Rating Scale, the Edmonton Symptom Assessment Scale (ESAS) or ESAS-HF, or the Integrated Palliative care Outcome Scale.
-
Breathlessness: repeat doses of opioids may be considered for the relief of dyspnoea; however, their effectiveness is not demonstrated.423,424 While using opioids, all patients should be guided about opioid side effects such as constipation and nausea, urinary retention, and mental status changes. Benzodiazepines may be considered as a second- or third-line treatment, when opioids and non-pharmacological measures have failed to control breathlessness. Increasing the inspired oxygen concentration may provide relief of dyspnoea.
-
Pain: non-pharmacologic management can be helpful. In addition, opioid, oxycodone, hydromorphone, and fentanyl are generally viewed as safe options and can be provided orally, intravenously, and transdermally, especially in the hospital or in patient palliative care or hospice setting.425
-
Anxiety and depression: adequate conventional treatment should be offered.
Proactive decisions and advanced planning with regard to palliative and end-of-life care discussions should be documented, regularly reviewed, and routinely communicated to all those involved in the patient’s care. Healthcare providers should make sure that patients’ and carer preferences are followed, wherever possible. They should also take into account that patients may choose not to, or may not be in a position to, express preferences (e.g. due to symptoms of depression or cognitive impairment).
11 Acute heart failure
11.1 Epidemiology, diagnosis and prognosis
AHF refers to rapid or gradual onset of symptoms and/or signs of HF, severe enough for the patient to seek urgent medical attention, leading to an unplanned hospital admission or an emergency department visit. Patients with AHF require urgent evaluation with subsequent initiation or intensification of treatment, including i.v. therapies or procedures. AHF is a leading cause of hospitalizations in subjects aged >65 years and is associated with high mortality and rehospitalization rates. In-hospital mortality ranges from 4% to 10%.426-429 Post-discharge 1-year mortality can be 25–30% with up to more than 45% deaths or readmission rates.104,427,428,430,431
AHF may be the first manifestation of HF (new onset) or, more frequently, be due to an acute decompensation of chronic HF. Compared to patients with acutely decompensated CHF, those with new onset HF may have a higher in-hospital mortality426 but have lower post-discharge mortality and rehospitalization rates.426,429,432,433 Specific extrinsic factors may precipitate, but not cause, AHF in patients with pre-existing cardiac dysfunction (Supplementary Table 16). Clinical severity and in-hospital trajectory are determined by the complex interplay between precipitants, the underlying cardiac substrate, and the patient’s comorbidities.
The diagnostic workup of AHF starts at the time of the first medical contact, and is continued throughout the initial patient pathway, aiming to identify the clinical presentation and to diagnose and manage any potentially reversible causes/precipitants/coexisting life-threatening conditions in a timely manner (Figure 6). Diagnostic tests are outlined at Table 20. In addition to clinical signs and symptoms, diagnostic workup includes ECG and echocardiography, if possible. Additional investigations, i.e. chest X-ray and lung ultrasound may be used to confirm AHF diagnosis, especially when NP testing is not available. Plasma NP levels (BNP or NT-proBNP or MR-proANP) should be measured if the diagnosis is uncertain and a point-of-care assay is available. Normal concentrations of NPs make the diagnosis of AHF unlikely. Cut-offs for acute HF are: BNP <100 pg/mL, NT-proBNP <300 pg/mL and MR-proANP <120 pg/mL.74,434-436 However, elevated NP values are associated with a wide range of cardiac and non-cardiac conditions (Table 6). Low concentrations can be detected in some patients with advanced decompensated end-stage HF, obesity, flash pulmonary oedema or right-sided AHF. Higher levels can be found in the patients with concomitant AF and/or reduced renal function.74
| Exam | Time of measurement | Possible findings | Diagnostic value for AHF | Indication |
|---|---|---|---|---|
| ECG | Admission, during hospitalization,a,b pre-discharge | Arrhythmias, myocardial ischaemia | Exclusion of ACS or arrhythmias | Recommended |
| Chest-X ray | Admission, during hospitalizationa | Congestion, lung infection | Confirmatory | May be considered |
| LUS | Admission, during hospitalization,a pre-discharge | Congestion | Confirmatory | May be considered |
| Echocardiography | Admission, during hospitalization,a pre-discharge | Congestion, cardiac dysfunction, mechanical causes | Major | Recommended |
| Natriuretic peptides (BNP, NT-proBNP, MR-proANP) | Admission, pre-discharge | Congestion | High negative predictive value | Recommended |
| Serum troponin | Admission | Myocardial injury | Exclusion of ACS | Recommended |
| Serum creatinine | Admission, during hospitalization,a pre-discharge | Renal dysfunction | None | Recommended for prognostic assessment |
| Serum electrolytes (sodium, potassium, chloride) | Admission, during hospitalization,a pre-discharge | Electrolyte disorders | None | Recommended for prognostic assessment and treatment |
| Iron status (transferrin, ferritin) | Pre-discharge | Iron depletion | None | Recommended for prognostic assessment and treatment |
| TSH | Admission | Hypo- hyperthyroidism | None | Recommended when hypo-hyperthyroidism is suspected |
| D-dimer | Admission | Pulmonary embolism | Useful to exclude pulmonary embolism | Recommended when pulmonary embolism is suspected |
| Procalcitonin | Admission | Pneumonia | Useful for diagnosis of pneumonia | May be done when pneumonia is suspected |
| Lactate | Admission, during hospitalizationa | Lactic acidosis | Useful to assess perfusion status | Recommended when peripheral hypoperfusion is suspected |
| Pulse oximetry and arterial blood gas analysis | Admission, during hospitalizationa | Respiratory failure | Useful to assess respiratory function | Recommended when respiratory failure is suspected |
- ACS = acute coronary syndrome; AHF = acute heart failure; BNP = B-type natriuretic peptide; ECG = electrocardiogram; LUS = lung ultrasound; MR-proANP = mid-regional pro-atrial natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide; TSH = thyroid-stimulating hormone.
- a Based on clinical conditions.
- b Continuous ECG monitoring can be considered based on clinical conditions.
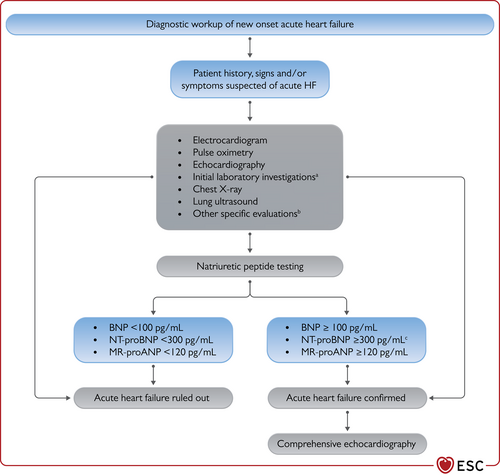
Diagnostic workup of new onset acute heart failure. ACS = acute coronary syndrome; BNP = B-type natriuretic peptide; CT = computed tomography; HF = heart failure; MR-proANP = mid-regional pro-atrial natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide; TSH = thyroid-stimulating hormone. aInitial laboratory exams include troponin, serum creatinine, electrolytes, blood urea nitrogen or urea, TSH, liver function tests as well as D-dimer and procalcitonin when pulmonary embolism or infection are suspected, arterial blood gas analysis in case of respiratory distress, and lactate in case of hypoperfusion. bSpecific evaluation includes coronary angiography, in case of suspected ACS, and CT in case of suspected pulmonary embolism. cRule-in values for the diagnosis of acute HF: >450 pg/mL if aged <55 years, >900 pg/mL if aged between 55 and 75 years and >1800 pg/mL if aged >75 years.434,435
Among other laboratory tests, troponin is useful for the detection of acute coronary syndrome (ACS) although elevated levels are detected in the vast majority of patients with AHF.437-439 Blood urea nitrogen or urea, serum creatinine, electrolytes (sodium, potassium, chloride), and antigen carbohydrate 125 may help tailor treatment.440,441 Detection of abnormal liver function identifies patients with a poor prognosis.442 Since both hypothyroidism and hyperthyroidism may precipitate AHF, thyroid-stimulating hormone (TSH) should be assessed in those with newly diagnosed AHF. Arterial blood gas analysis should be performed when a precise measurement of O2 and CO2 partial pressure is needed (i.e. patients with respiratory distress). Lactate and pH levels should be measured in patients with cardiogenic shock. D-dimer should be measured when acute pulmonary embolism is suspected. Procalcitonin may be used for the diagnosis of pneumonia and antibiotic therapy may have an indication when plasma levels are >0.2 μg/L. However, no impact of a strategy based on routine procalcitonin measurements on outcomes was shown in a prospective, controlled, trial.443 Pulse oximetry should be measured routinely at the time of first presentation of patients with AHF and continuous monitoring may be needed in the first hours or days.444,445
11.2 Clinical presentations
Four major clinical presentations can be described with possible overlaps between them (Table 21).1,426,446 Clinical presentations are mainly based on the presence of signs of congestion and/or peripheral hypoperfusion and require different treatments (Table 21).1,426-428,433,447,448
| Acute decompensated heart failure | Acute pulmonary oedema | Isolated right ventricular failure | Cardiogenic shock | |
|---|---|---|---|---|
| Main mechanisms |
|
|
RV dysfunction and/or pre-capillary pulmonary hypertension | Severe cardiac dysfunction |
| Main cause of symptoms | Fluid accumulation, increased intraventricular pressure | Fluid redistribution to the lungs and acute respiratory failure | Increased central venous pressure and often systemic hypoperfusion | Systemic hypoperfusion |
| Onset | Gradual (days) | Rapid (hours) | Gradual or rapid | Gradual or rapid |
| Main haemodynamic abnormalities |
|
|
|
|
| Main clinical presentations1,447 | Wet and warm OR Dry and cold | Wet and warmb | Dry and cold OR Wet and cold | Wet and cold |
| Main treatment |
|
|
|
|
- LV = left ventricular; LVEDP = left ventricular end-diastolic pressure; MCS = mechanical circulatory support; PCWP = pulmonary capillary wedge pressure; RV = right ventricular; RVEDP = right ventricular end-diastolic pressure; RRT = renal replacement therapy; SBP = systolic blood pressure.
- a May be normal with low cardiac output.
- b Wet and cold profile with need of inotropes and/or vasopressors may rarely occur.
11.2.1 Acute decompensated heart failure
Acute decompensated heart failure (ADHF) is the most common form of AHF, accounting for 50–70% of presentations.427,428,433 It usually occurs in patients with history of HF and previous cardiac dysfunction across the spectrum of LVEF and may include RV dysfunction. Distinct from the acute pulmonary oedema phenotype, it has a more gradual onset, and the main alteration is progressive fluid retention responsible for systemic congestion. Sometimes, congestion is associated with hypoperfusion.427 The objectives of treatment are identification of precipitants, decongestion, and in rare instances, correction of hypoperfusion (Figure 7).
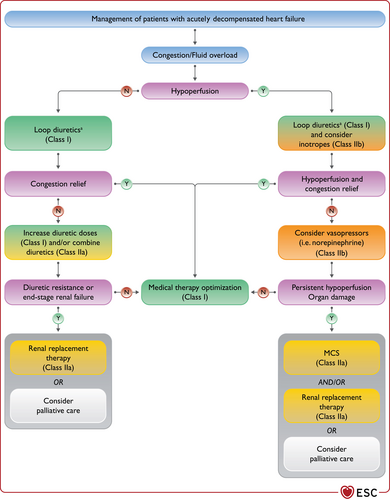
Management of acute decompensated heart failure. MCS = mechanical circulatory support. aAdequate diuretic doses to relieve congestion and close monitoring of diuresis is recommended (see Figure 13) regardless of perfusion status.
11.2.2 Acute pulmonary oedema
Acute pulmonary oedema is related to lung congestion. Clinical criteria for acute pulmonary oedema diagnosis include dyspnoea with orthopnoea, respiratory failure (hypoxaemia-hypercapnia), tachypnoea, >25 breaths/min, and increased work of breathing.449
Three therapies should be commenced, if indicated. First, oxygen, given as continuous positive airway pressure, non-invasive positive-pressure-ventilation and/or high-flow nasal cannula, should be started. Second, i.v. diuretics should be administered, and third, i.v. vasodilators may be given if systolic BP (SBP) is high, to reduce LV afterload (Figure 8). In a few cases of advanced HF, acute pulmonary oedema may be associated with low cardiac output and, in this case, inotropes, vasopressors, and/or MCS are indicated to restore organ perfusion.

Management of pulmonary oedema. MCS = mechanical circulatory support; RRT = renal replacement therapy; SBP = systolic blood pressure.
11.2.3 Isolated right ventricular failure
RV failure is associated with increased RV and atrial pressure and systemic congestion. RV failure may also impair LV filling, and ultimately reduce systemic cardiac output, through ventricular interdependence.450
Diuretics are often the first option of therapy for venous congestion. Noradrenaline and/or inotropes are indicated for low cardiac output and haemodynamic instability. Inotropes reducing cardiac filling pressures may be preferred (i.e. levosimendan, phosphodiesterase type III inhibitors). Since inotropic agents may aggravate arterial hypotension, they may be combined with norepinephrine if needed (Figure 9).450
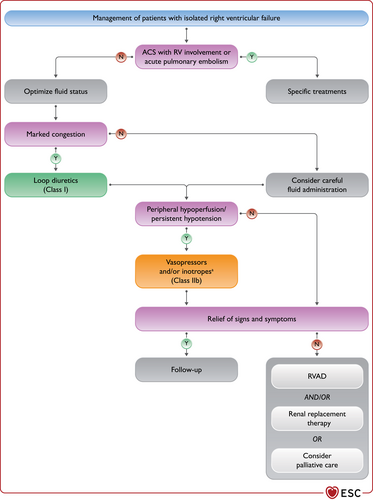
Management of right ventricular failure. ACS = acute coronary syndrome; RV = right ventricular; RVAD = right ventricular assist device. aInotropes alone in case of hypoperfusion without hypotension.
11.2.4 Cardiogenic shock
Cardiogenic shock is a syndrome due to primary cardiac dysfunction resulting in an inadequate cardiac output, comprising a life-threatening state of tissue hypoperfusion, which can result in multi-organ failure and death.451-453 Cardiac insult causing severe impairment of cardiac performance may be acute, as a result of the acute loss of myocardial tissue (acute MI, myocarditis) or may be progressive as seen in patients with chronic decompensated HF who may experience a decline in disease stability as a result of the natural progression of advanced HF and/or specific precipitants.427
Diagnosis of cardiogenic shock mandates the presence of clinical signs of hypoperfusion, such as cold sweated extremities, oliguria, mental confusion, dizziness, narrow pulse pressure. In addition, biochemical manifestations of hypoperfusion, elevated serum creatinine, metabolic acidosis and elevated serum lactate are present and reflect tissue hypoxia and alterations of cellular metabolism leading to organ dysfunction.438,454 Of note, hypoperfusion is not always accompanied by hypotension, as BP may be preserved by compensatory vasoconstriction (with/without pressor agents), albeit at the cost of impaired tissue perfusion and oxygenation.427,428,451,455
Management of cardiogenic shock should start as early as possible. Early identification and treatment of the underlying cause, concomitant with haemodynamic stabilization and management of organ dysfunction, are key components of its management (Figure 10, Supplementary text 11.1; Supplementary Figure 2).
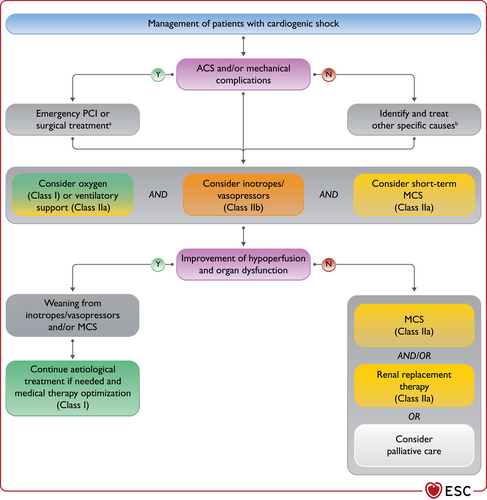
Management of cardiogenic shock. ACS = acute coronary syndrome; BTT = bridge to transplantation; MCS = mechanical circulatory support; PCI = percutaneous coronary intervention. aPCI in ACS, pericardiocentesis in tamponade, mitral valve surgery in papillary muscle rupture. In case of interventricular septum rupture, MCS as BTT should be considered. bOther causes include acute valve regurgitation, pulmonary embolism, infection, acute myocarditis, arrhythmia (see Figure 12).
11.3 Management
11.3.1 General aspects
Management can be subdivided in three stages (pre-hospital, in-hospital, and pre-discharge), having different goals and requiring different approaches (Figure 11).
Pre-hospital phase
In the pre-hospital setting, AHF patients should benefit from non-invasive monitoring, including pulse oximetry, BP, heart rate respiratory rate, and a continuous ECG, instituted within minutes of patient contact and in the ambulance if possible.306 Oxygen therapy may be given based on clinical judgment unless oxygen saturation is <90% in which case it should be administered. In patients with respiratory distress, respiratory rate >25 breaths/min, oxygen saturation <90%, non-invasive ventilation should be initiated.445,449 Although therapeutic tools may be available in the prehospital setting, whether more effective pre-hospital care would alter the clinical outcome remains to be proven in randomized clinical trials.456 Furthermore, pre-hospital management should not delay the rapid transfer of AHF patients to the most appropriate medical setting.456,457
In-hospital management
Diagnostic workup and appropriate pharmacological and non-pharmacological treatment must be started promptly and in parallel (Figure 12). AHF patients are triaged to the appropriate level of care according to the degree of haemodynamic instability and severity of the critical illness. Disposition decisions are important components of the initial phase of management (see Supplementary text 11.2 and Supplementary Tables 17–19).
The type and intensity of in-hospital monitoring depends on clinical severity, settings of care and in-hospital course (see Supplementary text 11.3). As AHF is a heterogeneous condition, management may differ according to the main clinical presentation. Management starts with the search for specific causes of AHF.1,306,431 These include ACS, a hypertensive emergency, rapid arrhythmias or severe bradycardia/conduction disturbance, acute mechanical causes such as acute valve regurgitation or acute pulmonary embolism, infection, including myocarditis, and tamponade (CHAMPIT) (Figure 12). After exclusion of these conditions, which need to be treated/corrected urgently, management of AHF differs according to the clinical presentations (Figures 7–10).
Pre-discharge phase
Details on this phase are shown in section 11.3.11.
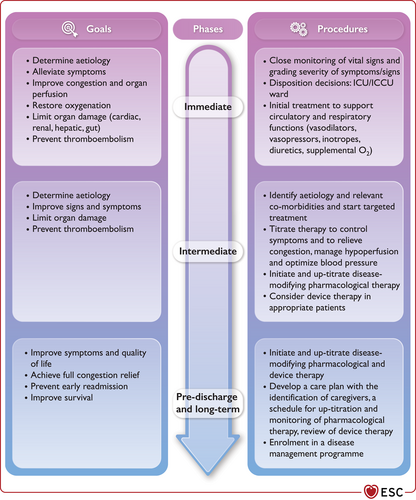
Stages of management of patients with acute heart failure. ICCU = intensive coronary care unit; ICU = intensive care unit.
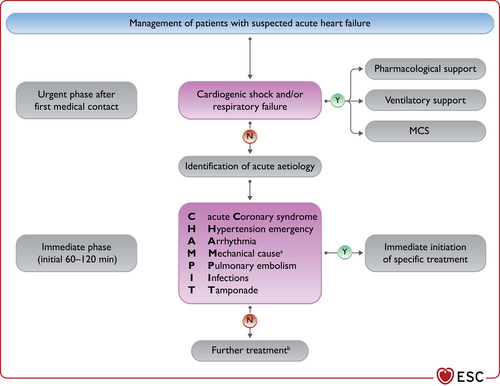
Initial management of acute heart failure. MCS = mechanical circulatory support. aAcute mechanical cause: myocardial rupture complicating acute coronary syndrome (free wall rupture, ventricular septal defect, acute mitral regurgitation), chest trauma or cardiac intervention, acute native or prosthetic valve incompetence secondary to endocarditis, aortic dissection or thrombosis. bSee Figures 7–10 for specific treatments according to different clinical presentations.
11.3.2 Oxygen therapy and/or ventilatory support
In AHF, oxygen should not be used routinely in non-hypoxaemic patients, as it causes vasoconstriction and a reduction in cardiac output.458 Oxygen therapy is recommended in patients with AHF and SpO2 <90% or PaO2 <60 mmHg to correct hypoxaemia. In chronic obstructive pulmonary disease (COPD), hyper-oxygenation may increase ventilation–perfusion mismatch, suppress ventilation and lead to hypercapnia. During oxygen therapy, acid-base balance and SpO2 should be monitored.
Non-invasive positive pressure ventilation, either continuous positive airway pressure and pressure support, improves respiratory failure, increases oxygenation and pH, and decreases the partial pressure of carbon dioxide (pCO2) and work of breathing. Although a large randomized trial had neutral results, meta-analyses suggest it may improve dyspnoea and reduce the need for intubation and mortality, compared with traditional oxygen therapy.1,459,460 Non-invasive positive pressure ventilation should be started as soon as possible in patients with respiratory distress (respiratory rate >25 breaths/min, SpO2 <90%) to improve gas exchange and reduce the rate of endotracheal intubation.449,460 The fraction of inspired oxygen (FiO2) should be increased up to 100%, if necessary, according to oxygen saturation level.
Blood pressure should be monitored regularly during non-invasive positive pressure ventilation. The increase in intrathoracic pressure with non-invasive positive pressure ventilation decreases venous return and right and left ventricular preload. It may also decrease cardiac output and BP and should therefore be used with caution in patients with reduced preload reserve and hypotension. The increase in pulmonary vascular resistance and RV afterload may also be detrimental in RV dysfunction.449
Intubation is recommended for progressive respiratory failure in spite of oxygen administration or non-invasive ventilation (Supplementary Table 20).449
11.3.3 Diuretics
Intravenous diuretics are the cornerstone of AHF treatment. They increase renal excretion of salt and water and are indicated for the treatment of fluid overload and congestion in the vast majority of AHF patients.
Loop diuretics are commonly used due to their rapid onset of action and efficacy. Data defining their optimal dosing, timing, and method of administration are limited. No difference in the primary efficacy outcome of patients’ symptoms global assessment was shown with a high-dose regimen, compared with a low-dose regimen, in the DOSE trial. However, there was a greater relief of dyspnoea, change in weight and net fluid loss (with no prognostic role for increases in serum creatinine) in the higher-dose regimen.461-463 High diuretic doses may cause greater neurohormonal activation and electrolyte abnormalities and are often associated with poorer outcomes, although a cause and effect relation cannot be proven by these retrospective analyses.464-467 Based on these observations, it may be appropriate, when starting i.v. diuretic treatment, to use low doses, to assess the diuretic response and increase the dose when that is insufficient.
Diuretic treatment should be started with an initial i.v. dose of furosemide, or equivalent dose of bumetanide or torasemide, corresponding to 1–2 times the daily oral dose taken by the patient before admission. If the patient was not on oral diuretics, a starting dose of 20–40 mg of furosemide, or a bolus of 10–20 mg i.v. torasemide, can be used.145,468 Furosemide can be given as 2–3 daily boluses or as a continuous infusion. Daily single bolus administrations are discouraged because of the possibility of post-dosing sodium retention.145,462 With continuous infusion, a loading dose may be used to achieve steady state earlier. Diuretic response should be evaluated shortly after start of diuretic therapy and may be assessed by performing a spot urine sodium content measurement after 2 or 6 h and/or by measuring the hourly urine output. A satisfactory diuretic response can be defined as a urine sodium content >50–70 mEq/L at 2 h and/or by a urine output >100–150 mL/h during the first 6 h.145,469 If there is an insufficient diuretic response, the loop diuretic i.v. dose can be doubled, with a further assessment of diuretic response.145 If the diuretic response remains inadequate, e.g. <100 mL hourly diuresis despite doubling loop diuretic dose, concomitant administration of other diuretics acting at different sites, namely thiazides or metolazone or acetazolamide, may be considered. However, this combination requires careful monitoring of serum electrolytes and renal function (Figure 13).145,470,471 This strategy, based on early and frequent assessment of diuretic response, allows starting treatment with relatively low doses of loop diuretics, with frequent dose adjustments that may be less likely to cause dehydration and increase in serum creatinine. The loop diuretic dose should be progressively decreased when a significant negative fluid balance has been obtained. However, it should be pointed out that this algorithm is entirely based on expert opinion, to date.145,462
Transition to oral treatment should be commenced when the patient’s clinical condition is stable. It is recommended that, after achievement of congestion relief, oral loop diuretics are continued at the lowest dose possible to avoid congestion.464,472 Care must also be taken to avoid patients being discharged from hospital with persistent congestion, as this is a major predictor of increased deaths and rehospitalizations.463,473 Hence, care should be taken to achieve adequate decongestion and establish an appropriate long-term diuretic dose before discharge.428,474
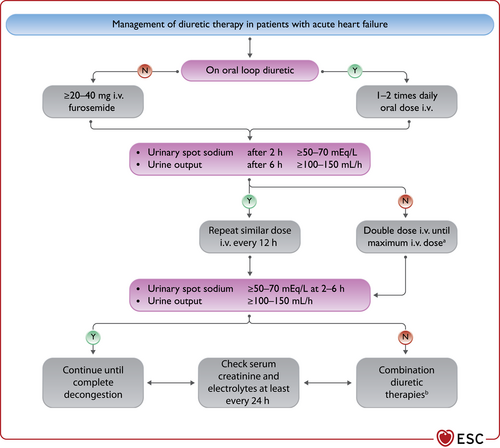
Diuretic therapy (furosemide) in acute heart failure. i.v. = intravenous. aThe maximal daily dose for i.v. loop diuretics is generally considered furosemide 400–600 mg though up to 1000 mg may be considered in patients with severely impaired kidney function. bCombination therapy is the addition to the loop diuretic of a diuretic with a different site of action, e.g. thiazides or metolazone or acetazolamide. Modified from 145.
11.3.4 Vasodilators
Intravenous vasodilators, namely nitrates or nitroprusside (Supplementary Table 21), dilate venous and arterial vessels leading to a reduction in venous return to the heart, less congestion, lower afterload, increased stroke volume and consequent relief of symptoms. Nitrates act mainly on peripheral veins whereas nitroprusside is more a balanced arterial and venous dilator.475,476 Because of their mechanisms of action, i.v. vasodilators may be more effective than diuretics in those patients whose acute pulmonary oedema is caused by increased afterload and fluid redistribution to the lungs in the absence or with minimal fluid accumulation.428,477-479 However, two recent randomized trials comparing usual care with early intensive and sustained vasodilation failed to show a beneficial effect of i.v. vasodilators vs. high-dose diuretics.480,481 No recommendation favouring a regimen based on vasodilator treatment vs. usual care can thus be given, to date.
Intravenous vasodilators may be considered to relieve AHF symptoms when SBP is >110 mmHg. They may be started at low doses and uptitrated to achieve clinical improvement and BP control. Nitrates are generally administered with an initial bolus followed by continuous infusion. However, they may also be given as repeated boluses. Nitroglycerine can be given as 1–2 mg boluses in severely hypertensive patients with acute pulmonary oedema.478 Care should be taken to avoid hypotension due to an excessive decrease in preload and afterload. For this reason, they should be used with extreme caution in patients with LVH and/or severe aortic stenosis. However, favorable effects were described in patients with LV systolic dysfunction and aortic stenosis when vasodilators were given with careful monitoring of haemodynamic parameters.482
11.3.5 Inotropes
Inotropes are still needed for treatment of patients with low cardiac output and hypotension (Table 22). They should be reserved for patients with LV systolic dysfunction, low cardiac output and low SBP (e.g. <90 mmHg) resulting in poor vital organ perfusion. However, they must be used with caution starting at low doses and uptitrating them with close monitoring.388,389
| Drug | Infusion rate |
|---|---|
| Dobutamine | 2–20 μg/kg/min (beta+) |
| Dopamine | 3–5 μg/kg/min; inotropic (beta+) >5 μg/kg/min: inotropic (beta+), vasopressor (alpha+) |
| Milrinone | 0.375–0.75 μg/kg/min |
| Enoximone | 5–20 μg/kg/min |
| Levosimendan | 0.1 μg/kg/min, which can be decreased to 0.05 or increased to 0.2 μg/kg/min |
| Norepinephrine | 0.2–1.0 μg/kg/min |
| Epinephrine | 0.05–0.5 μg/kg/min |
Inotropes, especially those with adrenergic mechanisms, can cause sinus tachycardia, increase ventricular rate in patients with AF, may induce myocardial ischaemia and arrhythmias, and increase mortality.388,389,431,479 Levosimendan or type-3-phosphodiesterase inhibitors may be preferred over dobutamine for patients on beta-blockers as they act through independent mechanisms.483,484 Excessive peripheral vasodilation and hypotension can be major limitations of type-3-phosphodiestaerase inhibitors or levosimendan, especially when administered at high doses and/or when commenced with a bolus dose.483,485
11.3.6 Vasopressors
Vasopressors used for the treatment of AHF are reported in Table 22.
Among drugs with a prominent peripheral arterial vasoconstrictor action, norepinephrine may be preferred in patients with severe hypotension. The aim is to increase perfusion to the vital organs. However, this is at the expense of an increase in LV afterload. Therefore, a combination of norepinephrine and inotropic agents may be considered, especially in patients with advanced HF and cardiogenic shock.
Some studies, though with limitations, support the use of norepinephrine as first choice, compared with dopamine or epinephrine. Dopamine was compared with norepinephrine as a first-line vasopressor therapy in patients with shock and was associated with more arrhythmic events and with a greater mortality in patients with cardiogenic shock but not in those with hypovolaemic or septic shock. Although the trial included 1679 patients, significance was seen only in a subgroup analysis of the 280 patients with cardiogenic shock and <10% of the patients had MI. As there were no data regarding revascularization, this limits the generalizability of the results.486 In another prospective randomized trial epinephrine was compared with norepinephrine in patients with cardiogenic shock due to acute MI.487 The trial was stopped prematurely due to a higher incidence of refractory shock with epinephrine. Epinephrine was also associated with higher heart rate and lactic acidosis. Despite limitations related to its relatively small sample size, short time of follow-up and lack of data regarding the maximum reached dose, the study suggests superior efficacy and safety with norepinephrine. These data are consistent with a meta-analysis including 2583 patients with cardiogenic shock showing a three-fold increase in the risk of death with epinephrine, compared with norepinephrine, in patients with cardiogenic shock.488 However, the lack of information about dose, duration of treatment, and aetiology, makes these results partially explorative.
11.3.7 Opiates
Opiates relieve dyspnoea and anxiety. They may be used as sedative agents during non-invasive positive pressure ventilation to improve patient adaptation. Dose-dependent side effects include nausea, hypotension, bradycardia, and respiratory depression. Retrospective analyses suggest that morphine administration is associated with a greater frequency of mechanical ventilation, prolonged hospitalization, more intensive care unit admissions, and increased mortality.489-492 Thus, routine use of opiates in AHF is not recommended although they may be considered in selected patients, particularly in case of severe/intractable pain or anxiety or in the setting of palliation.
Recommendations for the initial treatment of acute heart failure
| Recommendations | Classa | Levelb |
| Oxygen and ventilatory support | ||
| Oxygen is recommended in patients with SpO2 <90% or PaO2 <60 mmHg to correct hypoxaemia. | I | C |
| Intubation is recommended for progressive respiratory failure persisting in spite of oxygen administration or non-invasive ventilation.449 | I | C |
| Non-invasive positive pressure ventilation should be considered in patients with respiratory distress (respiratory rate >25 breaths/min, SpO2 <90%) and started as soon as possible in order to decrease respiratory distress and reduce the rate of mechanical endotracheal intubation.449 | IIa | B |
| Diuretics | ||
| Intravenous loop diuretics are recommended for all patients with AHF admitted with signs/symptoms of fluid overload to improve symptoms.145 | I | C |
| Combination of a loop diuretic with thiazide-type diuretic should be considered in patients with resistant oedema who do not respond to an increase in loop diuretic doses.145 | IIa | B |
| Vasodilators | ||
| In patients with AHF and SBP >110 mmHg, i.v. vasodilators may be considered as initial therapy to improve symptoms and reduce congestion.476-478,480,481 | IIb | B |
| Inotropic agents | ||
| Inotropic agents may be considered in patients with SBP <90 mmHg and evidence of hypoperfusion who do not respond to standard treatment, including fluid challenge, to improve peripheral perfusion and maintain end-organ function.388 | IIb | C |
| Inotropic agents are not recommended routinely, due to safety concerns, unless the patient has symptomatic hypotension and evidence of hypoperfusion.388,468,479 | III | C |
| Vasopressors | ||
| A vasopressor, preferably norepinephrine, may be considered in patients with cardiogenic shock to increase blood pressure and vital organ perfusion.486-488 | IIb | B |
| Other drugs | ||
| Thromboembolism prophylaxis (e.g. with LMWH) is recommended in patients not already anticoagulated and with no contraindication to anticoagulation, to reduce the risk of deep venous thrombosis and pulmonary embolism.495,496 | I | A |
| Routine use of opiates is not recommended, unless in selected patients with severe/intractable pain or anxiety.489,490 | III | C |
- AHF = acute heart failure; i.v. = intravenous; LMWH = low-molecular-weight heparin; PaO2 = partial pressure of oxygen; SBP = systolic blood pressure; SpO2 = transcutaneous oxygen saturation.
- a Class of recommendation.
- b Level of evidence.
11.3.8 Digoxin
Digoxin should be considered in patients with AF with a rapid ventricular rate (>110 b.p.m.) despite beta-blockers (see also section 12.1.1).151,493,494 It can be given in boluses of 0.25–0.5 mg i.v., if not used previously. However, in patients with comorbidities (i.e. CKD) or other factors affecting digoxin metabolism (including other drugs) and/or the elderly, the maintenance dose may be difficult to estimate theoretically and measurements of serum digoxin concentrations should be performed. Digitoxin is a potential alternative to digoxin and is currently being evaluated in a randomized placebo-controlled trial (ClinicalTrials.gov Identifier: NCT03783429).158
11.3.9 Thromboembolism prophylaxis
Thromboembolism prophylaxis with heparin (e.g. low-molecular-weight heparin) or another anticoagulant is recommended, unless contraindicated or unnecessary (because of existing treatment with oral anticoagulants).495,496
11.3.10 Short-term mechanical circulatory support
In patients presenting with cardiogenic shock, short-term MCS may be necessary to augment cardiac output and support end-organ perfusion. Short-term MCS can be used as a BTR, BTD or BTB.451-453 The initial improvements in cardiac output, BP and arterial lactate may be counterbalanced by significant complications. High-quality evidence regarding outcomes remains scarce. Hence, the unselected use of MCS in patients with cardiogenic shock is not supported and they require specialist multidisciplinary expertise for implantation and management, similar to that outlined for advanced HF centres (Supplementary text 11.4; Supplementary Table 22, see also section 10.2.2).377,497 Recent studies show that a ‘standardized team-based approach’ using predefined algorithms for early MCS implant coupled with close monitoring (invasive haemodynamics, lactate, markers of end-organ damage) may potentially translate into improved survival.498-500
The Intra-aortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK-II) trial showed no difference in 30-day, as well as in long-term, mortality between intra-aortic balloon pump (IABP) and OMT in patients with cardiogenic shock following acute MI who underwent early revascularization.501-503 According to these results, IABP is not routinely recommended in cardiogenic shock post-MI. However, it may still be considered in cardiogenic shock, especially if not due to ACS, and refractory to drug therapy, as a BTD, BTR, or BTB.
Other short-term MCS were compared with IABP in small, randomized trials and propensity-matched analyses with inconclusive results.504-508 Similarly, RCTs comparing extracorporeal membrane oxygenation (ECMO) with IABP or MT are lacking. A meta-analysis including only observational studies showed favourable outcomes in patients with cardiogenic shock or cardiac arrest treated with veno-arterial (VA)-ECMO compared to controls.509 VA-ECMO may also be considered in fulminant myocarditis and other conditions causing severe cardiogenic shock.510 Depending on the severity of myocardial dysfunction and/or concomitant mitral or aortic regurgitation, VA-ECMO may increase LV afterload with an increase in LV end-diastolic pressure and pulmonary congestion. In these cases, LV unloading is mandatory and can be achieved by means of transeptal/ventricular apex vent or adding an unloading device such as the Impella device.511,512
Recommendations for the use of short-term mechanical circulatory support in patients with cardiogenic shock
| Recommendations | Classa | Levelb |
| Short-term MCS should be considered in patients with cardiogenic shock as a BTR, BTD, BTB. Further indications include treatment of the cause of cardiogenic shock or long-term MCS or transplantation. | IIa | C |
| IABP may be considered in patients with cardiogenic shock as a BTR, BTD, BTB, including treatment of the cause of cardiogenic shock (i.e. mechanical complication of acute MI) or long-term MCS or transplantation.451 | IIb | C |
| IABP is not routinely recommended in post-MI cardiogenic shock.501-503 | III | B |
- BTB = bridge to bridge; BTD = bridge to decision; BTR = bridge to recovery; IABP = intra-aortic balloon pump; MCS = mechanical circulatory support; MI = myocardial infarction.
- a Class of recommendation.
- b Level of evidence.
11.3.11 Pre-discharge assessment and post-discharge management planning
A significant proportion of patients with AHF are discharged with minimal or no weight loss and, more importantly, persistent congestion.428,473 Persistent congestion before discharge is associated with a higher risk of readmission and mortality.427,473 Treatment, including diuretic dose, should therefore be optimized in order to keep the patient free of congestion.
In those admitted with ADHF, oral OMT should be continued, except for possible dose reduction or withdrawal if there is haemodynamic instability (symptomatic hypotension), severely impaired renal function or hyperkalaemia. Once haemodynamic stabilization is achieved with i.v. therapy, treatment should be optimized before discharge.468 Treatment optimization has three major aims. First, to relieve congestion. Second, to treat comorbidities, such as iron deficiency, that have an impact on post-discharge outcome.513 Third, to initiate, or restart oral, OMT with beneficial effects on outcome. Doses may be uptitrated before discharge and/or in the early post-discharge phase.
Studies have shown that such optimization of medical treatment is associated with a lower risk of 30-day readmission, although prospective randomized trials have not been performed, to date.103,468,514 Retrospective analyses show that discontinuation or dose reduction of beta-blocker therapy during an AHF hospitalization is associated with worse outcomes.515 Initiation of ARNI in recently hospitalized stable patients with HFrEF, including those who are ACE-I/ARB naïve, is safe and may be considered in this setting.106,107 Safety and better outcome have also been recently shown in a prospective randomized trial with sotagliflozin in diabetic patients hospitalized for HF, irrespective of their LVEF.136
It is recommended to have one follow-up visit within 1 to 2 weeks after discharge.516,517 Components of this follow-up visit should include monitoring of signs and symptoms of HF, assessment of volume status, BP, heart rate, and laboratory measurements including renal function, electrolytes, and possibly NPs. Iron status and hepatic function should also be assessed when not done before discharge. Based on clinical evaluation and laboratory exams, further optimization and/or initiation of disease-modifying treatment for HFrEF should occur. Retrospective studies show that such an approach is associated with lower 30-day readmission rates although prospective randomized trials have not been performed, to date.514,517-519
12 Cardiovascular comorbidities
12.1 Arrhythmias and conduction disturbances
12.1.1 Atrial fibrillation
AF and HF frequently coexist.520,521 They can cause or exacerbate each other through mechanisms such as structural cardiac remodelling, activation of neurohormonal systems, and rate-related LV impairment.520-524 The proportion of patients with HF who develop AF increases with age and HF severity. When AF causes HF the clinical course seems more favourable than with other causes of HF (so called tachycardiomyopathy).525 In contrast, development of AF in patients with chronic HF is associated with worse prognosis, including stroke and increased mortality.526,527
Recommendations for pre-discharge and early post-discharge follow-up of patients hospitalized for acute heart failure
| Recommendations | Classa | Levelb |
| It is recommended that patients hospitalized for HF be carefully evaluated to exclude persistent signs of congestion before discharge and to optimize oral treatment.428,473 | I | C |
| It is recommended that evidence-based oral medical treatment be administered before discharge.103,514 | I | C |
| An early follow-up visit is recommended at 1–2 weeks after discharge to assess signs of congestion, drug tolerance and start and/or uptitrate evidence-based therapy.518,519 | I | C |
| Ferric carboxymaltose should be considered for iron deficiency, defined as serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT <20%, to improve symptoms and reduce rehospitalizations.513 | IIa | B |
- HR = heart failure; TSAT = transferrin saturation.
- a Class of recommendation.
- b Level of evidence.
- (1)
Identification and treatment of possible causes or triggers of AF
- (2)
Management of HF
- (3)
Prevention of embolic events
- (4)
Rate control
- (5)
Rhythm control
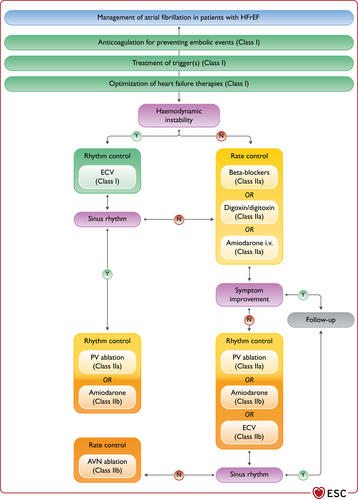
Management of atrial fibrillation in patients with heart failure with reduced ejection fraction. AF = atrial fibrillation; AVN = atrioventricular node; ECV = electrical cardioversion; HF = heart failure; i.v. = intravenous; PV = pulmonary vein. Colour code for classes of recommendation: Green for Class of recommendation I; Yellow for Class of recommendation IIa; Orange for Class of recommendation IIb; Red for Class of recommendation III (see Table 1 for further details on classes of recommendation).
Identification of triggers and management of heart failure
Potential causes or precipitating factors such as hyperthyroidism, electrolyte disorders, uncontrolled hypertension, mitral valve disease, and infection should be identified and corrected.
Worsening congestion due to AF should be managed with diuretics. Congestion relief may reduce sympathetic drive and ventricular rate and increase the chance of spontaneous return to SR. The presence of AF may reduce or abolish the prognostic benefits of beta-blockers and renders ivabradine ineffective.12,125 Some treatments for HF decrease the risk of developing AF, including ACE-I, slightly, and CRT, probably.7,528
Prevention of embolic events
Unless contraindicated, an oral, long-term anticoagulant is recommended in all patients with HF and paroxysmal, persistent, or permanent AF. Direct-acting oral anticoagulants (DOACs) are preferred for the prevention of thromboembolic events in patients with AF and without severe mitral stenosis and/or mechanical valve prosthesis, as they have similar efficacy to vitamin K antagonists (VKAs) but a lower risk of intracranial haemorrhage.529
LA appendage closure can be considered in patients with HF and AF who have a contraindication to oral anticoagulation though data from randomized trials have not included patients with contraindications to oral anticoagulants.530,531
Rate control
Data regarding rate control are not conclusive for the patients with AF and HF. A strategy of lenient rate control, defined by a resting heart rate <110 b.p.m., was compared to a strategy of strict rate control, defined by a heart rate <80 b.p.m. at rest and <110 b.p.m. during moderate exercise, in RACE II and in a pooled analysis of RACE and AFFIRM.152,532 The studies showed no differences in outcome between the two strategies. However, only 10% of the patients in RACE II and 17% of those in the pooled analysis had a history of HF hospitalization or NYHA class II–III, respectively.152,532 Higher heart rates are associated with worse outcomes in observational studies.533,534 Thus, a lenient rate control is an acceptable initial approach with, however, treatment targeting a lower heart rate in case of persistent symptoms or cardiac dysfunction likely related to tachycardia (e.g. tachycardia-induced cardiomyopathy).7,535
Beta-blockers can be used for rate control in patients with HFrEF or HFmrEF because of their established safety in these patients (see section 5.3.2).7,535,536 Digoxin or digitoxin can be considered when the ventricular rate remains high, despite beta-blockers, or when beta-blockers are contraindicated or not tolerated.151,494,537 It may therefore be considered also an alternative to beta-blockers. For patients with NYHA class IV and/or haemodynamic instability, i.v. amiodarone can be considered to reduce ventricular rate.538 For HFpEF, there is a paucity of evidence to demonstrate efficacy of any agent. The RATE-AF trial compared digoxin with bisoprolol in patients with persistent AF and NYHA class II–IV symptoms. Compared with bisoprolol, digoxin had the same effect on QOL at 6 months (primary endpoint) and a better effect on EHRA and NYHA functional class.537 Only 19% of the patients had LVEF <50% so that most of the patients can be considered as having HFmrEF or HFpEF.537
AV node ablation can be considered in patients with poor ventricular rate control despite medical treatment not eligible for rhythm control by catheter ablation or in patients with biventricular pacing.7,539-541
Rhythm control
Urgent electrical cardioversion is recommended in the setting of acute worsening HF in patients presenting with rapid ventricular rates and haemodynamic instability, after consideration of the thromboembolic risk. Cardioversion should be considered also to improve symptoms in patients who have persistent and symptomatic AF, despite optimal pharmacological management. In patients who do not receive chronic therapy with oral anticoagulant and with AF onset >48 h, at least 3 weeks of therapeutic anticoagulation or a transoesophageal echocardiography is needed before cardioversion.7 When pharmacological cardioversion is preferred, amiodarone is the drug of choice as other antiarrhythmic drugs (i.e. propafenone, flecainide, dronedarone) are associated with worse outcomes in HFrEF.186,535,542-545 Amiodarone can help maintain HF patients in SR after cardioversion.546,547
Trials including patients with HF and comparing rate control and rhythm control strategies with the latter based on antiarrhythmic drugs failed to show any benefit of one strategy over the other.548-551 More recently, EAST-AFNET 4, enrolling patients with early AF, 28.6% with HF, was stopped early after a median follow-up of 5.1 years for a lower occurrence of the primary outcome of death, stroke, or hospitalization for worsening HF or ACS in the patients assigned to early rhythm control vs. those assigned to usual care.552 However, the patients assigned to the rhythm control strategy had a closer follow-up, which may have influenced their better outcome. Catheter ablation was performed in a minority of the patients in the rhythm control arm (19.4%).552
LA catheter ablation was compared with MT, rate or rhythm control strategy, in 363 patients with persistent or paroxysmal AF, LVEF <35% and an implanted device (ICD or CRT-D) enrolled in the CASTLE-AF trial.553 The primary endpoint of all-cause death or HF hospitalizations occurred in fewer patients in the ablation group vs. the MT group, 51 patients (28.5%) vs. 82 (44.6%) [hazard ratio (HR); 95% confidence interval (CI), 0.62; 0.43–0.87; P = 0.007]. Also, other endpoints, all-cause or CV death or worsening HF, were reduced by catheter ablation.553 This trial suggests that catheter ablation can improve the prognosis of patients with HFrEF. However, it enrolled a highly selected population, 363 of 3013 patients, was not blinded, had crossovers between the two treatment strategies and the number of events observed was low: 24 (13.4%) vs. 46 (25.0%) all-cause deaths and 37 (20.7%) vs. 66 (35.9%) HF hospitalizations in the ablation and MT groups, respectively.553
The CABANA trial was an investigator-initiated, open-label, multicentre, randomized trial enrolling 2204 patients with symptomatic AF. The trial failed to show a benefit of AF ablation strategy over medical care on the primary composite endpoint of death, disabling stroke, serious bleeding, or cardiac arrest in the overall population.554 In an analysis of the 778 patients (35%) with NYHA class symptoms >II, the primary outcome occurred in 34 patients (9.0%) in the catheter ablation group vs. 49 (12.3%) in the drug therapy group (HR; 95% CI, 0.64; 0.41–0.99).555 However, also in this trial, the number of events was small and HF was defined based only on symptoms with LVEF available in 73% of the patients and >50% and 40–49% in 79% and 11.7% of the cases, respectively.555 Both CASTLE-AF and CABANA showed a highly significant effect of catheter ablation on patients’ symptoms.553-555
Two other prospective trials enrolled patients with HFrEF and persistent AF, who were randomized to catheter ablation or MT in one trial (AMICA trial, n = 140), and to catheter ablation or amiodarone in the other one (AATAC trial, n = 203).556,557 The first trial failed to show any difference in the LVEF increase between the two groups.556 The second trial showed superiority of catheter ablation with respect of AF recurrence, the primary endpoint, with also a reduction in unplanned hospitalizations and mortality.557 In contrast with the AMICA trial,556 but in accordance with CASTLE-AF,553 AATAC also showed a benefit of catheter ablation on LVEF.557
In conclusion, there is insufficient evidence in favour of a strategy of rhythm control with antiarrhythmic drugs vs. rate control in patients with HF and AF.548-552 The results of randomized trials with catheter ablation vs. MT showed a consistent improvement in symptoms whereas the results on mortality and hospitalization were obtained with a relatively small number of events not permitting to draw definitive conclusions.152,549-551,553-555,558
Recommendations for the treatment of atrial fibrillation in patients with heart failure
| Recommendations | Classa | Levelb |
| Anticoagulation | ||
| Long-term treatment with an oral anticoagulant is recommended in all patients with AF, HF, and CHA2DS2-VASc score ≥2 in men or ≥3 in women.7 | I | A |
| DOACs are recommended in preference to VKAs in patients with HF, except in those with moderate or severe mitral stenosis or mechanical prosthetic heart valves.529,559 | I | A |
| Long-term treatment with an oral anticoagulant should be considered for stroke prevention in AF patients with a CHA2DS2-VASc score of 1 in men or 2 in women.7,560 | IIa | B |
| Rate control | ||
| Beta-blockers should be considered for short- and long-term rate control in patients with HF and AF.536 | IIa | B |
| Digoxin should be considered when the ventricular rate remains high, despite beta-blockers, or when beta-blockers are contraindicated or not tolerated.537 | IIa | C |
| Cardioversion | ||
| Urgent ECV is recommended in the setting of acute worsening of HF in patients presenting with rapid ventricular rates and haemodynamic instability. | I | C |
| Cardioversion may be considered in patients in whom there is an association between AF and worsening of HF symptoms despite optimal medical treatment.7,542 | IIb | B |
| AF catheter ablation | ||
| In cases of a clear association between paroxysmal or persistent AF and worsening of HF symptoms, which persist despite MT, catheter ablation should be considered for the prevention or treatment of AF.553-555,558 | IIa | B |
- AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female) (score); DOAC = direct-acting oral anticoagulant; ECV = electrical cardioversion; HF = heart failure; MT = medical therapy; VKA = vitamin K antagonist.
- a Class of recommendation.
- b Level of evidence.
12.1.2 Ventricular arrhythmias
Ventricular arrhythmias may be a complication, and in some instances, a cause of HF. Frequent ventricular premature beats (VPBs) may lead to reversible systolic dysfunction. Possible factors may include dyssynchrony and abnormal calcium handling.561
Initial management of ventricular arrhythmias in HF should include correction of potential precipitants (including electrolyte abnormalities, particularly hypo/hyperkalaemia, and pro-arrhythmic drugs) as well as the optimization of HF drug therapy. Although ischaemia may be a triggering factor, revascularization has not been shown to reduce risk of ventricular arrhythmias.562
Amiodarone is effective also for suppression of ventricular arrhythmias. However, it does not reduce the incidence of sudden cardiac death or overall mortality.161 For patients with premature ventricular contraction (PVC)-induced CMP, amiodarone administration may be considered to reduce recurrent arrhythmias and improve symptoms and LV function, although its side effects should be taken into consideration. Other drugs are discussed in Supplementary text 12.1.
Radiofrequency ablation of VPBs may improve LV function and, possibly, outcomes in patients with tachycardiomyopathy when VPBs contribute to LV dysfunction.563 A sustained reduction in the baseline PVC burden has been associated with a lower risk of cardiac mortality, cardiac transplantation, or hospitalization for HF during follow-up.564,565
12.1.3 Symptomatic bradycardia, pauses and atrio-ventricular block
Indications for pacemaker therapy do not differ in patients with HF from those with other CV disease. There is ample evidence that RV pacing may have an adverse effect on LV systolic function leading, in the long term, to HF.566 Patients with HFrEF requiring frequent ventricular pacing, e.g. with AV block or slow AF, and who have systolic dysfunction, should be implanted with CRT rather than a standard pacemaker to avoid adverse outcomes, as shown in the BLOCK-HF (Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block) trial.216 In the quest for a more physiological alternative to RV pacing, physiological pacing is being increasingly adopted.567 In a non-randomized comparison of 304 consecutive patients with His bundle pacing and 433 consecutive patients with RV pacing, the former group had less HF hospitalization and a trend in reduced mortality.568 Although the technique is promising, more data are needed to confirm its role.
12.2 Chronic coronary syndromes
CAD is the most common cause of HF in industrialized, middle-income, and increasingly in low-income, countries. It should be considered as possible cause of HF in all patients presenting with new onset HF.
The diagnostic workup of patients with HF and chronic coronary syndromes (CCS) is reported in the recent 2019 ESC Guidelines on CCS.5 Patients with HF should be carefully evaluated to assess signs and/or symptoms of CCS. Clinical and family histories, physical examination, ECG and imaging tests are recommended.5 Documentation of ischaemia using non-invasive and invasive tests can be difficult in patients with HF because of the possible exercise intolerance and the effects of increased end-diastolic LV pressures. Coronary angiography or CTCA can be performed to establish the presence and extent of CAD and evaluate the potential indication for revascularization (see section 4.3).5
12.2.1 Medical therapy
Beta-blockers are the mainstay of therapy in patients with HFrEF and CAD because of their prognostic benefit.116-120,569 Ivabradine should be considered as an alternative to beta-blockers (when contraindicated) or as additional anti-anginal therapy in patients in SR whose heart rate is ≥70 b.p.m.139,570 Other anti-anginal drugs (e.g. amlodipine, felodipine, nicorandil, ranolazine, and oral or transdermal nitrates) are effective for treating symptoms, although data about their effects on outcomes are neutral or lacking.5,571-575 Trimetazidine seems to have additive effects, such as improvement of LV function and exercise capacity, in patients with HFrEF and CCS already on beta-blockers.576-578 Trimetazidine and other anti-anginal drugs may be considered in patients with HF and angina despite beta-blocker and/or ivabradine. Short-acting nitrates should be used with caution in patients with HF as they cause hypotension. Diltiazem and verapamil increase HF-related events in patients with HFrEF and are contraindicated.579
An algorithm for the use of anti-anginal medications in patients with HFrEF is reported in Figure 15.
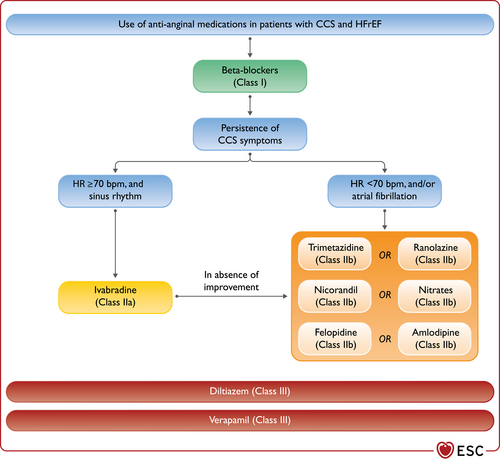
Algorithm for the medical treatment of chronic coronary syndrome in patients with heart failure with reduced ejection fraction. CCS = chronic coronary syndrome; HFrEF = heart failure with reduced ejection fraction; HR = heart rate. Colour code for classes of recommendation: Green for Class of recommendation I; Yellow for Class of recommendation IIa; Orange for Class of recommendation IIb; Red for Class of recommendation III (see Table 1 for further details on classes of recommendation).
Beta-blockers, long-acting nitrates, calcium channel blockers (CCBs), ivabradine, ranolazine, trimetazidine, nicorandil and their combinations should be considered in HFpEF for angina relief but without a foreseen benefit on HF and coronary end points.
Low-dose of rivaroxaban [2.5 mg twice daily (b.i.d.)] did not add prognostic benefit in patients with HFrEF and CCS in the COMMANDER-HF trial, a study to assess the effectiveness and safety of rivaroxaban in reducing the risk of death, MI or stroke in participants with HF and CAD following an episode of decompensated HF.580 This trial included patients with HFrEF and a recent episode of worsening HF occurring within 21 days from the time of enrolment. These patients are at high risk of HF-related events and these were the main cause of deaths and hospitalizations in the trial. Rivaroxaban had no effect on these events. In contrast, in a non-pre-specified subgroup analysis of the COMPASS trial, low dose of rivaroxaban, on top of aspirin, was associated with a reduction in ischaemic events in patients with HF, mainly HFmrEF or HFpEF.581 Based on these data, low-dose rivaroxaban may be considered in patients with CAD (or peripheral artery disease) and HF, LVEF >40% and SR, when at high risk of stroke and with a low haemorrhagic risk.
12.2.2 Myocardial revascularization
Data on the benefit of myocardial revascularization in patients with HF are limited.
STICH compared coronary artery bypass grafting (CABG) with MT in patients with CAD, amenable by CABG, and with reduced LV function (EF ≤35%). At a median follow-up of 56 months, there was no significant difference between the CABG group and the MT group in the rate of death from any cause, primary outcome of the trial.89 The extended follow-up report showed a significant reduction of death in the CABG group vs. the control group (58.9% vs. 66.1%; HR 0.84; 95% CI, 0.73–0.97; P = 0.02) over 10 years.582 CV death and the combined endpoint of all-cause death or hospitalization for CV causes were also significantly reduced after CABG at 10 years of follow-up.582 Post hoc analyses of the STICH trial suggested that neither myocardial viability, angina, nor ischaemia were related with outcomes after revascularization.92,93,583 The Heart Failure Revascularisation Trial (HEART) was under-powered, with only 138 of the planned 800 patients to be enrolled, and failed to show differences in outcomes between HF patients receiving CABG or MT.584
There are currently no reported RCTs comparing percutaneous coronary intervention (PCI) with MT in patients with HFrEF. However, the REVIVED-BCIS2 trial has finished recruitment (ClinicalTrials.gov Identifier: NCT01920048).585 There are also no randomized studies comparing PCI with CABG as such randomized trials excluded patients with HFrEF. In one prospective registry, including 4616 patients with multivessel disease and HFrEF, propensity-score matched comparisons showed similar survival (mean follow-up 2.9 years) in PCI vs. CABG group with PCIs associated with a higher risk of MI, particularly in patients with incomplete revascularization, and CABG associated with a higher risk of stroke.586 A propensity-matched analysis showed a significantly lower risk of death or major CV events in diabetic patients with LV dysfunction and multivessel disease treated with CABG compared with PCI.587 CABG was associated with better outcome than PCI also in patients with moderate or severe LV dysfunction and left main or complex coronary disease.588,589 Two meta-analyses confirmed that CABG is associated with better outcomes, including mortality, MI, and repeated revascularization, compared with PCI and/or MT.590,591
12.3 Valvular heart disease
12.3.1 Aortic stenosis
Aortic stenosis may cause or worsen HF by increasing LV afterload and causing LV hypertrophy and remodelling.592 When HF symptoms occur in patients with severe aortic stenosis, prognosis is extremely poor. No MT for aortic stenosis can improve outcomes. HF medical treatment should be given to all HF patients with symptomatic severe aortic stenosis. Care must be taken using vasodilators to avoid hypotension. Importantly, possible improvement of symptoms after MT should not delay intervention.
In the presence of suspected symptomatic and severe high-gradient aortic stenosis (valve area ≤1 cm², mean gradient ≥40 mmHg), other causes of high flow status must be excluded and corrected (i.e. anaemia, hyperthyroidism, arteriovenous shunts) before proceeding to aortic valve intervention.593 An aortic valve intervention is recommended in patients with HF symptoms and severe, high-gradient aortic stenosis, regardless of LVEF. Management of patients with low-flow low-gradient aortic stenosis is reported in Figure 16.593
Recommendations for myocardial revascularization in patients with heart failure with reduced ejection fraction
| Recommendations | Classa | Levelb |
| CABG should be considered as the first-choice revascularization strategy, in patients suitable for surgery, especially if they have diabetes and for those with multivessel disease.582,588,589,591 | IIa | B |
| Coronary revascularization should be considered to relieve persistent symptoms of angina (or an angina-equivalent) in patients with HFrEF, CCS, and coronary anatomy suitable for revascularization, despite OMT including anti-anginal drugs. | IIa | C |
| In LVAD candidates needing coronary revascularization, CABG should be avoided, if possible. | IIa | C |
| Coronary revascularization may be considered to improve outcomes in patients with HFrEF, CCS, and coronary anatomy suitable for revascularization, after careful evaluation of the individual risk to benefit ratio, including coronary anatomy (i.e. proximal stenosis >90% of large vessels, stenosis of left main or proximal LAD), comorbidities, life expectancy, and patient’s perspectives. | IIb | C |
| PCI may be considered as an alternative to CABG, based on Heart Team evaluation, considering coronary anatomy, comorbidities, and surgical risk. | IIb | C |
- CABG = coronary artery bypass graft; CCS = chronic coronary syndrome; HFrEF = heart failure with reduced ejection fraction; LAD = left anterior descending artery; LVAD = left ventricular assist device; OMT = optimal medical therapy; PCI = percutaneous coronary intervention.
- a Class of recommendation.
- b Level of evidence.
Intervention is recommended in patients with a life expectancy >1 year, avoiding futility. Transcatheter aortic valve implantation (TAVI) has been shown to be non-inferior to surgical aortic valve replacement (SAVR) in reducing clinical events (including mortality and disabling stroke) in patients at high and intermediate risk for surgery.594-601 In low-risk patients, mean age in the RCTs comparing TAVI and SAVR was >70 years and follow-up was restricted to 2 years. Therefore, SAVR is recommended in patients aged <75 years and at low surgical risk (STS-PROM score or EuroSCORE II <4%), whereas TAVI is recommended in those aged >75 years or at high/prohibitive surgical risk (STS-PROM score or EuroSCORE II >8%). In all the other cases, the choice between TAVI and SAVR should be made by the Heart Team, weighing the pros and cons of each procedure, according to age, life expectancy, individual patient preference and other features including clinical and anatomical aspects. Aortic valve interventions should be performed only in centres having both interventional cardiology and cardiac surgery services on site and a structured collaborative Heart Team approach.
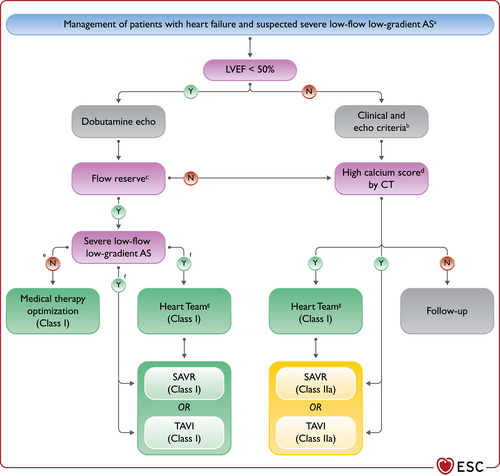
Management of patients with severe low-flow low-gradient aortic stenosis and heart failure. AS = aortic stenosis; CT = computed tomography; EuroSCORE II = European System for Cardiac Operative Risk Evaluation II; LVEF = left ventricular ejection fraction; OMT = optimal medical therapy; SAVR = surgical aortic valve replacement; STS-PROM = Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI = transcatheter aortic valve implantation. aValve area ≤1 cm², peak velocity <4.0 m/s, mean gradient <40; stroke volume index ≤35 mL/m². bAge >70 years, typical symptoms without other explanations, left ventricular hypertrophy or reduced left ventricular longitudinal function, mean gradient 30–40 mmHg, valve area ≤0.8 cm², stroke volume index ≤35 mL/m² assessed by techniques other than standard Doppler. cFlow reserve is defined as stroke volume index increase >20%. dAS is very likely if calcium score is ≥3000 in men and ≥1600 in women. AS is likely if calcium score is ≥2000 in men and ≥1200 in women. AS is unlikely if calcium score is <1600 in men and <800 in women. eIncrease in valve area to >1.0 cm² in response to flow increase (flow reserve) during dobutamine echo. fIncrease in mean gradient to at least 40 mmHg without significant change in valve area in response to flow increase (flow reserve) during dobutamine echo. gSAVR is recommended in patients aged <75 years and low surgical risk (STS-PROM score or EuroSCORE II <4%), whereas TAVI in those aged >75 years or at high/prohibitive surgical risk (STS-PROM score or EuroSCORE II >8%). In all the other cases, the choice between TAVI and SAVR is recommended to be decided by the Heart Team, weighing the pros and cons of each procedure, according to age, life expectancy, individual patient preference and features including clinical and anatomical aspects. Colour code for classes of recommendation: Green for Class of recommendation I; Yellow for Class of recommendation IIa (see Table 1 for further details on classes of recommendation).
Balloon aortic valvuloplasty may be considered in highly symptomatic patients with AHF (i.e. cardiogenic shock) as bridge to TAVI or SAVR, or in advanced HF as BTR or DT.
12.3.2 Aortic regurgitation
Severe aortic regurgitation can lead to progressive LV dilation with subsequent dysfunction, HF, and poor prognosis.
MT can improve HF symptoms in patients with severe aortic regurgitation. In particular, inhibitors of the RAAS can be useful.602 Beta-blockers should be cautiously used as they prolong diastole and may worsen aortic regurgitation.
Aortic valve surgery is recommended in patients with severe aortic regurgitation and HF symptoms regardless of LVEF.593,603,604 In case of high or prohibitive surgical risk, TAVI has been used to treat also aortic regurgitation.605
12.3.3 Mitral regurgitation
Primary (organic) mitral regurgitation
Primary mitral regurgitation (MR) is caused by abnormalities of the valve apparatus and can cause HF.
Surgery, preferably repair, is recommended in patients with severe primary MR and HF symptoms. If surgery is contraindicated or considered at high risk, then percutaneous repair may be considered.593,606
Secondary (functional) mitral regurgitation
Secondary mitral regurgitation (SMR) is mostly a disease of the left ventricle. It can also be caused by mitral annulus enlargement due to LA dilation.607 Moderate or severe SMR is associated with an extremely poor prognosis in patients with HF.608,609 The assessment of MR aetiology and severity should be performed by an experienced echocardiographer applying a multi-parametric approach, and ideally in stable patient conditions, after optimization of medical and resynchronization therapies. Being SMR a dynamic condition, echocardiographic quantification during exercise may be helpful in patients with moderate SMR at rest and symptoms during physical activity.610 Early referral of patients with HF and moderate or severe MR to a multidisciplinary Heart Team, including HF specialists, is recommended for assessment and treatment planning. The Heart Team has to verify, first of all, that the patient is on optimal therapy, including CRT, when indicated (Figure 17).
In patients with severe SMR and HFrEF requiring revascularization, mitral valve surgery and CABG should be considered. Isolated mitral valve surgery may be considered in symptomatic patients with severe SMR despite optimal therapy and low surgical risk.593
Two randomized trials, MITRA-FR and COAPT, evaluated the effectiveness of percutaneous edge-to-edge mitral valve repair plus OMT compared to OMT alone, in symptomatic patients with reduced LVEF (15–40% in MITRA-FR and 20–50% in COAPT) and moderate-to-severe or severe SMR [effective regurgitant orifice area (EROA) ≥ 20 mm2 in MITRA-FR and EROA ≥ 30 mm2 in COAPT].611-613 MITRA-FR failed to show any benefit from the intervention on all-cause mortality or HF hospitalization at 12 months (primary endpoint; HR 1.16, 95% CI 0.73–1.84) and at 24 months.611,612 In contrast, COAPT showed a significant reduction in hospitalization for HF at 24 months (primary endpoint; HR 0.53, 95% CI 0.40–0.70) and mortality (secondary endpoint; HR 0.62, 95% CI 0.46–0.82).613 Differences in patient selection, concomitant MT, echocardiographic assessment, procedural issues and severity of SMR in relation to the degree of LV dilatation may be responsible for the diverging results of the MITRA-FR and COAPT trials.614-616 Thus, percutaneous edge-to-edge mitral valve repair should be considered for outcome improvement only in carefully selected patients who remain symptomatic (NYHA class II–IV) despite OMT, with moderate-to-severe or severe SMR (EROA ≥30 mm2), favourable anatomical conditions, and fulfilling the inclusion criteria of the COAPT study (i.e. LVEF 20–50%, LV end-systolic diameter <70 mm, systolic pulmonary pressure <70 mmHg, absence of moderate or severe RV dysfunction, absence of severe TR, absence of haemodynamic instability) (Figure 17).616,617
Percutaneous edge-to-to edge mitral valve repair may also be considered to improve symptoms in patients with advanced HF, severe SMR and severe symptoms despite OMT. In these patients, cardiac transplantation or LVAD implantation must also be considered.377,618
Other percutaneous mitral valve repair systems, such as indirect annuloplasty, are available for treatment of SMR. This approach has a shorter learning curve and lesser technical requirements than percutaneous edge-to-to edge mitral valve repair and does not preclude different procedures once it is performed. A sham-controlled randomized trial testing a transcatheter indirect mitral annuloplasty device met its primary endpoint of mitral regurgitant volume reduction with reverse LV and LA remodelling at 12 months.619 Further studies confirmed favourable results on LA volumes and LV remodelling with trends towards improvement in mean 6MWT distance and symptoms and a reduction in HF hospitalizations in an IPD meta-analysis.620-623 Transcatheter mitral valve replacement is also emerging as a possible alternative option, but randomized trials are still lacking.624
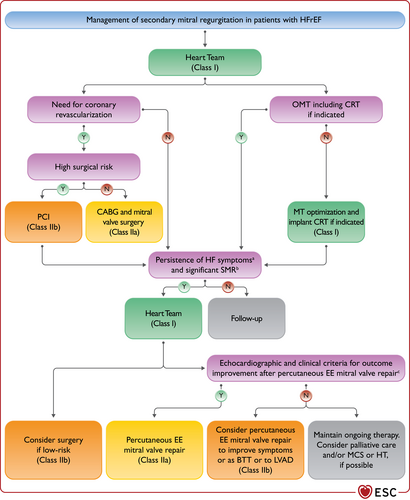
Management of secondary mitral regurgitation in patients with heart failure with reduced ejection fraction. BTT = bridge to transplantation; CABG = coronary artery bypass graft; CRT = cardiac resynchronization therapy; EE = edge-to-edge; EROA = effective regurgitant orifice area; HF = heart failure; LVAD = left ventricular assist device; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; MCS = mechanical circulatory support; MT = medical therapy; NYHA = New York Heart Association; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; SMR = secondary mitral regurgitation; TR = tricuspid regurgitation. aNYHA class II–IV. bModerate-to-severe or severe (EROA ≥30 mm2). cAll of the following criteria must be fulfilled: LVEF 20–50%, LVESD <70 mm, systolic pulmonary pressure <70 mmHg, absence of moderate or severe right ventricular dysfunction or severe TR, absence of haemodynamic instability.613 Colour code for classes of recommendation: Green for Class of recommendation I; Yellow for Class of recommendation IIa; Orange for Class of recommendation IIb (see Table 1 for further details on classes of recommendation).
Mitral valve interventions are not recommended in patients with a life expectancy of <1 year due to extra-cardiac conditions.593
Recommendations for the management of valvular heart disease in patients with heart failure
| Recommendations | Classa | Levelb |
| Aortic stenosis | ||
| Aortic valve intervention, TAVI or SAVR, is recommended in patients with HF and severe high-gradient aortic stenosis to reduce mortality and improve symptoms.595 | I | B |
| It is recommended that the choice between TAVI and SAVR be made by the Heart Team, according to individual patient preference and features including age, surgical risk, clinical, anatomical and procedural aspects, weighing the risks and benefits of each approach.593 | I | C |
| Secondary mitral regurgitation | ||
| Percutaneous edge-to-edge mitral valve repair should be considered in carefully selected patients with secondary mitral regurgitation, not eligible for surgery and not needing coronary revascularization, who are symptomaticc despite OMT and who fulfil criteriad for achieving a reduction in HF hospitalizations.613 | IIa | B |
| In patients with HF, severe secondary mitral regurgitation and CAD who need revascularization, CABG and mitral valve surgery should be considered. | IIa | C |
| Percutaneous edge-to-edge mitral valve repair may be considered to improve symptoms in carefully selected patients with secondary mitral regurgitation, not eligible for surgery and not needing coronary revascularization, highly symptomatic despite OMT and who do not fulfil criteria for reducing HF hospitalization.618 | IIb | C |
- CABG = coronary artery bypass graft; CAD = coronary artery disease; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; NYHA = New York Heart Association; OMT = optimal medical therapy; SAVR = surgical aortic valve replacement; TAVI = transcatheter aortic valve implantation; TR = tricuspid regurgitation.
- a Class of recommendation.
- b Level of evidence.
- c NYHA class II–IV.
- d All of the following criteria must be fulfilled: LVEF 20–50%, LVESD <70 mm, systolic pulmonary pressure <70 mmHg, absence of moderate or severe right ventricular dysfunction or severe TR, absence of haemodynamic instability.613
12.3.4 Tricuspid regurgitation
Tricuspid regurgitation (TR) can be caused by or be a consequence of RV dysfunction and HF. The management of HF with TR includes MT (i.e. diuretics, neurohormonal antagonists). Transcatheter therapy and surgery may be considered in selected cases.593 A multidisciplinary Heart Team, including HF specialists, should be considered for assessment and treatment planning.
Tricuspid valve surgery is recommended in patients with severe TR requiring left-sided cardiac surgery. It should be also considered in patients with moderate TR and tricuspid annulus dilatation requiring left-sided cardiac surgery and in symptomatic patients with isolated severe TR.593 However, surgery in isolated TR is burdened by high in-hospital mortality (8.8%) although the advanced stage of HF may have influenced these data.625 Transcatheter techniques have recently emerged as potential treatment options of TR. Preliminary results show improvement in TR severity and symptoms with low complication rates.626 Further prospective studies are needed to show the prognostic impact of these treatments in HF patients.
12.4 Hypertension
Arterial hypertension is a leading risk factor for the development of HF. Almost two-thirds of HF patients have a past history of hypertension.104,627 Clinical trials evaluating antihypertensive strategies and BP targets in patients with HF and hypertension have not been performed.
Treatment of HFrEF is similar in hypertensive and normotensive patients. Recommended medications, including neurohormonal antagonists and diuretics, lower also BP. Lifestyle modifications, such as weight loss, reduced sodium intake, and increased physical activity, are useful adjunctive measures.4 Uncontrolled hypertension in patients with HFrEF is rare, provided the patient is receiving OMT at recommended doses for HF. If further BP lowering is required, in absence of signs of fluid overload, amlodipine and felodipine have been shown to be safe in HFrEF and may be considered.571,572 Non-dihydropyridine CCBs (diltiazem and verapamil) and centrally acting agents, such as moxonidine, are contraindicated as they are associated with worse outcomes.628 Alpha-blockers have no effects on survival and are therefore not indicated.143 They can be used for the treatment of concomitant prostatic hyperplasia but should be withdrawn in cases of hypotension.
Hypertension is the most important cause of HFpEF, with a prevalence of 60% to 89%.39 Patients with HFpEF also frequently have an exaggerated hypertensive response to exercise and may present with hypertensive acute pulmonary oedema.629,630 Antihypertensive agents, including ACE-I, ARBs, beta-blockers, CCB, and diuretics reduce the incidence of HF.631,632 Reducing BP leads also to LVH regression, the degree of which depends on the class of drug used.4 ARBs, ACE-I, and CCBs cause more effective LVH regression than beta-blockers or diuretics.633 Poorly controlled hypertension may precipitate episodes of decompensation. Causes of secondary hypertension, such as renal vascular or parenchymal disease, primary aldosteronism and obstructive sleep apnoea (OSA), should be ruled out or, if confirmed, considered for treatment. Treatment of hypertension is an important issue in patients with HFpEF, but the optimal treatment strategy is uncertain. The treatment strategy used in HFrEF should also be considered in HFpEF.4
BP targets are uncertain in both HFrEF and HFpEF. However, evaluation of patient’s age and comorbidities (i.e. diabetes, CKD, CAD, valvular heart disease and stroke) can be helpful to personalize the BP target.4 Every effort should be made to reach target doses of evidence-based medications in HFrEF patients, despite slight hypotension.4,634 Conversely, in HFpEF patients with LVH and limited preload reserve, hypotension should be avoided.
12.5 Stroke
HF and stroke frequently coexist because of an overlap of shared risk factors and subsequent mechanisms.520,635 A higher risk of stroke is present also in HF patients in SR.39,427,636–638 AF confers an additional risk and patients with HF and AF have a five-fold increased risk compared to the control population.520,635,639
As a temporal trend, the incidence of stroke is higher in the first 30 days after HF diagnosis or an episode of HF decompensation and decreases in the first 6 months following the acute event.638,640 Patients with stroke and HF have higher mortality, more severe neurological deficits and longer hospital stays than those without HF.638,641 Similarly, patients with HF and stroke have a higher mortality than patients without stroke.641 In COMMANDER-HF, 47.5% of strokes were either disabling, 16.5%, or fatal, 31%.638
Patients with HF and concomitant AF, including paroxysmal AF, have a CHA2DS2-VASc score of at least 1 and have therefore an indication to anticoagulation. The indication to antithrombotic strategies in patients with HF and SR is controversial. In the Warfarin and Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, warfarin reduced ischaemic stroke as compared with aspirin, but increased major haemorrhages and did not influence the primary endpoint of ischaemic stroke, intracerebral haemorrhage, or death.642 Meta-analyses confirm an increased risk of bleeding outweighing ischaemic stroke prevention in placebo-controlled trials in patients with HFrEF and SR.643 In COMMANDER-HF, rivaroxaban 2.5 mg b.i.d. did not improve the composite outcome of all-cause mortality, MI or stroke, nor did it favourably influence HF-related deaths or HF hospitalizations.580 There are no data to support a routine strategy of anticoagulation in patients with HFrEF in SR who do not have history of paroxysmal AF. However, low-dose rivaroxaban may be considered in patients with concomitant CCS or peripheral artery disease, a high risk of stroke and no major haemorrhagic risk (see section 12.2).
Patients with visible intraventricular thrombus or at high thrombotic risk, such as those with history of peripheral embolism or some patients with PPCM or LV non-compaction (LVNC), should be considered for anticoagulation.3,644-646
13 Non-cardiovascular comorbidities
13.1 Diabetes
Treatment of HF is similar in patients with and without diabetes.6,647 Conversely, antidiabetic drugs differ in their effects in patients with HF and preference must be given to drugs that are both safe and reduce HF-related events.6,647,648
The SGLT2 inhibitors canagliflozin, dapagliflozin, empagliflozin, ertugliflozin and sotagliflozin were studied in patients with established CV disease in the EMPA-REG OUTCOME and VERTIS-CV trials, with established CV disease or CV risk factors in the CANVAS and DECLARE-TIMI 58 trials, and with CKD and CV risk in the SCORED trial, respectively.294-298 A small proportion of patients had a history of HF. Empagliflozin and canagliflozin reduced the primary composite endpoint of major CV adverse events, including CV death or non-fatal MI or non-fatal stroke, and HF hospitalizations in EMPA-REG OUTCOME and CANVAS, respectively.294,295 Empagliflozin also reduced all-cause death or CV death alone.294 The effects on the primary endpoint were driven by the reduction in HF-related events.294,295 In DECLARE-TIMI 58, dapagliflozin did not reduce major CV events but reduced the co-primary efficacy endpoint of CV death or HF hospitalization and HF hospitalization alone.296 In VERTIS-CV, neither the primary major CV event endpoint nor the key secondary outcome of CV death or HF hospitalization were reduced significantly by ertugliflozin, although there was a statistically significant reduction in HF hospitalization and repeated hospitalizations.298,649 In SCORED, sotagliflozin reduced CV deaths and HF hospitalizations.297 In a meta-analysis of these trials and one further trial in patients with CKD (CREDENCE), overall SGLT2 inhibitors reduced HF and CV hospitalization by 22%.650 SGLT2 inhibitors were well tolerated, although they may cause genital fungal skin infections and, rarely, diabetic ketoacidosis.294-296 Trial results with dapagliflozin and empagliflozin in patients with HFrEF, with or without diabetes, and with the SGLT1/2 inhibitor sotagliflozin in patients with type 2 diabetes stabilized after hospitalization for acute HF or within 3 days after discharge, further support the administration of these agents (see section 5.3.5 and section 11.3.11).108,109,136
EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, and VERTIS-CV also showed a reduction in worsening renal function, end-stage renal disease or death from renal causes, with SGLT2 inhibitors.
Based on these results, the SGLT inhibitors canagliflozin, dapagliflozin, empagliflozin, ertugliflozin or sotagliflozin are recommended to prevent HF and CV death and worsening kidney function in patients with type 2 diabetes and CV disease and/or CV risk factors, or CKD. Dapagliflozin and empagliflozin are also indicated for the treatment of patients with type 2 diabetes and HFrEF (see section 5.3.5 and section 11.2.4) and sotagliflozin was shown to reduce CV deaths and HF rehospitalizations in patients recently hospitalized for HF.6,297,647,648,651
Metformin is thought to be safe in patients with HF, compared with insulin and sulfonylureas, based on observational studies.652,653 However, it is not recommended in patients with an eGFR <30 mL/min/1.73 m2 or hepatic impairment because of the risk of lactic acidosis. It has not been studied in controlled outcome trials, to date.6,647
Regarding dipeptidyl peptidase-4 (DPP-4) inhibitors, HF hospitalizations were increased by 27% in one trial with saxagliptin in patients with diabetes.654 However, no difference over placebo for HF events was found with alogliptin, sitagliptin, and linagliptin.655-657 Vidagliptin was associated with an increase in LV volumes and a numerically greater number of deaths and CV events in a small trial in patients with diabetes and HF.658 Overall, the effects on mortality or CV events were neutral in the DPP-4 inhibitor trials and meta-analyses.659,660 These drugs are therefore not recommended to reduce CV events in diabetic patients with HF.
Glucagon-like peptide-1 (GLP-1) receptor agonists reduce the risk of MI, stroke, and CV death in patients with diabetes, although probably do not reduce incident HF.6,661 Liraglutide had no effect on LVEF, increased heart rate, and increased serious cardiac events in a randomized placebo-controlled trial in 241 patients with HFrEF with and without diabetes.662 Neutral results on the primary endpoint were found in another trial in 300 patients with a numerical increase in deaths and HF hospitalizations, compared with placebo.663 GLP-1 receptor agonists are therefore not recommended for the prevention of HF events.
Insulin is needed in patients with type 1 diabetes and to control hyperglycaemia in some patients with type 2 diabetes, especially when beta-cell function is exhausted. It is a sodium-retaining hormone and concern has been raised that it may exacerbate fluid retention in patients with HF. However, in a RCT that included patients with type 2 diabetes, impaired glucose tolerance, or impaired fasting glucose, insulin did not increase the risk of incident HF.664 Use of insulin was associated with poorer outcomes in retrospective analyses of randomized trials and administrative databases.665,666 If insulin is needed in a patient with HF, the patient should be monitored for evidence of worsening of HF after treatment initiation.
Sulfonylureas were associated with a higher risk of HF events in some analyses.667,668 Therefore, they are not a preferred treatment in patients with HF and, if needed, patients should be monitored for evidence of worsening of HF after treatment initiation.6,647 Thiazolidinediones (glitazones) cause sodium and water retention and an increased risk of worsening HF and hospitalization.669 They are contraindicated in patients with HF.
Recommendations for the treatment of diabetes in heart failure
| Recommendations | Classa | Levelb |
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with T2DM at risk of CV events to reduce hospitalizations for HF, major CV events, end-stage renal dysfunction, and CV death.294-298 | I | A |
| SGLT2 inhibitors (dapagliflozin, empagliflozin, and sotagliflozin) are recommended in patients with T2DM and HFrEF to reduce hospitalizations for HF and CV death.108,109,136 | I | A |
- CV = cardiovascular; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; SGLT2 = sodium-glucose co-transporter 2; T2DM = type 2 diabetes mellitus.
- a Class of recommendation.
- b Level of evidence.
13.2 Thyroid disorders
Assessment of thyroid function is recommended in all patients with HF as both hypo- and hyperthyroidism may cause or precipitate HF.670 Subclinical hypothyroidism and isolated low triiodothyronine levels were associated with poorer outcomes in observational studies in patients with HF.671,672 Treatment of thyroid disorders should be guided by general endocrine guidelines. There are no randomized trials evaluating the efficacy of thyroid replacement therapy in subclinical hypothyroidism, but there is a general agreement to correct it when the TSH is >10 mIU/L, particularly in patients <70 years. Correction may also be considered at lower TSH levels (7–10 mIU/L).673-675
13.3 Obesity
Obesity is a risk factor for hypertension and CAD and is also associated with an increased risk of HF. There is possibly a stronger association with HFpEF.259,676-678 Once obese patients have HF, an obesity paradox has been described such that overweight or mildly/moderately obese patients have a better prognosis than leaner patients, particularly compared with those who are underweight.679,680 However, other variables may influence this relationship and the obesity paradox is not observed in patients with diabetes.681,682 Second, BMI does not take into account body composition, e.g. the relation between lean skeletal muscle mass and fat mass. Obese patients who are fit and have a preserved skeletal muscle mass have better prognosis than obese sarcopenic patients.683 Waist circumference or the waist-to-hip ratio, measuring visceral obesity, is less influenced by muscle mass and may have a stronger relationship with outcomes than BMI, especially in female patients.684,685
Body fat has a major impact on the diagnostic and prognostic value of multiple parameters. Obese patients with HF have lower NP concentrations due to increased expression of clearance receptors and augmented peptide degradation by the adipose tissue.74 Peak oxygen consumption adjusted for body weight underestimates exercise capacity in obese patients and an adjustment for lean body mass should be used for risk stratification.96
Obesity may be a major cause of HFpEF and obese HFpEF patients display several pathophysiologic mechanisms that differ from non-obese patients with HFpEF.259,676-678,686 Caloric restriction and exercise training had additive beneficial effects on exercise capacity and QOL of patients with obesity and HFpEF in a randomized trial.338
13.4 Frailty, cachexia, sarcopenia
Frailty is a multidimensional dynamic state, independent of age, that makes the individual more vulnerable to the effect of stressors.687 HF and frailty are two distinct yet commonly associated conditions. The assessment of frailty in patients with HF is crucial as it is associated with both unfavourable outcomes and reduced access to, and tolerance of, treatments. Several tools have been proposed for frailty screening and assessment in different chronic conditions, including HF. The HFA of ESC has developed a HF-specific tool based on four major domains, clinical, psycho-cognitive, functional, and social.687
Frailty is more prevalent in patients with HF than in the general population and may occur in up to 45% of the patients, according to a recent meta-analysis.688,689 Patients with HF are up to six times more likely to be frail, and frail people have a significantly increased risk of developing HF.690,691 Frailty is associated with a higher risk of death, hospitalizations, and functional decline as well as with a longer duration of hospital stay.692-694 The treatment of frailty in HF should be multifactorial and targeted to its main components and may include physical rehabilitation with exercise training, nutritional supplementation as well as an individualized approach to treating comorbidities.687
Cachexia is defined as a ‘complex metabolic syndrome associated with underlying illness and characterized by loss of muscle with or without loss of fat mass’.695 Its major clinical feature is a >5% oedema-free body weight loss during the previous 12 months or less.695,696 Cachexia is a generalized wasting process that may coexist with frailty and may occur in 5–15% of patients with HF, especially those with HFrEF and more advanced disease status. It is associated with reduced functional capacity and decreased survival.696-699 As it is associated with other chronic diseases, such as cancer, alternative, non-cardiac causes for cachexia should always be investigated.700
Sarcopenia is defined by the presence of low muscle mass together with low muscle function, strength, or performance.699 It is usually identified by an appendicular skeletal muscle mass, defined as the sum of the muscle mass of the four limbs, 2 standard deviation below the mean of a healthy reference group aged 18–40 years with a cut-off value of 7.26 kg/m² for men.689,701,702 It occurs physiologically with aging. However, it is accelerated by chronic diseases, such as cancer and HF. Sarcopenia can be found in 20–50% of patients with HFrEF and is often associated with frailty and increased morbidity and mortality. It is a major determinant of outcomes outweighing the effect of body weight and BMI.685,699,702,703 So far, the most effective strategy for sarcopenia treatment is resistance exercise training, possibly combined with a protein intake of 1–1.5 g/kg/day.699,704 Drug treatments, including anabolic compounds like testosterone, growth hormone, ghrelin receptor agonists, were tested in small studies, showing favourable results mostly in terms of exercise capacity and muscle strength.698,704-706 There are no data showing a favourable impact of sarcopenia treatment on outcomes. However, exercise training has favourable effects in patients with HF (see section 9.4).95,324-330
13.5 Iron deficiency and anaemia
Iron deficiency and anaemia are common in patients with HF, being independently associated with reduced exercise capacity, recurrent HF hospitalizations, and high CV and all-cause mortality.707,708 According to the World Health Organization criteria, anaemia is defined as a haemoglobin concentration <12 g/dL in women and <13 g/dL in men. In patients with HF, iron deficiency is defined as either a serum ferritin concentration <100 ng/mL or 100–299 ng/mL with transferrin saturation (TSAT) <20%.709-711 Ferritin tissue expression and concentration in the peripheral blood is increased by inflammation and several disorders such as infection, cancer, liver disease, and HF itself. Hence, higher cut-off values have been applied for the definition of iron deficiency in patients with HF.710-712 Another marker reflecting depleted intracellular iron can be high serum soluble transferrin receptors, which derives from proteolysis of the membrane transferrin receptor. Its synthesis is increased in case of iron deficiency and is not affected by inflammation. High serum soluble transferrin receptors identify patients at high risk of death beyond standard prognostic variables.712,713 However, its applicability for iron supplementation therapy has not been demonstrated yet.
Iron deficiency, which can be present independently of anaemia, is present in up to 55% of chronic HF patients and in up to 80% of those with AHF.714-717 It may be caused by increased loss, reduced intake or absorption (i.e. malnutrition, gut congestion) and/or impaired iron metabolism caused by the chronic inflammatory activation of HF, although the exact cause of iron deficiency in HF remains unknown. Iron deficiency may impair functional capacity, precipitate circulatory decompensation, promote skeletal muscle dysfunction, and is associated with frailty, irrespective of anaemia.717-719
It is recommended that all patients with HF are regularly screened for anaemia and iron deficiency with full blood count, serum ferritin concentration, and TSAT. The detection of anaemia and/or iron deficiency should prompt appropriate investigation to define their cause.
Darbepoetin-alpha failed to reduce all-cause death or HF hospitalization and increased the risk of thromboembolic events in the only large-scale randomized trial in patients with HFrEF and mild to moderate anaemia.720 As a result, erythropoietin stimulating agents are not indicated for the treatment of anaemia in HF.
Recommendations for the management of anaemia and iron deficiency in patients with heart failure
| Recommendations | Classa | Levelb |
| It is recommended that all patients with HF be periodically screened for anaemia and iron deficiency with a full blood count, serum ferritin concentration, and TSAT. | I | C |
| Intravenous iron supplementation with ferric carboxymaltose should be considered in symptomatic patients with LVEF <45% and iron deficiency, defined as serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT <20%, to alleviate HF symptoms, improve exercise capacity and QOL.721,723,725 | IIa | A |
| Intravenous iron supplementation with ferric carboxymaltose should be considered in symptomatic HF patients recently hospitalized for HF and with LVEF <50% and iron deficiency, defined as serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT <20%, to reduce the risk of HF hospitalization.513 | IIa | B |
- HF = heart failure; LVEF = left ventricular ejection fraction; QOL = quality of life; TSAT = transferrin saturation.
- a Class of recommendation.
- b Level of evidence.
RCTs have shown that iron supplementation with i.v. ferric carboxymaltose is safe and improves symptoms, exercise capacity, and QOL of patients with HFrEF and iron deficiency.721-724 Meta-analyses of RCTs showed also a reduction in the risk of the combined endpoints of all-cause death or CV hospitalization, CV death or HF hospitalization, CV death or recurrent CV or HF hospitalizations.725,726 The favourable effects of iron supplementation were independent from anaemia coexistence.727 In AFFIRM-AHF, patients hospitalized for HF with LVEF <50% and concomitant iron deficiency were randomized to i.v. ferric carboxymaltose or placebo, repeated at 6- and then 12-week intervals if indicated according to repeat iron studies.513 Administration of ferric carboxymaltose did not significantly reduce the primary composite outcome of total HF hospitalizations and CV death at 52 weeks (rate ratio 0.79, 95% CI 0.62–1.01, P = 0.059). However, it reduced the composite endpoint of first HF hospitalization or CV death (HR 0.80, 95% CI 0.66–0.98, P = 0.030) and total HF hospitalizations (rate ratio 0.74, 95% CI 0.58–0.94, P = 0.013).513 Therefore, iron supplementation with i.v. ferric carboxymaltose should be considered for the improvement of symptoms, exercise capacity, and QOL in patients with HF and LVEF <45%. It should also be considered for the reduction of HF rehospitalizations in patients with LVEF <50% recently hospitalized for worsening HF. Ongoing trials are expected to provide more evidence on the effects of ferric carboxymaltose in patients with HFpEF. In addition, large outcomes trials with other iron formulations are ongoing in HFrEF, HFpEF, and AHF.728 Oral iron therapy is not effective in iron repletion and did not improve exercise capacity in patients with HFrEF and iron deficiency.729 It is therefore not recommended for the treatment of iron deficiency in the patients with HF.
13.6 Kidney dysfunction
CKD and HF frequently coexist.472,708,730 They share common risk factors, such as diabetes or hypertension. CKD may worsen CV function causing hypertension and vascular calcification. HF may worsen renal function, through the effects of neurohormonal and inflammatory activation, increased venous pressure and hypoperfusion. Oxidative stress and fibrosis likely play a major role as pathogenic mechanisms in HF with CKD.731,732
While CKD and worsening renal function both appear more common in HFpEF as compared to in HFmrEF and HFrEF, perhaps due to shared pathophysiological mechanisms, they appear to be less associated with worse outcomes in HFpEF than in HFmrEF and HFrEF.733,734
CKD is a major independent determinant of increased mortality and morbidity in HF.472,735-737 However, there are settings in which changes in serum creatinine are not associated with worse outcomes. When RAAS inhibitors, ARNI or SGLT2 inhibitors are started, the initial decrease in the glomerular filtration pressure may decrease GFR and increase serum creatinine. However, these changes are generally transient and occur despite improvement in patient outcomes and slower worsening of renal function in the long term. For instance, in EMPEROR-Reduced, the placebo-corrected eGFR dip induced by empagliflozin at week 4 was of 2.4 mL/min/1.73 m² for patients with CKD and 2.7 mL/min/1.73 m² for those without CKD, corresponding to a decrease from baseline of 5.2% and 3.8%, respectively. This was followed by a slower slope of eGFR decline and by a reduced rate of the composite kidney outcome with empagliflozin vs. placebo, with no difference between patients with or without CKD at baseline.109,738
Thus, with respect to the initiation of RAAS inhibitors, ARNI or SGLT2 inhibitors, a transient decrease in renal function should not prompt their interruption. An increase in serum creatinine of <50% above baseline, as long as it is <266 μmol/L (3 mg/dL), or a decrease in eGFR of <10% from baseline, as long as eGFR is >25 mL/min/1.73 m², can be considered as acceptable (see section 5.3 and Supplementary Table 8). Also, with respect to diuretic therapy, small and transient rises in serum creatinine during treatment of acute HF are not associated with poorer outcomes when the patient is free of congestion.108,109,461-463,472,730,738-741
Randomized trials have shown that patients with HF and concomitant CKD are at higher risk of events but the beneficial effects of MT are similar, if not greater, than in the patients with normal renal function.206,472,742,743 Beta-blockers reduce mortality in HFrEF patients with moderate (eGFR 45–59 mL/min/1.73 m²) and moderately severe (eGFR 30–44 mL/min/1.73 m²) renal dysfunction, whereas limited evidence is available regarding patients with severe renal impairment (eGFR <30 mL/min/1.73 m²).744 Sacubitril/valsartan, compared with enalapril, led to a slower decline in renal function, despite a slight increase in the urinary albumin/creatinine ratio, and improved CV outcomes to a similar extent in patients with CKD vs. the others in PARADIGM-HF.127 SGLT2 inhibitors lead to a slower decline in renal function, compared with placebo, both in patients with HFrEF and in those with CKD.108,109,738,739,745 The improvement in cardiac output after CRT or LVAD implantation may be associated with, at least, a transient improvement in renal function.472,746,747 The benefits of ICDs may be reduced in patients with severe renal dysfunction because of the competing risk of non-arrhythmic causes of death.748-750
There is little direct evidence to support any recommendations for treatment of HF patients with severe CKD as to date, RCTs excluded patients with advanced stages of CKD, i.e. eGFR <30 mL/min/1.73 m2 (Supplementary Table 23). Cut-off values for inclusion were lower in recent trials: 25 mL/min/1.73 m2 in DAPA-CKD, 20 mL/min/1.73 m² in EMPEROR-Reduced and GALACTIC-HF, and 15 mL/min/1.73 m2 in VICTORIA, respectively.109,141,738,739,751 Despite differences in baseline characteristics between patients with severely impaired renal function and the others, no interaction between drug effects and renal function was noted in subgroup analysis of these trials.109,141,739,751
13.7 Electrolyte disorders: hypokalaemia, hyperkalaemia, hyponatraemia, hypochloraemia
Electrolyte disturbances are frequent in patients with HF and may often be iatrogenic.752 Serum potassium levels have a U-shaped relation with mortality with the lowest risk of death within a relatively narrow range of 4 to 5 mmol/L.753-759
Hypokalaemia is defined as serum potassium <3.5 mmol/L and may occur in up to 50% of patients with HF.760 Hypokalaemia is often induced by loop and thiazide diuretic administration. It may cause lethal ventricular arrhythmias and increase CV mortality. Its treatment includes the use of RAAS inhibitors, potassium-sparing diuretics, and prescription of oral potassium supplements (i.e. potassium chloride tablets). When oral administration is not possible, potassium supplementation by infusion may be necessary (20 to 40 mmol of potassium in 250–1000 mL of normal saline). Potassium-rich solution should be infused at slow rate through a large vein using a venous catheter.
Hyperkalaemia is defined as serum potassium >5 mmol/L and can be classified as mild (>5.0 to <5.5 mmol/L), moderate (5.5 to 6.0 mmol/L), or severe (>6.0 mmol/L).761 It is associated with an increased risk of hospitalization and death.753,754,756,757,762,763 Hyperkalaemia can be associated with the administration of RAAS inhibitors, CKD and increased absorption.761 Among patients with HF, the prevalence of hyperkalaemia at any given time among patients with HF appears to be less than 5%,758 but the incidence is much higher, at up to 40% in chronic HF and 73% in CKD over follow-up durations of approximately 1 year.754,757,758,764-766 In PARADIGM-HF, treatment with sacubitril/valsartan was associated with lower risk of severe hyperkalaemia, compared with enalapril.128 Life-threatening hyperkalaemia requires immediate treatment with a combination of calcium carbonate and/or sodium bicarbonate, insulin, with or without glucose, and beta adrenoceptor agonists [e.g. salbutamol, off-label use in some European Union (EU) countries]. These agents favour potassium entry into the cells and do not increase potassium excretion. Thus, they only provide temporary benefit and rebound hyperkalaemia can occur after few hours. Loop diuretics can be administered to facilitate potassium loss.
Potassium binders bind to potassium in the gastrointestinal tract reducing its absorption. They can be used for acute and chronic potassium lowering. They include sodium polystyrene sulfonate, calcium polystyrene sulfonate, and the much better tolerated patiromer sorbitex calcium and sodium zirconium cyclosilicate (SZC). Sodium polystyrene sulfonate is still indicated in anuric or severely oliguric patients, but it should not be used in the medium or long term as it may cause severe gastrointestinal side effects, including bowel necrosis.761 Patiromer or SZC increase faecal potassium excretion and act mainly in the colon. Both compounds are effective in normalizing elevated potassium levels, maintaining normokalaemia over time and preventing the recurrence of hyperkalaemia and can be considered for treatment of hyperkalaemia767-769 (see Supplementary Table 24).
Renal dysfunction and hyperkalaemia are the major causes of underuse of RAAS inhibitors, particularly MRA, in clinical practice.343,754,759,770-772 Administration of the potassium-lowering agents, patiromer or SZC, may allow their initiation or uptitration in a larger proportion of patients. This hypothesis was tested in double-blind, placebo-controlled, randomized trials with patiromer or placebo administration to patients with CKD and hyperkalaemia, or discontinuation of RAAS inhibitors for hyperkalaemia, and with an indication for spironolactone for HF and/or resistant hypertension. Patiromer was more likely to lower serum potassium and decreased episodes of hyperkalaemia than spironolactone initiation and uptitration.773-776 The ongoing RCT DIAMOND (NCT03888066) is testing the impact on clinical outcomes of a strategy based on patiromer administration, compared with placebo, in patients with HFrEF who are hyperkalaemic while on RAAS inhibitors or with a history of hyperkalaemia with subsequent reduction or discontinuation of a RAAS inhibitor777,778 (see Supplementary text 13.1).
Hyponatraemia is defined as a serum sodium concentration lower than 136 mmol/L. It is common in HF and may be present in up to 30% of patients admitted to hospital with HF. It reflects neurohormonal activation and is a powerful independent marker of poor outcomes in patients with acute or chronic HF.779,780
Severe hyponatraemia may cause neurologic symptoms (seizures, obtundation, delirium) due to cerebral oedema and may require immediate treatment with hypertonic saline with serum sodium increases by 1–2 mmol/L per hour, though less than 8 mmol/L in 24 h as a more rapid correction increases the risk of myelinolysis. Intravenous treatment is not required when hyponatraemia is less severe, e.g. >124 mmol/L, and in the absence of symptoms. As the pathogenesis of hyponatraemia in HF is dilutional, e.g. caused by water retention induced by increased vasopressin secretion, treatment is based on water restriction or vasopressin antagonists. Fluid restrictions to less than 800–1000 mL/day may be indicated to achieve a negative water balance and treat hyponatraemia. Water restriction was associated with improved QOL in a small, randomized study but with only slight increases in serum sodium in an observational registry.781,782 Tolvaptan, an orally active selective arginine vasopressin V2 receptor antagonist, can be considered to increase serum sodium and diuresis in patients with persistent hyponatraemia and congestion. However, no effects on outcomes have been shown in RCTs783-786 (see Supplementary text 13.1). The infusion of hypertonic saline combined with loop diuretics was associated with an increase in serum sodium levels and greater diuretic efficacy in small trials and observational studies.787-789
Hypochloraemia (<96 mmol/L) is a powerful independent predictor of mortality in patients with acute and chronic HF.440,790-793 Serum chloride may have a direct role in the control of renin secretion and the response to loop or thiazide diuretics.440,794 The carbonic anhydrase inhibitor acetazolamide increases chloride reabsorption causing a greater bicarbonate and sodium excretion in the proximal tubule of the nephron. It can increase serum chloride levels and diuresis in patients with severe HF at risk of diuretic resistance.145,795 It is currently being tested in a multicentre randomized study in decompensated HF.470
13.8 Lung disease, sleep-disordered breathing
Overall, COPD affects about 20% of patients with HF and has a major impact on symptoms and outcomes.796–798 Due to the overlap in symptoms and signs, the differentiation between HF and COPD may be difficult. Pulmonary function testing with spirometry is recommended as the first diagnostic tool and should be considered in patients with suspected COPD. For adequate interpretation, it should be performed in stable and euvolaemic patients to avoid congestion related to obstructive pulmonary function patterns. If there is uncertainty about the reversibility of airflow obstruction, pneumology referral for more sophisticated tests (bronchodilatatory test, bronchial provocation tests, diffusion capacity) is warranted.799,800
Treatment of HF is generally well tolerated in COPD.801 Beta-blockers can worsen pulmonary function in individual patients but are not contraindicated in either COPD or asthma, as stated in the Global initiative for chronic Obstructive Lung Disease (GOLD) and the Global INitiative for Asthma (GINA), respectively.802,803 GINA states that asthma should not be regarded as an absolute contraindication to the use of cardioselective beta-blockers (bisoprolol, metoprolol succinate, or nebivolol) with consideration of relative risks and benefits. In clinical practice, starting with low doses of cardioselective beta-blockers combined with close monitoring for signs of airway obstruction (wheezing, shortness of breath with lengthening of the expiration) should be encouraged. Although not tested in HF patients, inhaled corticosteroids and beta-adrenergic agonists do not seem to increase CV events, including HF, in patients at high risk.804,805 Moreover, optimal COPD management can improve cardiac function.806
Sleep-disordered breathing occurs in more than one third of patients with HF and is even more prevalent in patients with AHF. The most common types are: central sleep apnoea (CSA, similar to Cheyne-Stokes respiration), OSA, and a mixed pattern of the two. CSA and OSA have been shown to be associated with a worse prognosis in HF. OSA is associated with an increased risk of incident HF in men. CSA is the most common form of sleep-disordered breathing in HFrEF, and HFrEF is the most common cause of CSA.807,808
Patients with HF can be investigated for sleep-disordered breathing. History taking should involve partners. Questionnaires are instrumental in identifying patients at risk. Home monitoring can usually identify and distinguish the type of sleep apnoea. However, overnight polysomnography remains the definitive investigation.808 The use of adaptive servo-ventilation in patients with HFrEF and predominantly CSA is not recommended, based on the results of SERVE-HF, which was neutral regarding the composite primary endpoint of death from any cause or lifesaving CV intervention, but showed an increase in both all-cause mortality and CV mortality with adaptive servo-ventilation.809 Transvenous phrenic nerve stimulation was tested in a prospective, multicentre, randomized trial involving 151 patients with CSA.810 The primary efficacy endpoint was the reduction of the apnoea-hypopnoea index from baseline to 6 months and was achieved by a larger percentage of patients with the active treatment. Other measurements of sleep quality and QOL were improved and no difference in any safety endpoint was found between active treatment and control.810 Similar results were observed in the 96 patients with HF.811
Patients with HFrEF being considered for a sleep-disordered breathing treatment with positive pressure airway mask must undergo formal sleep study to document the predominant type of sleep apnoea (central vs. obstructive). When sleep-disordered breathing is caused by OSA, nocturnal hypoxaemia can be treated with nocturnal oxygen supplementation, continuous positive airway pressure, bi-level positive airway pressure, and adaptive servo-ventilation. However, none of these interventions has been shown to have beneficial effects on outcomes in HF.808 When sleep-disordered breathing is caused by CSA, positive pressure airway masks are contraindicated in HFrEF patients.809 In these patients, implantable phrenic nerve stimulation can be considered for symptomatic relief.
13.9 Hyperlipidaemia and lipid-modifying therapy
Two large RCTs, including mainly patients with HFrEF, as well as a meta-analysis of 24 RCTs, showed no benefit of statin treatment on CV mortality or stroke in patients with HFrEF.812,813 A reduction in HF hospitalizations as well as a small reduction in MI was observed in a meta-analysis of the CORONA and GISSI-HF trials.814-816 Based on current evidence, routine administration of statins in patients with HF without other indications for their use (e.g. CAD) is not recommended. Because there is no evidence of harm in patients on statin treatment after the occurrence of HF, there is no need for statin discontinuation for patients already treated.
13.10 Gout and arthritis
Hyperuricemia is a common finding in patients with CHF with a prevalence up to 50%.817,818 Hyperuricemia may be caused or aggravated by diuretic treatment and it is related to symptoms, exercise capacity, severity of diastolic dysfunction and long-term prognosis.818,819 For every 1 mg/dL increase in serum uric acid levels the risk of all-cause mortality and of HF hospitalization increases by 4% and 28%, respectively.820 Both febuxostat and allopurinol reduce uric acid levels. However, allopurinol was associated with a lower rate of all-cause death and CV death, compared with febuxostat, in a prospective, multicentre, double-blind, non-inferiority trial enrolling 6190 patients with gout and CV disease, 20% with HF, with a median follow-up of 32 months.821 Allopurinol is therefore recommended as the first-choice urate-lowering drug in HF patients with no contraindication. There is no evidence that uric acid-lowering treatment has beneficial effects on LV function, symptoms or outcomes of patients with HF.822-824
With respect to treatment of acute gout attacks, non-steroidal anti-inflammatory drugs (NSAIDs) can worsen renal function and precipitate acute HF decompensation. Colchicine should be preferred as it is associated with less side effects.825 However, it, too, should be used with caution in patients with severe renal dysfunction and is contraindicated in patients on dialysis. An increase in ventricular vulnerability was shown in experimental models.826
Arthritis is a common comorbidity and is a common cause of both self-taken and prescribed NSAIDs. These agents are relatively contraindicated as they may precipitate acute decompensation in patients with HF.827 Rheumatoid arthritis is associated with a two- to three-fold increase in the risk of HF and this increased risk is independent of ischaemic heart disease, suggesting a direct role in HF pathophysiology.828,829 The safety of disease-modifying drugs used for the treatment of rheumatoid arthritis has not been established in HF. High doses of anti-tumour necrosis factor alpha agents were associated with worsening HF in initial trials and should be used with caution. No adverse effects were noted with lower doses.830-832
13.11 Erectile dysfunction
Erectile dysfunction is a serious problem in HF patients due to its association with CV risk factors, comorbidities (e.g. diabetes), life-style (e.g. inactivity), and treatment (e.g. drugs).833 In the general population, the prevalence of erectile dysfunction is estimated to be 50% in men aged ≥60, but erectile dysfunction can be present in up to 81% of cardiac patients across different cultures and ethnic groups.834 Optimal assessment should include both questions assessing the presence of erectile dysfunction and factors that can be related to erectile dysfunction. Numerous classes of CV drugs, particularly diuretics and beta-blockers, have been implicated causing erectile dysfunction. However, the relationships between many contemporary CV drugs and erectile dysfunction is not clear.835 For the treatment of erectile dysfunction, phosphodiesterase type 5 inhibitors are generally safe and effective in patients with compensated HF.835,836 No studies have shown one agent to be more effective or safer than the others. However, phosphodiesterase type 5 inhibitors should not be used in patients receiving nitrates and nitrates should not be administered to patients within 24 h of sildenafil or vardenafil administration or within 48 h of tadalafil administration.835
13.12 Depression
Depression affects 20% of patients with HF and is severe in half of them. Its occurrence is higher in women and it is associated with worse clinical status and a poor prognosis.837–839 Screening using a validated questionnaire is recommended when there is a clinical suspicion of depression. The Beck Depression Inventory and Cardiac Depression Scale are the tools formally validated for the assessment of depression in patients with HF. Other questionnaires (e.g. Geriatric Depression Scale, Hamilton Depression Scale, Hospital Anxiety and Depression Scale) can also be used.838,839
There is still no consensus on the best therapy for HF patients with depression. Psychosocial intervention may improve depressive symptoms but has no effect on prognosis of depressed patients with HF.840 Depressive symptoms may improve with selective serotonin reuptake inhibitors but trials specifically designed to assess the effect of these drugs in patients with HF and depression have failed to show any significant benefit over placebo on both symptoms and outcomes.841,842 Interestingly, patients improved also in the placebo arm showing the importance of better care in these patients. Both trials showed the safety of sertraline and escitalopram, respectively.841,842 Tricyclic antidepressants should be avoided for the treatment of depression in HF as they may cause hypotension, worsening HF, and arrhythmias.838,839
13.13 Cancer
HF occurs in patients with cancer as a result of the interaction among anticancer therapy, cancer itself, and patients’ CV background (risk factors and coexisting CV disease).843-847 Several anticancer therapies may cause HF directly, thorough their cardiotoxic effects (Table 23), or, indirectly, through other mechanisms, such as myocarditis, ischaemia, systemic or pulmonary hypertension, arrhythmias or valve disease.845,846,848-853 HF, in turn, may affect cancer outcomes by depriving patients of effective anticancer therapies.700 Some epidemiological and experimental evidence suggests a further reciprocal interaction between cancer and HF with some, though not all, studies showing a higher incidence rate of cancer in patients with HF.854-859
| Cancer therapy | Indication |
|
Anthracycline chemotherapy (doxorubicin, epirubicin, daunorubicin, idarubicin) |
Breast cancer, lymphoma, acute leukaemia, sarcoma |
|
HER2-targeted therapies (trastuzumab, pertuzumab, trastuzumab emtansine T-DM1, lapatinib, neratinib, tucatinib) |
HER2+ breast cancer HER2+ gastric cancer |
|
VEGF inhibitors TKIs (sunitinib, pazopanib, sorafenib, axitinib, tivozanib, cabozantinib, regorafenib, lenvatinib, vandetinib) and antibodies (bevacizumab, ramucirumab) |
VEGF TKIs: renal cancer, hepatocellular cancer, thyroid cancer, colon cancer, sarcoma, GIST Antibodies: breast cancer, ovarian cancer, gastric cancer, gastro-oesophageal cancer, colon cancer |
|
Multi-targeted kinase inhibitors: second and third generation BCR-ABL TKIs (ponatinib, nilotinib, dasatinib, bosutinib) |
Chronic myeloid leukaemia |
|
Proteasome inhibitors (carfilzomib, bortezomib, ixazomib) Immunomodulatory drugs (lenalidomide, pomalidomide) |
Multiple myeloma |
|
Combination RAF and MEK inhibitors (dabrafenib+trametinib, vemurafenib+cobimetinib, encorafenib+ binimetinib) |
RAF mutant melanoma |
|
Androgen deprivation therapies GnRH agonists (goserelin, leuprorelin) Antiandrogrens (abiraterone) |
Prostate cancer, breast cancer |
|
Immune checkpoint inhibitors: anti-programmed cell death 1 inhibitors (nivolumab, pembrolizumab) anti-cytotoxic T-lymphocyte-associated protein 4 inhibitor (ipilimumab) anti-programmed death-ligand 1 inhibitors (avelumab, atezolizumab, durvalumab) |
Melanoma (metastatic and adjuvant) Metastatic renal cancer, non-small cell lung cancer, small cell lung cancer, refractory Hodgkin’s lymphoma, metastatic triple negative breast cancer, metastatic urothelial cancer, liver cancer, MMR-deficient cancer |
- GIST = gastrointestinal stromal tumour; GnRH = gonadotropin-releasing hormone; HER2 = human epidermal growth factor receptor 2; MEK = mitogen-activated protein kinase; MMR = mismatch repair; TKI = tyrosine kinase inhibitor; VEGF = vascular endothelial growth factor.
The prevention of HF in patients with cancer undergoing potential cardiotoxic therapies requires careful patient’s assessment and management before, during, and after cancer therapy, preferably in the context of an integrated Cardio-Oncology service (Figure 18).846,860,861 A CV baseline risk assessment for all patients scheduled to receive potentially cardiotoxic cancer therapies using the HFA-ICOS risk assessment is advisable.847 Baseline CV risk assessment forms have been developed for different potentially cardiotoxic cancer therapies. History of HF or CMP characterizes patients as being at very high risk or at high risk for all cancer therapies, except anti-androgen treatments for prostate cancer. An LVEF <50% is an additional factor for high-risk patients and elevated levels of NPs or troponin at baseline are additional criteria of medium risk for most of the cancer treatments.847
During cancer treatment with potential cardiotoxic therapies, LV systolic function can be monitored through echocardiography. Chemotherapy should be reconsidered and treatment with an ACE-I and a beta-blocker (preferably carvedilol) should be started in patients who develop LV systolic dysfunction, defined as 10% or more absolute reduction in LVEF to a value below 50%.845,862-865 Global longitudinal strain can detect cardiac dysfunction at an earlier stage.866,867 A ≥12% relative reduction in global longitudinal strain was compared with an LVEF decline in a prospective randomized trial in high-risk patients undergoing potentially cardiotoxic chemotherapy. Compared to treatment based on LVEF, treatment based on changes in global longitudinal strain led to the same decrease in LVEF (primary endpoint) but with fewer patients who developed cardiac dysfunction at the end of the study, thus suggesting usefulness of global longitudinal strain for the early detection of cardiotoxicity.868 Promising results for the early detection of cardiac dysfunction have also been obtained through monitoring of biomarkers, such as NPs and troponin.869,870 Patients on immunotherapy with immune checkpoint inhibitors are at increased risk of myocarditis and should be monitored for related symptoms and signs and by weekly assessment of cardiac troponin during at least the first 6 weeks of therapy and managed accordingly.871
Timing of the imaging procedures and biomarkers assessment depend on the anticancer treatment and patient’s risk profile (Figure 18).866 In general, all patients scheduled for potential cardiotoxic therapies must undergo a baseline evaluation that would define the level of risk for cardiotoxicity (low, medium, or high) and the intensity of monitoring and follow-up during and after cancer treatment.866 Cancer survivors exposed to potentially cardiotoxic therapies should be periodically monitored in the long term as HF may develop several years after cancer therapy.866,872
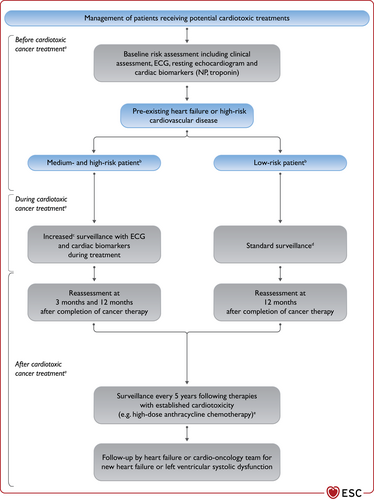
Management of patients with cancer and heart failure. ECG = electrocardiogram; HER2 = human epidermal growth factor receptor 2; HF = heart failure; HFA = Heart Failure Association; ICOS = International Cardio-Oncology Society; MEK = mitogen-activated protein kinase; NP = natriuretic peptide; VEGF = vascular endothelial growth factor. aAnthracycline chemotherapy, trastuzumab and HER2 targeted therapies, VEGF inhibitors, proteasome inhibitors, combination RAF+MEK inhibitors. bLow, medium and high risk may be calculated using the HFA-ICOS baseline cardiovascular risk proformas.847 cIncreased surveillance is intended between 1 and 4 weeks. dStandard surveillance is intended every 3 months. e5 yearly surveillance at follow-up = clinical review every 5 years with history, examination, NP and troponin levels, and echocardiogram.866
Recommendations for the management of patients with cancer and heart failure
| Recommendations | Classa | Levelb |
| It is recommended that cancer patients at increased risk for cardiotoxicity, defined by a history or risk factors of CV disease, previous cardiotoxicity or exposure to cardiotoxic agents, undergo CV evaluation before scheduled anticancer therapy, preferably by a cardiologist with experience/interest in Cardio-Oncology. | I | C |
| Treatment with an ACE-I and a beta-blocker (preferably carvedilol) should be considered in cancer patients developing LV systolic dysfunction, defined as a 10% or more decrease in LVEF and to a value lower than 50%, during anthracycline chemotherapy.862,863 | IIa | B |
| A baseline CV risk assessment should be considered in all cancer patients scheduled to receive a cancer treatment with the potential to cause heart failure.847,866 | IIa | C |
- ACE-I = angiotensin-converting enzyme inhibitor; CV = cardiovascular; LV = left ventricular; LVEF = left ventricular ejection fraction.
- a Class of recommendation.
- b Level of evidence.
13.14 Infection
Infective disorders may worsen HF symptoms and be a precipitant factor for AHF.873,874 Severe sepsis and pneumonia can cause myocardial injury and depress cardiac function leading to cardiac dysfunction and HF and this risk is greater in patients with a history of HF.874-876 More recently, the coronavirus disease 2019 (COVID-19) pandemic has emerged as a major cause of morbidity and mortality as well as of HF decompensation.874,877-879 Specific guidance is available.880 General recommendations related to infections are given in Table 24.
| Patients with HF are at increased risk of infections and have poorer outcomes once infected. |
| Telemonitoring avoids the risks of infections caused by close contact. It is useful during pandemic conditions. |
| Telemonitoring may be implemented for patients’ follow-up in pandemic conditions. |
| During pandemics, HF patients should be screened for infection at the time of hospitalization, in case of urgent admissions, or before elective hospitalizations. |
| Careful assessment of fluid status, in addition to clinical signs of HF, is mandatory during hospitalization in patients with concomitant sepsis. Repeated measures of inferior vena cava diameter and collapsibility by echocardiography may be used to assess fluid status. |
| OMT (including beta-blocker, ACE-I, ARB or ARNI, MRA and SGLT2 inhibitors), should be continued in chronic HF patients whenever BP and haemodynamic conditions permit and considering drug interaction with infection related therapies and side effect profile. |
- ACE-I = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BP = blood pressure; HF = heart failure; MRA = mineralocorticoid receptor antagonist; OMT = optimal medical therapy; SGLT2 = sodium-glucose co-transporter 2.
Influenza vaccination is associated with a reduced risk of all-cause death in patients with HF in observational studies and retrospective analyses.881-883 Influenza and pneumococcal vaccination, as well as COVID-19 vaccination, when available, should be considered in patients with HF.880,884
14 Special conditions
14.1 Pregnancy
14.1.1 Pregnancy in pre-existing heart failure
Women with pre-existing HF have a higher risk of pregnancy-related CV complications including HF decompensation. Moderate- and high-risk patients according to the modified World Health Organization (mWHO) class III–IV should be referred to a specialist centre with a multidisciplinary Pregnancy Heart Team.885 An algorithm for the management of HF patients before and during pregnancy is reported in Figure 19.
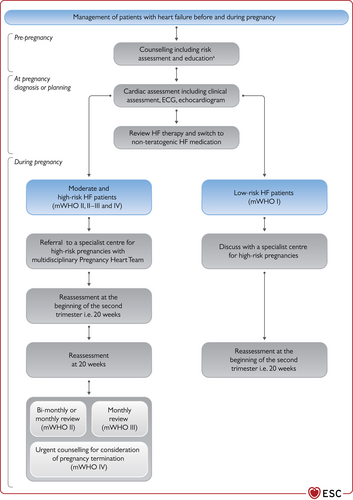
Management of patients with heart failure before and during pregnancy. ECG = electrocardiogram; HF = heart failure; mWHO = modified World Health Organization. aAdvice regarding contraception, HF medication, to contact HF specialist when planning pregnancy.
Pre-pregnancy management includes the modification of existing HF medications to avoid foetal harm. ACE-Is, ARBs, ARNI, MRAs, ivabradine, and SGLT2 inhibitors are contraindicated and should be stopped prior to conception with close clinical and echocardiographic monitoring. Beta-blockers should be continued and switched to beta-1-selective blockers (bisoprolol, metoprolol succinate). Hydralazine, oral nitrates and methyldopa can be started if required. Therapeutic anticoagulation with low-molecular-weight heparin (LMWH), in the first and last trimesters, and VKAs, with the usual target international normalized ratios (INRs) or LMWH for the second trimester, is recommended for patients with HF and AF. DOACs should be avoided.885
Assessment of patients with HF in pre-pregnancy or at presentation with a new pregnancy should include a clinical assessment (symptoms, clinical examination, BP, SaO2), ECG and resting echocardiography. The modalities of delivery should be planned by cardiologists, obstetricians, and anaesthesiologists around 35 weeks in a multidisciplinary Pregnancy Heart Team. Bimonthly assessments for women in mWHO II–III, and monthly assessments for women with pre-existing HF in mWHO III, must be performed. Women with advanced HF (LVEF <30%, NYHA class III–IV) in mWHO IV who are pregnant can be referred to a specialist centre for counselling regarding any consideration of termination of pregnancy. The decision regarding modalities of delivery can be planned by cardiologists, obstetricians, and anaesthesiologists around 35 weeks in a multidisciplinary Pregnancy Heart Team and discussed with the patient.885
14.1.2 New heart failure presenting during pregnancy
The increased demands on ventricular function due to the increased circulating volume and cardiac output of pregnancy can unmask pre-existing, but previously undiagnosed, causes of HF such as CMPs and valve diseases.886 Symptoms are more likely to occur in the second trimester when the demand for an increased cardiac output is the highest. Severe emotionally stressful episodes during pregnancy and delivery can also cause Takotsubo syndrome.885,886
PPCM presents as HF secondary to LV systolic dysfunction, usually shown by an LVEF <45%, occurring towards the end of pregnancy (third trimester) or in the months following delivery without any other identifiable cause. The majority of PPCM cases are diagnosed in post-partum. The prevalence ranges from 1:100 in Nigeria to 1:1000 in South Africa and 1:1500 in Germany.644 Prospective large cohort studies report a 6-month mortality ranging from 2.0% in Germany to 12.6% in a 206 PPCM patient cohort from South Africa.644
PPCM frequently presents with acute HF but may also present with ventricular arrhythmias and/or cardiac arrest. An LVEF <30%, marked LV dilatation, LV end-diastolic diameter >6.0 cm, and RV involvement are associated with adverse outcomes.644 Cardiac recovery may occur in the first 3–6 months though it may be delayed to up to 2 years. Recovery rates vary among regions, from 75% to less than 50%.887-889
Assessment and management of pregnant patients presenting with HF depends upon the clinical setting and severity of presentation. Detailed cardiac assessment with echocardiography, NP levels, foetal ultrasound and foetal monitoring is recommended. In cases of new HF or if there is diagnostic uncertainty non-contrast CMR may be considered.
Milder cases can be treated with oral diuretics, beta-blockers, hydralazine and oral nitrates. Pregnant women presenting with signs of acute HF require urgent hospital admission. In case of PPCM presenting with severe HF and cardiogenic shock requiring inotropic or vasopressor support, transfer to an advanced HF centre, where ECMO, LVAD and/or cardiac transplantation can be performed, is recommended. Urgent delivery by caesarean section (irrespective of gestation) should be considered with MCS immediately available.
Adrenergic agents (dobutamine, adrenaline) may have detrimental effects.890 When a PPCM patient is haemodynamically unstable, levosimendan or MCS may be considered. LVAD implantation as a BTT or BTR should be considered in refractory cases of cardiogenic shock.644 Bromocriptine has been proposed for patients with acute PPCM to reduce the production of a cleaved 16 kDa prolactin fragment, which may contribute to the pathophysiology of PPCM. Bromocriptine was tested in a randomized trial in 63 patients comparing its long-term, 8 weeks, with its short-term, 1-week, treatment. It was associated with recovery of LV function, with no difference between the two regimens and in line with the results of a previous international PPCM registry.891,892 Bromocriptine may be considered for treatment of PPCM. Untoward effects of treatment, including deep venous thrombosis and cessation of lactation, must be considered if it is initiated. It should therefore be accompanied by prophylactic (or therapeutic) anticoagulation.
14.2 Cardiomyopathies
14.2.1 Epidemiology and diagnosis
CMPs can be either inherited (genetic/familial) and/or acquired. They can also be accelerated by disease modifiers.893-895 They are a heterogeneous group of diseases and are major causes of HF.896 DCM has an estimated prevalence of 1 in 250 to 1 in 500 of the general population, HCM ranges between 1 in 500 to 1 in 5000, and AC is estimated to be present in around 1 in 1000 to 1 in 5000 persons.896,897
Direct causes of CMPs include pathogenic gene variants (mutations), toxins, auto-immunity, storage diseases, infections, and tachyarrhythmias. Disease modifiers, conditions that may aggravate or trigger a CMP, include epigenetic factors and acquired modifiers, such as pregnancy and most CV comorbidities. It is important to consider this key interaction between genetic and acquired causes during the diagnostic workup.898 Identification of an acquired cause of the CMP does not exclude an underlying pathogenic gene variant, whereas the latter may require an additional acquired cause and/or disease modifier to become manifest clinically. The commonest causes and disease modifiers are shown in Table 25.
| Cause | Disease modifier | Phenotype | |
|---|---|---|---|
| Genetic mutations | |||
| LMNA | x | DCM | |
| TTN | x | x | DCM, (HCM) |
| RBM20 | x | DCM | |
| MYH7 | x | DCM, HCM | |
| MYPC | x | DCM, HCM | |
| TNNT | x | DCM, HCM | |
| PLN | x | DCM, HCM, AC | |
| DSP | x | x | AC, DCM, myocarditis |
| SCN5a | x | x | AC, (DCM) |
| Tropomyosin-1 | x | DCM | |
| Haemochromatosis (HFE gene, C282Y) | x | HCM, DCM | |
| Galactosidase-A (Fabry disease) | x | HCM | |
| Neuromuscular disorders | |||
| Duchenne muscular dystrophy, Becker muscular dystrophy, myotonic dystrophy | x | DCM | |
| Syndromic disorders | |||
| Mitochondrial X-linked mutations | x | DCM | |
| Acquired diseases | |||
| Infection (viruses) | x | x | Myocarditis, DCM |
| Immuno-mediated diseases (rheumathoid arthritis, systemic lupus erythematosus, dermatomyositis) | x | x | Myocarditis, DCM |
| Toxic (alcohol, amphetamines, cocaine) | x | x | DCM, myocarditis |
| Drugs (anthracyclines, trastuzumab, immune checkpoint inhibitors) | x | x | DCM, myocarditis |
| Overload (haemochromoatosis) | x | x | HCM, DCM |
| Peripartum (pregnancy) | x | x | DCM |
| Comorbidities with possible interactions with the gene mutations and an effect on phenotype and outcome | |||
| Tachy-arrhythmias | x | x | DCM |
| Diabetes mellitus | x | x | DCM, HCM |
| Hypertension | x | x | DCM, HCM |
| Hypo- and hyperthyroidism | x | DCM, HCM, myocarditis | |
- AC = arrhythmogenic cardiomyopathy; DCM = dilated cardiomyopathy; DSP = desmoplakin; HCM = hypertrophic cardiomyopathy; LMNA = lamin A/C; MYH7 (gene) = myosin heavy chain 7; MYPC = myosin-binding protein C; PLN = phospholamban; RBM20 = ribonucleic acid binding motif 20; SCN5a = sodium channel alpha unit 5; TTN = titin; TNNT = troponin-T.
The key elements of the diagnostic workup for all patients with HF and CMP are reported in Table 26.893,895,896,899,900 Specifics aspects of diagnosis and treatment are summarized in Tables 27–29. Clinical history, laboratory tests, and imaging are the first-line investigations. Echocardiography is central for the diagnosis and monitoring of HCM, DCM, and AC. CMR imaging provides more detailed morphological and prognostic information and should be performed at baseline. The prevalence of gene mutations may vary according to the morphological phenotype or the underlying acquired cause. Gene mutations occur in up to 40% of DCM, 60% of HCM, and 15% in chemotherapy-induced, alcoholic or peripartum CMPs.896,899,901-906 The prevalence of genetic mutations is over 10% also in non-familial DCM.899,907 Finding a pathogenic gene variant in a patient with CMP allows better prediction of the disease outcome and progression, may contribute to the indications for device implantation and inform genetic counselling for families.
| History including detailed questions on any systemic disease, toxic agents (chemotherapy, alcohol, drugs), and familial history of cardiac or neuromuscular disease, or sudden cardiac death in family members at young age (<50 years). |
| Laboratory exams including cardiac and muscular enzymes, liver and renal function, haemoglobin, white blood cell count (including differential white blood cell count to detect eosinophilia), natriuretic peptides, thyroid function tests, iron status, and markers of systemic auto-immune disease (hsCRP, anti-nuclear antibodies, soluble IL-2 receptor). |
| Standard 12-lead ECG and echocardiography to detect arrhythmias and assess cardiac structure and function and concomitant abnormalities. |
| Invasive coronary angiography or CTCA to rule out significant CAD in patients with cardiac dysfunction. |
| CMR imaging with T1 and T2 sequencing and LGE to visualize structural changes, storage, infiltration, inflammation, fibrosis and scarring. |
| Genetic counselling and genetic testing should be performed depending on age, family history, cardiac phenotype. |
| 24 or 48-hour ambulatory ECG monitoring to detect atrial and ventricular arrhythmias. |
- CAD = coronary artery disease; CMR = cardiac magnetic resonance; CTCA = computed tomography coronary angiography; ECG = electrocardiogram; hsCRP = high-sensitivity C-reactive protein; IL-2 = interleukin-2; LGE = late gadolinium enhancement.
Endomyocardial biopsy (EMB) with immunohistochemical quantification of inflammatory cells remains the gold standard investigation for the identification of cardiac inflammation. It may confirm the diagnosis of auto-immune disease in patients with DCM and suspected giant cell myocarditis, eosinophilic myocarditis, vasculitis and sarcoidosis.894,908 It may also help for the diagnosis of storage diseases, including amyloid or Fabry disease, if imaging or genetic testing does not provide a definitive diagnosis (see also section 14.6). EMB might be considered also in HCM if genetic or acquired causes cannot be identified. The risks and benefits of EBM should be evaluated and this procedure should be reserved for specific situations where its results may affect treatment.
14.2.2 Treatment
The current pharmacological treatment of HF in DCM, HCM, or AC patients does not differ from general HF management, except for peculiar aspects reported in Tables 27–29. A pilot randomized study, TRED-HF, investigated the possibility of withdrawing medical treatment in those patients with non-ischaemic DCM who had had partial to complete recovery of LVEF (>40%). However, relapse of DCM within 6 months was observed in 44% of patients, and rapid LV remodelling with early tissue and functional changes, even amongst patients who did not relapse, was found.272,909
| Diagnostic criteria and definitions895,896 |
|
| Genetic counselling and testing893,895,899,917 |
|
| Endomyocardial biopsy97,908,918-920 |
|
| Therapeutic options896,918 |
| HF treatment for HFrEF (see sections 5 and 6) |
| LMNA, RBM20, PLN and FLN mutation. Higher risk of sudden cardiac death: early indication for primary prevention by ICD implantation should be considered (guided by risk factors as detailed).921 |
| TTN mutation. Higher rate of LV reverse remodeling (in up to 70%), but a higher risk of atrial and ventricular tachyarrhythmias. |
| Lyme disease (Borrelia). Treatment with doxycycline. |
| Chagas disease (Trypanosoma cruzi). Specific treatment according to current recommendations.922,923 |
| Auto-immune/inflammatory. Consider immunosuppressive therapy in giant cell myocarditis, eosinophilic myocarditis, sarcoidosis or vasculitis, and in highly selected patients with increased cardiac inflammation of unknown origin based upon multidisciplinary counselling (cardiology and immunology). |
- BAG3 = Bcl2-associated athanogene 3; CAD = coronary artery disease; CMR = cardiac magnetic resonance; CMV = cytomegalovirus; DCM = dilated cardiomyopathy; DNA = deoxyribonucleic acid; ECG = electrocardiogram; FLN = filamin; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; HHV = human herpes virus; HIV = human immunodeficiency virus; HLA-DR = human leukocyte antigen-DR isotype; HNDC = hypokinetic non-dilated cardiomyopathy; ICD = implantable cardioverter-defibrillator; LMNA = lamin A/C; LV = left ventricular; LVEF = left ventricular ejection fraction; MHC = myosin heavy chain; MYPC = myosin-binding protein C; mRNA = messenger ribonucleic acid; NSVT = non-sustained ventricular tachycardia; PCR = polymerase chain reaction; PLN = phospholamban; RBM20 = ribonucleic acid binding motif 20; RNA = ribonucleic acid; rtPCR = reverse transcriptase polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SLE = systemic lupus erythematosus; TNNT = troponin-T; TTN = titin.
- a This list of genes is not exhaustive, and will change over time, with increasing knowledge of the pathogenicity. Contact the genetic department to ask which core panel of genes they are using.
- b Risk factors in patients with a confirmed LMNA mutation: NSVT during ambulatory ECG monitoring, LVEF <45% at first evaluation, male sex and non-missense mutations (insertion, deletion, truncations, or mutations affecting splicing).
In a phase 3, randomized, double-blind, placebo-controlled trial (EXPLORER-HCM), treatment with mavacamten improved exercise capacity, LVOT obstruction, NYHA functional class, and health status in patients with obstructive HCM. This offers the possibility of disease-specific treatment for inherited CMPs. 910
ICD implantation should be considered for patients with DCM, HCM, or AC (see section 6).896,911-913 The strength of the indication varies according to the clinical risk factors for sudden cardiac death with higher priority being given to those patients with significant LGE on CMR, younger age, or with a specific familial/genetic phenotype (Tables 27-29). Risk models for the prediction of ICD benefits were applied to the patients enrolled in DANISH and may help for the indication to ICD implantation in DCM.166,914 Treatment of HCM and AC, including indications for ICD, are detailed in previous documents.896,897,900,913,915,916
| Definition896,897,924 |
|
| Differential diagnosis |
|
| Genetic counselling and testing |
|
| Endomyocardial biopsy |
| Indication. It may be considered when the baseline clinical assessment suggests cardiac inflammation or storage disease which cannot be diagnosed by other means897 (see also Section 14.6). |
| Therapeutic options896,897,924 |
With LVOTO
|
Symptomatic without LVOTO
|
| Indication to ICD |
|
| Amyloidosis. See section 14.6 and Figure 21. |
- AV = atrio-ventricular; BAG3 = Bcl2-associated athanogene 3; ECG = electrocardiogram; HCM = hypertrophic cardiomyopathy; ICD = implantable cardioverter-defibrillator; LGE = late gadolinium enhancement; LMNA = lamin A/C; LV = left ventricular; LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract; LVOTO = left ventricular outflow tract obstruction; MHC = myosin heavy chain; MYPC = myosin-binding protein C; NSVT = non-sustained ventricular tachycardia; NYHA = New York Heart Association; OMT = optimal medical therapy; PLN = phospholamban; RMB20 = ribonucleic acid binding motif 20; RV = right ventricular; TNNT = troponin-T; TTN = titin.
- a The list of genes is not exhaustive, and will change over time, with increasing knowledge of the pathogenicity. Contact the genetic department to ask which core panel of genes they are using.
| Definition |
|
| Diagnosis934 |
| Based upon the evaluation of a combination of the genetic factors (most cases autosomal dominant desmosomal mutations), documentation of ventricular arrhythmias and imaging criteria (echocardiography and MRI) of RV dysplasia with the fibrofatty replacement either or not confirmed by EMB. Specific ECG abnormalities can be present or absent. |
| Genetic counselling/testing899,913 |
|
| Endomyocardial biopsy |
| It should be reserved to highly selected cases after all non-invasive studies have been assessed. Fibrofatty replacement with or without replacement type fibrosis at RV septal biopsies are the characteristic findings. EMB has low sensitivity for the diagnosis of AC in cases of focal distribution. |
| Therapeutic options |
|
- AC = arrhythmogenic cardiomyopathy; BAG3 = Bcl2-associated athanogene 3; CMR = cardiac magnetic resonance; DCM = dilated cardiomyopathy; DSC2 = desmocollin 2; DSG2 = desmoglein 2; DSP = desmoplakin; ECG = electrocardiogram; EMB = endomyocardial biopsy; FLN = filamin; FLNC = filamin C; HF = heart failure; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; JUP = junction plakoglobin; KCNH2 = potassium voltage-gated channel subfamily H member 2; KCNQ1 = potassium voltage-gated channel subfamily Q member 1; LDB3 = LIM domain binding 3; LMNA = lamin A/C; LV = left ventricular; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; NKX2-5 = NK2 transcription factor related, locus 5; PLN = phospholamban; PKP2 = plakophilin 2; RMB20 = ribonucleic acid binding motif 20; RV = right ventricular; SCN5A = sodium channel alpha subunit 5; TMEM43 = transmembrane protein 43; TNNT = troponin-T; TRPM4 = transient receptor potential cation channel subfamily M member 4.
14.3 Left ventricular non-compaction
LVNC is a very rare congenital CMP characterized by endomyocardial trabeculations that increase in number and prominence. In most cases, including when the condition is caused by mutations in the MYH7 or MYBPC3 gene, LVNC is inherited in an autosomal dominant pattern.645,938,939 A clear overlap exists in families with DCM and HCM phenotypes. Quite commonly individuals with features of LVNC are found in families where other affected relatives have typical HCM or DCM. Therefore, LVNC is not treated as a separate disease entity, but as a separate rare presentation of a genetic susceptibility to either HCM or DCM.940
14.4 Atrial disease
14.4.1 Definition
Atrial disease, also termed atrial failure or myopathy, can be defined as a complex of subclinical structural, electrophysiological, and functional changes that affect the atria with the potential to produce clinical consequences.607,941,942 It has been suggested that atrial disease links the pathophysiology of HF, especially HFpEF, with AF, as they often coexist, are closely inter-related and share common risk factors.607,686,943,944
14.4.2 Diagnosis
Atrial size and function can be evaluated by multimodality imaging including two- and three-dimensional echocardiography, myocardial deformation, computed tomography (CT) and CMR.945 Cardiac biomarkers, including high-sensitivity cardiac troponins and NPs, may assess pathophysiological aspects of atrial disease.946-948 The increased levels of NPs in AF may also be an indicator of an underlying atrial disease.943,949 A comprehensive characterization of atrial disease combining clinical, imaging, biochemical and molecular features is, however, still lacking.
14.4.3 Management
Atrial disease is an emerging therapeutic target in the prevention of AF, systemic thromboembolism, and perhaps HFpEF.950 As atrial disease appears to result from the intersection of shared risk factors and comorbidities predisposing to both AF and HF, diabetes mellitus, hypertension, obesity, smoking, and physical inactivity may be of paramount importance for its development.7,951 Effective management of HF and AF (see section 12.1.1), as well as treatment of mitral regurgitation (see section 12.3.3), may also be important to counteract atrial disease progression.
14.5 Myocarditis
14.5.1 Epidemiology and diagnosis
The incidence of acute myocarditis is estimated to be 1.5 million cases per year globally.952 The contribution of myocarditis as a cause of HF varies by age and region from approximately 0.5% to 4.0%.919,953 Chronic, EMB proven, inflammation can be found in 9% to 30% of adult patients with a DCM.919,954 The most frequent potential aetiologies triggering acute myocarditis in Europe are reported in Table 30.
| Infectious | |
| Viral | Parvovirus B19, human herpes virus-6, Epstein-Barr virus, enteroviruses, (coxsackievirus, adenovirus), CMV, HIV, SARS-CoV-2 |
| Others | Borrelia, Coxiella burnetii (Q-fever) |
| Systemic disease | |
| Auto-immune and others | Sarcoidosis, giant cell myocarditis, eosinophilic myocarditis, SLE, ANCA-positive vasculitis, rheumatoid arthritis, any other auto-immune disease |
| Toxic | |
| Medications | Immune check point inhibitors, anthracyclines, clozapine, adrenergic drugs, 5-fluorouracil |
| Other agents | Alcohol, amphetamines, cocaine |
- ANCA = antineutrophil cytoplasmic antibody; CMV = cytomegalovirus; HIV = human immunodeficiency virus; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SLE = systemic lupus erythematosus.
The clinical presentation of acute myocarditis may vary from mild symptoms to cardiogenic shock. Workup for the diagnosis of acute myocarditis in patients with HF is reported in Table 31 and Figure 20. Specific criteria about biopsies and CMR are reported in Tables 32 and 33.
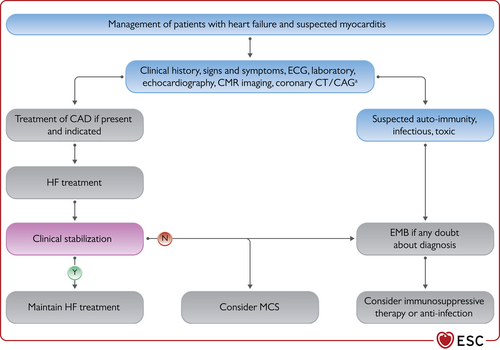
Management of patients with heart failure and acute myocarditis. ACS = acute coronary syndrome; CAD = coronary artery disease; CAG = coronary artery angiogram; CMR = cardiac magnetic resonance; CT = computed tomography; ECG = electrocardiogram; EMB = endomyocardial biopsy; HF = heart failure; MCS = mechanical circulatory support. aTo exclude CAD/ACS.
| Definition of suspected acute myocarditis | |||
| Clinical presentation + ≥1 mandatory diagnostic test being positive (by preference CMR) in the absence of significant coronary artery, valvular or congenital heart disease, or other causes. | |||
| Sensitivity | Specificity | ||
| Clinical presentation | |||
| Acute/new onset chest pain, dyspnoea, signs of left and/or right HF, and/or unexplained arrhythmias or aborted sudden death. | Low | Low | |
| Mandatory diagnostic tests | |||
| ECG | New and dynamic ST-T abnormalities, including pseudo-infarct ST segment elevation, atrial or ventricular arrhythmias, AV blocks, QRS abnormalities. | High | Low |
| Laboratory tests | Intermediate | Low | |
| Echocardiography | New structural or function abnormalities, regional wall motion abnormalities or global ventricular dysfunction without ventricular dilatation or with, generally mild, dilatation, increased wall thickness due to myocardial oedema, pericardial effusion, intracardiac thrombi, not explained by other conditions (e.g., CAD, ACS or valvular heart disease). | High | Low |
| CMR | Oedema, inflammation and fibrosis detection, quantification and localization through T1 and T2 mapping, extracellular volume assessment and LGE (see Table 33).956,957 | High | Intermediate |
| Additional diagnostic tests | |||
| Coronary angiography or CTCA | Excludes significant CAD or ACS in clinically suspected myocarditis. | High | High |
| Endomyocardial biopsy | For diagnosis and indication to specific treatment (see Table 32). | Intermediate | High |
| Cardiac PET | May be useful in patients who cannot undergo CMR or with suspected systemic autoimmune disease or cardiac sarcoidosis.920,958 | Low | Low |
| Additional laboratory test | Skeletal muscle enzymes, liver and renal function, natriuretic peptides, thyroid function tests, iron status, markers of systemic autoimmune disease. | Low | Low |
| CRP elevated in 80–90% patients.920,955 | Intermediate | Low | |
|
Low | Low | |
- ACS = acute coronary syndrome; AV = atrio-ventricular; CAD = coronary artery disease; CMR = cardiac magnetic resonance; CMV = cytomegalovirus; CRP = C-reactive protein; CTCA = computed tomography coronary angiography; ECG = electrocardiogram; EMB = endomyocardial biopsy; HF = heart failure; HIV = human immunodeficiency virus; IgG = immunoglobulin G; LGE = late gadolinium enhancement; PCR = polymerase chain reaction; PET = positron emission tomography; QRS = Q, R and S waves (combination of three of the graphical deflections); SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; ST = ST segment of the electrocardiogram; ST-T = ST segment and T wave of the electrocardiogram.
|
|
|
|
- CMR = cardiac magnetic resonance; CMV = cytomegalovirus; DNA = deoxyribonucleic acid; EMB = endomyocardial biopsy; HHV = human herpes virus; HIV = human immunodeficiency virus; HLA-DR = human leucocyte antigen-DR isotype; mRNA = messenger ribonucleic acid; PCR = polymerase chain reaction; PET = positron emission tomography; RNA = ribonucleic acid; rtPCR = reverse transcriptase polymerase chain reaction; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
|
|
- CAD = coronary artery disease; CMR = cardiac magnetic resonance; ECG = electrocardiogram; LGE = late gadolinium enhancement.
- a It takes at least 3 months before CMR signs of oedema (secondary to inflammation in the acute phase/baseline) disappear. At 6 months, T1 or T2 signs of oedema should have disappeared if inflammation would be completely absent. Still, the absence of T1 or T2 oedema does not exclude chronic low-grade inflammation.
14.5.2 Treatment
Hospitalization for at least 48 h may be useful for patients with acute myocarditis and HF, especially when troponins are elevated and when cardiac dysfunction, and/or arrhythmias are present at initial presentation.
Despite the lack of evidence in the specific setting of acute myocarditis, treatment of HFrEF is recommended in the presence of systolic LV dysfunction. Immunosuppression is only indicated in selected cases of acute myocarditis (Table 34). Once cardiac enzymes decrease, arrhythmias are absent, and cardiac systolic dysfunction is stabilized, standard HF therapy should be continued for at least 6 months (see also Figure 20).
| HF therapy should be started if LV systolic dysfunction is present at presentation and should be continued for at least 6 months upon complete functional recovery (EF >50%).919,920 |
| Immunosuppression for at least 6–12 months is required in acute myocarditis with clinical or EMB evidence of auto-immune disease, including giant cell myocarditis, vasculitis or sarcoidosis.98,918-920,954,955,962 |
| Immunosuppression is not advised on a routine basis in acute myocarditis without clinical or EMB-based evidence of auto-immune disease.918 Initial empirical administration of i.v. corticosteroids may be taken into consideration in cases of high suspicion of immune-mediated myocarditis especially if complicated by acute HF, malignant arrhythmias and/or high degree AV block.955,963 |
| Intense sporting activities should be avoided as long as symptoms, cardiac enzymes elevated or ECG/imaging abnormalities are present and last for at least 6 months since complete recovery.937 |
| Yearly follow-up for at least 4 years, with an ECG and echocardiography, is needed as acute myocarditis may lead to DCM in up to 20% of cases. |
- AV = atrio-ventricular; DCM = dilated cardiomyopathy; ECG = electrocardiogram; EF = ejection fraction; EMB = endomyocardial biopsy; HF = heart failure; i.v. = intravenous; LV = left ventricular.
Immunosuppression has been considered for treatment of patients with chronic cardiac inflammation at EMB and no evidence of active viral infection.919,920 This was associated with an improvement in cardiac function in small studies and with better outcomes in a retrospective observational study.954,964,965 Prospective trials with old or newer immunosuppressive/immunomodulatory drugs are needed. A placebo-controlled trial testing the effects of immunoadsorption with i.v. immunoglobulins on LV function is ongoing and other treatment options are being tested.920
14.6 Amyloidosis
14.6.1 Epidemiology and diagnosis
CA or amyloid cardiomyopathy is still an underdiagnosed cause of HF.896,966,967 The two most prevalent forms of CA are light chain immunoglobulin (AL) and transthyretin (ATTR) amyloidosis. ATTR includes the wild-type (>90% of cases), and the hereditary or variant type (<10% of cases). It is estimated that 6% to 16% of all patients with unexplained LVH or HFpEF at hospitalization or severe aortic stenosis undergoing aortic valve replacement, aged above 65 years, may have wtTTR-CA.968-973
Diagnosis and treatment of CA were recently reviewed.974 Age >65 years and HF along with a LV wall thickness >12 mm at echocardiography are major criteria for the suspicion of CA.974 Criteria for a suspicion of CA and to confirm diagnosis are reported in Table 35, Supplementary Table 25, and Figure 21.974,975 Cardiac imaging and EMB or extra-cardiac biopsy are needed for the diagnosis of AL-CA in patients with abnormal haematological tests (Figure 21). Technetium-labelled 99mTc-PYP or DPD or HMDP scintigraphy with planar and SPECT imaging has a specificity and positive predictive value for TTR-CA of up to 100%.976 In contrast, CMR has a sensitivity and specificity of 85% and 92%, respectively.967,977 The hereditary form should be excluded by genetic testing. EMB is the gold standard for the diagnosis of TTR-CA with nearly 100% sensitivity and specificity if specimens are collected from >4 multiple sites and tested for amyloid deposits by Congo red staining.967 However, a biopsy is not needed with a grade 2–3 positivity of scintigraphy with SPECT (Figure 21).974
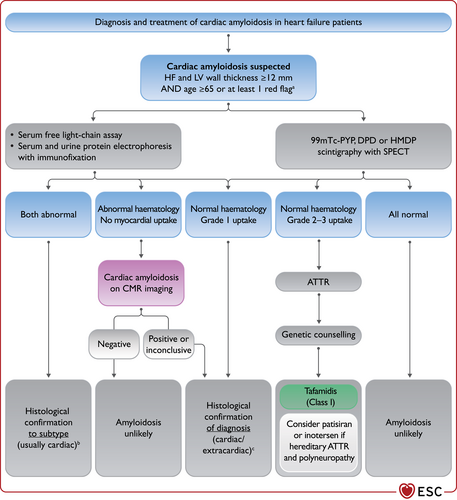
Diagnosis and treatment of cardiac amyloidosis in heart failure patients. Based on.974 ATTR = transthyretin amyloidosis; CMR = cardiac magnetic resonance; DPD = 3,3-diphosphono1,2-propanodicarboxylic acid; HF = heart failure; HMDP = hydroxymethylene diphosphonate; LV = left ventricular; SPECT = single-photon emission computed tomography; 99mTc-PYP = technetium-labelled 99mTc-pyrophosphate. aRed flags are listed in Table 35. bGenerally requires endomyocardial biopsy for a diagnosis of the cardiac subtype. cRequires biopsy that may be cardiac or abdominal.
| Type | Red Flag | TTR | AL |
|---|---|---|---|
| Extracardiac | Polyneuropathy | X | X |
| Dysautonomia | X | X | |
| Skin bruising | X | ||
| Macroglossia | X | ||
| Deafness | X | ||
| Bilateral carpal tunnel syndrome | X | ||
| Ruptured biceps tendon | X | ||
| Lumbar spinal stenosis | X | ||
| Vitreous deposits | Xa | ||
| Family history | Xa | ||
| Renal insufficiency | X | ||
| Proteinuria | X | ||
| Cardiac | Clinical Hypotension or normotensive if previously hypertensive | X | X |
|
ECG Pseudo-infarct ECG pattern Low/decreased QRS voltage to degree of LV thickness AV conduction disease |
X X X | X X X | |
| Laboratory Disproportionally elevated NT-proBNP to degree of HF Persisting elevated troponin levels | X X | X X | |
|
Echocardiography Granular sparkling of myocardium Increased right ventricular wall thickness Increased AV valve thickness Pericardial effusion Reduced longitudinal strain with apical sparing pattern |
X X X X X | X X X X X | |
|
CMR Subendocardial LGE Elevated native T1 values Increased extracellular volume Abnormal gadolinium kinetics |
X X X X |
X X X X |
- AL = light chain immunoglobulin; AV = atrio-ventricular; CA = cardiac amyloidosis; CMR = cardiac magnetic resonance; ECG = electrocardiogram; HF = heart failure; LGE = late gadolinium enhancement; LV = left ventricular; NT-proBNP = N-terminal pro-B-type natriuretic peptide; QRS = Q, R and S waves (combination of three of the graphical deflections); TTR = transthyretin.
- Modified from974.
- a Hereditary TTR-CA.
14.6.2 Therapy of amyloidosis and heart failure
Maintenance of euvolaemia is central to management but is challenging due to the markedly reduced ventricular capacitance.978 If HF symptoms are present, a loop diuretic, possibly with an MRA, may be given, but orthostatic hypotension may cause intolerance. Beta-blockers, digitalis, ACE-I, ARBs, or ARNI may not be well tolerated because of hypotension, and their place in CA treatment is unsettled. Their withdrawal must be often considered due to hypotension and/or bradycardia.974,975 CCB should be avoided as they may cause severe hypotension and fatigue, or form complexes with amyloid.967
Amyloid infiltration of the atrial wall leads to atrial myopathy and electromechanical dissociation with high embolic risk. Patients with CA and history of AF should receive anticoagulation. There is no evidence to support anticoagulation for patients in SR, yet.7,979 Amiodarone is the preferred antiarrhythmic agent.974
Therapy of AL-CA is based on treatment of the underlying haematological problem with chemotherapy or autologous stem-cell transplant.
TTR stabilization and reduction of its production are the basis of TTR-CA treatment. Liver and/or cardiac transplantation can be considered only in end-stage disease of familial TTR-CA. Tafamidis reduced all-cause mortality and CV hospitalizations in cardiac or non-cardiac biopsy-proven hereditary and wtTTR-CA, mainly in those patients with NYHA class I and II at baseline. Functional improvement occurred within 6 months, whereas the decrease in mortality took nearly 2 years to occur.980,981 Intravenous patisiran, a small RNA interfering molecule, or subcutaneous inotersen, antisense oligonucleotide against TTR, may be considered in those patients with combined hTTR-polyneuropathy and CA (Figure 21).982,983 Off-label use of diflunisal may be considered in wtTTR-CA in combination with a proton pump inhibitor.984
14.7 Iron overload cardiomyopathy
Iron overload results either from genetically determined increased intestinal iron absorption in the context of hereditary haemochromatosis (primary iron overload) or from multiple blood transfusions required for the management of haematological conditions such as beta-thalassaemia (secondary iron overload).985 In iron overload, the iron binding capacity of transferrin is saturated and non-transferrin-bound iron enters cardiomyocytes through L-type calcium channels, causing oxidative myocardial damage.986 Additional iron-induced complications, such as liver disease and endocrine abnormalities, further contribute to cardiac deterioration.987,988 The end result is the development of iron overload cardiomyopathy (IOCM), which may have either a restrictive or a dilated phenotype, the former potentially evolving to the latter as the disease advances. Myocardial iron deposition can be accurately estimated by the CMRT2* technique; T2* values are correlated with left and right ventricular systolic function and predict the development of iron-induced HF or arrhythmias.985 Prevention of IOCM is successfully accomplished with iron chelators, including deferoxamine, deferiprone, and deferasirox, while established IOCM may be completely reversed by intensified and combined iron chelation therapy.986
Recommendations for the treatment of transthyretin amyloidosis-cardiac amyloidosis
| Recommendations | Classa | Levelb |
| Tafamidis is recommended in patients with genetic testing proven hTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality.980 | I | B |
| Tafamidis is recommended in patients with wtTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality.980 | I | B |
- CA = cardiac amyloidosis; CMP = cardiomyopathy; CV = cardiovascular; hTTR = hereditary transthyretin; NYHA = New York Heart Association; wtTTR = wild-type transthyretin.
- a Class of recommendation.
- b Level of evidence.
14.8 Adult congenital heart disease
The management of ACHD has been reviewed in detail in a recent ESC guideline.989 HF is a common problem affecting 20–50% of the ACHD population, and an important cause of death.990 The pathophysiology of cardiac dysfunction in ACHD is often different from non-congenital (acquired) heart disease, in particular in those with: a systemic right ventricle (RV), a failing subpulmonary ventricle, a single ventricle,989 surgery-related injury, chronic pressure/volume overload in systemic and sub-pulmonary ventricles, and those with hypertrophy or non-compaction induced by gene mutations. Therefore, extrapolation of current HF treatment guidelines to ACHD patients is not always appropriate. In addition, the few available data on HF treatments in ACHD patients are often inconclusive and are derived from small patient cohorts. As a consequence, ACHD specific recommendations are mostly based on clinical experience or position statements.991
Importantly, ACHD patients with HF should be referred to expert centres. The general principles of management, while awaiting transfer to specialist centres, is summarized in Table 36.
| ACHD patients with chronic HF should be referred to specialized centres. |
| Specific guidelines for medical treatment of chronic HF in ACHD are lacking, and practitioners should follow the current guidelines for medical treatment of HF. It remains unknown whether the long-term use of neurohormonal modulators affects clinical outcomes and prognosis in ACHD. |
| Sacubitril/valsartan may decrease morbidity,992-994 however, no recommendation can be made at this moment based on the retrospective or anecdotical nature of these observations. |
| Co-morbidities in HF such as diabetes mellitus, AF, CSA, iron deficiency, and cachexia should be treated according to specific recommendations reported in this document. |
| In a biventricular circulation, patients with an impaired systemic LV should be treated with conventional HF therapy; this may also be considered in symptomatic patients with a failing systemic right ventricle. |
| Diuretics are recommended to control symptoms of fluid retention. |
| Treatment of symptomatic patients with a failing single ventricle in a Fontan circulation, or in the case of a persistent right-to-left shunt, should always be carefully initiated, taking the labile balance of ventricular preload and systemic afterload into account. |
| CRT may be a therapeutic option in ACHD patients with HF, but evidence in this specific setting is still lacking. Efficacy of CRT will depend on the underlying structural and functional substrate, such as anatomy of the systemic ventricle (left, right, or functionally single), presence and degree of structural systemic AV valve regurgitation, primary myocardial disease or scarring, and type of electrical conduction delay.989 |
| Treatment of acute HF in ACHD patients should be in an expert centre, with proper knowledge of inotropes, the availability of extracorporeal membrane oxygenation, and advanced bridging techniques.989,995 |
| Timely evaluation for transplantation by ACHD HF specialists in a transplant centre with ACHD expertise is recommended. |
| Ventricular assist devices can bridge patients to transplantation, or in a subgroup of patients, may be an option as destination therapy. |
- ACHD = adult congenital heart disease; AF = atrial fibrillation; AV = atrioventricular; CRT = cardiac resynchronization therapy; CSA = central sleep apnoea; HF = heart failure; LV = left ventricular.
15 Key messages
- (1)
Patients with HF are classified based on their LVEF. Those with a LVEF between 41% and 49% are defined as ‘mildly reduced LVEF’ (HFmrEF).
- (2)
Measurement of NPs and echocardiography have key roles in the diagnosis of HF.
- (3)
ACE-I or ARNI, beta-blockers, MRA, and SGLT2 inhibitors are recommended as cornerstone therapies for patients with HFrEF.
- (4)
ICDs are recommended in selected patients with HFrEF of an ischaemic aetiology and should be considered in those with a non-ischaemic aetiology.
- (5)
CRT-P/D is recommended in those patients with HFrEF, in sinus rhythm, with a LBBB ≥150 ms and should be considered in those with a LBBB ≥130–149 ms or non-LBBB ≥150 ms.
- (6)
Advanced HF strategies (heart transplantation/MCS) may be appropriate in selected patients.
- (7)
ACE-I/ARNI, beta-blockers, and MRA may be considered in patients with HFmrEF.
- (8)
The diagnosis of HFpEF requires objective evidence of cardiac structural, or functional abnormalities as well as elevated plasma NP concentrations consistent with the presence of LV diastolic dysfunction and raised LV filling pressures. A diastolic stress test is recommended when these markers are equivocal.
- (9)
To date, no treatment has been shown to reduce mortality and morbidity in patients with HFpEF.
- (10)
It is recommended that all patients with HF be enrolled in a multidisciplinary HF-MP.
- (11)
Exercise is recommended for all patients who are able, to improve exercise capacity and QOL, and reduce HF hospitalization.
- (12)
Patients with advanced HF refractory to medical/device therapy and who do not have absolute contraindications should be referred for consideration of heart transplantation. MCS should also be considered as BTT or DT in selected patients.
- (13)
Four major clinical presentations of acute HF may occur: ADHF, acute pulmonary oedema, RV failure, and cardiogenic shock.
- (14)
Treatment of acute HF is based on diuretics for congestion, inotropes, and short- term MCS for peripheral hypoperfusion.
- (15)
Patients hospitalized for HF should be carefully evaluated to exclude persistent signs of congestion. Oral treatment should be optimized before discharge.
- (16)
In addition to oral anticoagulation, a strategy of rhythm control including catheter ablation should be considered in patients whose symptoms and/or cardiac dysfunction are associated with AF.
- (17)
SAVR or TAVI, as advised by the Heart Team, are recommended in patients with symptomatic severe aortic valve stenosis.
- (18)
Patients with isolated significant SMR and COAPT criteria should be considered for percutaneous edge-to-edge repair, whereas those with SMR and CAD, who need revascularization, should be considered for surgery.
- (19)
It is recommended that patients with type II diabetes are treated with SGLT2 inhibitors.
- (20)
Patients should be periodically screened for anaemia and iron deficiency and i.v. iron supplementation with ferric carboxymaltose should be considered in symptomatic patients with LVEF <45% and iron deficiency, and in patients recently hospitalized for HF and with LVEF ≤50% and iron deficiency.
16 Gaps in evidence
- (1)
Definition and epidemiology
-
Further research into the underlying characteristics, pathophysiology, and diagnosis of HFmrEF and HFpEF
-
Consensus about normal values/ranges of EF
-
Better phenotyping of HFpEF
-
More information on the incidence and prevalence of ‘recovered LV’ systolic function
-
- (2)
Diagnosis
-
Definitive studies on the role of biomarkers, focusing on their additive value in the diagnosis of HF
-
More randomized studies on screening for HF in asymptomatic subjects that may translate into improved outcomes
-
Studies on biomarkers showing the impact on outcome of their measurements for the identification of subjects at risk of developing HF as well as to guide treatment in patients with HF
-
Validated diagnostic protocols for the diagnosis of HFmrEF and HFpEF
-
- (3)
Pharmacotherapy of CHF
-
Pragmatic studies on the order of adding disease-modifying drugs for HFrEF
-
Specific therapies for HFmrEF and HFpEF and, likely, their different phenotypes
-
More data and prospective clinical trials of HFrEF therapies in patients with eGFR <30 mL/min/1.73 m2
-
Further evidence from prospective RCTs for the treatment of specific HF phenotypes: myocarditis, cardiotoxicity, inherited CMPs, PPCM, amyloidosis
-
Management strategies and therapies for ‘recovered LV’ systolic function
-
More evidence on the effects of fluid restriction, dietary salt restriction, and nutrition
-
- (4)
Devices and interventions
-
Indications for ICDs in specific subgroups of HFmrEF/HFpEF and optimal selection of ICD candidates in HFrEF, including patients with ischaemic and non-ischaemic cardiomyopathy
-
More research on CRT efficacy in AF
-
Further prospective randomized studies showing the impact on outcomes of AF ablation strategies compared to OMT in HF patients
-
Further research on the percutaneous treatment of valve heart disease and its impact on patients’ outcomes and QOL
-
Larger RCTs on CCM and baroreceptor stimulation in HFrEF
-
- (5)
Disease management
-
The role of remote monitoring strategies in HF in the post COVID-19 era
-
Studies on optimal models for follow-up of stable HF patients
-
Studies to determine specific options for palliative care
-
- (6)
Advanced HF
-
Better definition of risk profiles according to INTERMACS and other classifications
-
RCTs to establish the effects on outcomes of long-term MCS in hospitalized patients as well as in ambulatory outpatients (for instance INTERMACS 4–6 profiles)
-
Advances in long-term MCS, including strategies to reduce the risk of bleeding, thromboembolic events, and infection
-
Advances in medical treatment for the many patients who cannot undergo MCS or heart transplantation including development of treatment strategies, novel inotropes or myotropes for patients with advanced HF
-
- (7)
AHF
-
Better definition and classification of patient phenotypes to facilitate improved treatment
-
Evidence-based use of imaging techniques and biomarkers that have an impact on patients’ clinical course
-
Development of better strategies for congestion relief, including monitoring of diuretic administration, and/or to improve organ perfusion
-
Identification of treatments with an impact on post-discharge outcomes
-
New devices for short-term MCS
-
Definition of evidence-based treatment options and therapeutic algorithms for patients with cardiogenic shock
-
- (8)
CV comorbidities
-
RCTs showing best strategies for the treatment of ventricular arrhythmias
-
RCTs to establish the role of coronary revascularization procedures in different patient subsets
-
RCTs to establish the impact on patients’ outcomes and/or QOL of percutaneous treatment of mitral or tricuspid valve disease in patients with HF
-
- (9)
Non-CV comorbidities
-
RCTs addressing cachexia and/or sarcopenia and/or frailty and showing the impact of treatment on QOL and/or outcome
-
RCTs of medical therapies or devices in patients with severe CKD and HF
-
RCTs showing the effects on outcomes of medical treatment of electrolyte abnormalities
-
RCTs showing the effects on outcomes of treatment of CSA
-
Prospective studies showing the impact on outcomes and/or QOL of early diagnosis, better prevention and treatment of cardiotoxicity of cancer therapies
-
Better treatment of infections and prevention of cardiac injury with infection
-
- (10)
Special conditions
-
RCTs of treatment for PPCM
-
Better phenotyping of CMPs through genetic testing, biomarkers and imaging modalities, and tailoring of therapy
-
RCTs of treatment of different types of myocarditis, including immunosuppressive therapies
-
RCTs of new treatments of different forms of cardiac amyloid
-
Better definition and treatment of LA myopathy.
-
17 ‘What to do’ and ‘what not to do’ messages from the guidelines
| Recommendations | Classa | Levelb |
| Recommendations for the diagnosis of chronic HF | ||
| BNP/NT-proBNP.c | I | B |
| 12-lead ECG. | I | C |
| Transthoracic echocardiography. | I | C |
| Chest radiography (X-ray). | I | C |
| Routine blood tests for comorbidities (including full blood count, urea and electrolytes, thyroid function, fasting glucose and HbA1c, lipids. Iron studies (TSAT and ferritin). | I | C |
| CMR is recommended for the assessment of myocardial structure and function in those with poor echocardiogram acoustic windows. | I | C |
| CMR is recommended for the characterization of myocardial tissue in suspected infiltrative disease, Fabry disease, inflammatory disease (myocarditis), LV non-compaction, amyloid, sarcoidosis, iron overload/haemochromatosis. | I | C |
| Invasive coronary angiography is recommended in patients with angina despite pharmacological therapy or symptomatic ventricular arrhythmias. | I | B |
| Cardiopulmonary exercise testing is recommended as a part of the evaluation for heart transplantation and/or MCS. | I | C |
| Right heart catheterization is recommended in patients with severe HF being evaluated for heart transplantation or MCS. | I | C |
| Recommendations for the treatment of HFrEF | ||
| ACE-I is recommended for patients with HFrEF to reduce the risk of HF hospitalization and death. | I | A |
| Beta-blocker is recommended for patients with stable HFrEF to reduce the risk of HF hospitalization and death. | I | A |
| MRA is recommended for patients with HFrEF to reduce the risk of HF hospitalization and death. | I | A |
| Dapagliflozin or empagliflozin are recommended for patients with HFrEF to reduce the risk of HF hospitalization and death. | I | A |
| Sacubitril/valsartan is recommended as a replacement for an ACE-I in patients with HFrEF to reduce the risk of HF hospitalization and death. | I | B |
| Diuretics are recommended in patients with HFrEF with signs and/or symptoms of congestion to alleviate HF symptoms, improve exercise capacity and reduce HF hospitalizations. | I | C |
| An ARBc is recommended to reduce the risk of HF hospitalization and CV death in symptomatic patients unable to tolerate an ACE-I or ARNI (patients should also receive a beta-blocker and an MRA). | I | B |
| The addition of an ARB (or renin inhibitor) to the combination of an ACE-I and an MRA is not recommended in patients with HF, because of the increased risk of renal dysfunction and hyperkalaemia. | III | C |
| An ICD is recommended to reduce the risk of sudden death and all-cause mortality in patients who have recovered from a ventricular arrhythmia causing haemodynamic instability, and who are expected to survive for >1 year with good functional status, in the absence of reversible causes or unless the ventricular arrhythmia has occurred <48 h after a MI. | I | A |
| An ICD is recommended to reduce the risk of sudden death and all-cause mortality in patients with symptomatic HF (NYHA class II–III) of an ischaemic aetiology (unless they have had an MI in the prior 40 days—see below), and an LVEF ≤35% despite ≥3 months of OMT, provided they are expected to survive substantially longer than 1 year with good functional status. | I | A |
| ICD implantation is not recommended within 40 days of an MI as implantation at this time does not improve prognosis. | III | A |
| ICD therapy is not recommended in patients in NYHA class IV with severe symptoms refractory to pharmacological therapy unless they are candidates for CRT, a VAD, or cardiac transplantation. | III | C |
| CRT is recommended for symptomatic patients with HF in SR with a QRS duration ≥150 ms and LBBB QRS morphology and with LVEF ≤35% despite OMT in order to improve symptoms and reduce morbidity and mortality. | I | A |
| CRT rather than RV pacing is recommended for patients with HFrEF regardless of NYHA class or QRS width who have an indication for ventricular pacing for high degree AV block in order to reduce morbidity. This includes patients with AF. | I | A |
| CRT is not recommended in patients with a QRS duration <130 ms who do not have an indication for pacing due to high-degree AV block. | III | A |
| Recommendations for the treatment of HFmrEF and HFpEF | ||
| Diuretics are recommended in patients with congestion and HFmrEF in order to alleviate symptoms and signs. | I | C |
| Screening for, and treatment of, aetiologies, and CV and non-CV comorbidities is recommended in patients with HFpEF (see relevant sections of this document). | I | C |
| Diuretics are recommended in congested patients with HFpEF in order to alleviate symptoms and signs. | I | C |
| Recommendations for the prevention of chronic HF | ||
| Treatment of hypertension is recommended to prevent or delay the onset of HF, and to prevent HF hospitalizations. | I | A |
| Treatment with statins is recommended in patients at high risk of CV disease or with CV disease in order to prevent or delay the onset of HF, and to prevent HF hospitalizations. | I | A |
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with diabetes at high risk of CV disease or with CV disease in order to prevent HF hospitalizations. | I | A |
| Counselling against sedentary habit, obesity, cigarette smoking, and alcohol abuse is recommended to prevent or delay the onset of HF. | I | C |
| Other recommendations for the management of chronic HF | ||
| It is recommended that HF patients are enrolled in a multidisciplinary HF management programme to reduce the risk of HF hospitalization and mortality. | I | A |
| Self-management strategies are recommended to reduce the risk of HF hospitalization and mortality. | I | A |
| Either home-based and/or clinic-based programmes improve outcomes and are recommended to reduce the risk of HF hospitalization and mortality. | I | A |
| Exercise is recommended for all patients who are able in order to improve exercise capacity and QOL, and reduce HF hospitalization.d | I | A |
| Recommendations for treatment of patients with advanced HF | ||
| Patients being considered for long-term MCS must have good compliance, appropriate capacity for device handling and psychosocial support. | I | C |
| Heart transplantation is recommended for patients with advanced HF, refractory to medical/device therapy and who do not have absolute contraindications. | I | C |
| Recommendations for treatment of patients with acute HF | ||
| Oxygen is recommended in patients with SpO2 <90% or PaO2 <60 mmHg to correct hypoxaemia. | I | C |
| Intubation is recommended for progressive respiratory failure persisting in spite of oxygen administration or non-invasive ventilation. | I | C |
| Intravenous loop diuretics are recommended for all patients with AHF admitted with signs/symptoms of fluid overload to improve symptoms. | I | C |
| Thromboembolism prophylaxis (e.g. with LMWH) is recommended in patients not already anticoagulated and with no contraindication to anticoagulation, to reduce the risk of deep venous thrombosis and pulmonary embolism. | I | A |
| Inotropic agents are not recommended routinely, due to safety concerns, unless the patient has symptomatic hypotension and evidence of hypoperfusion. | III | C |
| Routine use of opiates is not recommended, unless in selected patients with severe/intractable pain or anxiety. | III | C |
| IABP is not routinely recommended in post-MI cardiogenic shock. | III | B |
| Recommendations for management of patients after HF hospitalization | ||
| It is recommended that patients hospitalized for HF be carefully evaluated to exclude persistent signs of congestion before discharge and to optimize oral treatment. | I | C |
| It is recommended that evidence-based oral medical treatment be administered before discharge. | I | C |
| An early follow-up visit is recommended at 1–2 weeks after discharge to assess signs of congestion, drug tolerance, and start and/or uptitrate evidence-based therapy. | I | C |
| Recommendations for treatment of patients with HF and AF | ||
| Long-term treatment with an oral anticoagulant is recommended in all patients with AF, HF, and CHA2DS2-VASc score >2 in men or >3 in women. | I | A |
| DOACs are recommended in preference to VKAs in patients with HF, except in those with moderate or severe mitral stenosis or mechanical prosthetic heart valves. | I | A |
| Urgent ECV is recommended in the setting of acute worsening of HF in patients presenting with rapid ventricular rates and haemodynamic instability. | I | C |
| Treatment with the anti-arrhythmic agents flecainide, encainide, disopyramide, dronedarone, and D-sotalol is not recommended due to safety concerns. | III | A |
| Diltiazem or verapamil are not recommended in patients with HFrEF, as they increase the risk of HF worsening and HF hospitalization. | III | C |
| Recommendations for treatment of patients with HF and aortic stenosis | ||
| Aortic valve intervention, TAVI or SAVR, is recommended in patients with HF and severe high-gradient aortic stenosis to reduce mortality and improve symptoms. | I | B |
| It is recommended that the choice between TAVI and SAVR be made by the Heart Team, according to individual patient preference and features including age, surgical risk, clinical, anatomical and procedural aspects, weighing the risks and benefits of each approach. | I | C |
| Recommendations for treatment of patients with HF and diabetes | ||
| SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, sotagliflozin) are recommended in patients with T2DM at risk of CV events to reduce hospitalizations for HF, major CV events, end-stage renal dysfunction, and CV death. | I | A |
| SGLT2 inhibitors (dapagliflozin, empagliflozin, and sotagliflozin) are recommended in patients with T2DM and HFrEF to reduce hospitalizations for HF and CV death. | I | A |
| Thiazolidinediones (glitazones) are not recommended in patients with HF, as they increase the risk of HF worsening and HF hospitalization. | III | A |
| The DPP-4 inhibitor saxagliptin is not recommended in patients with HF. | III | B |
| Recommendations for treatment of patients with HF and iron deficiency | ||
| It is recommended that all patients with HF be periodically screened for anaemia and iron deficiency with a full blood count, serum ferritin concentration, and TSAT. | I | C |
| Treatment of anaemia in HF with erythropoietin-stimulating agents is not recommended in the absence of other indications for this therapy. | III | B |
| Recommendation for treatment of patients with HF and sleep apnoea | ||
| Adaptive servo-ventilation is not recommended in patients with HFrEF and a predominant CSA because of an increased all-cause and CV mortality. | III | A |
| Recommendation for treatment of patients with HF and arthritis | ||
| NSAIDs or COX-2 inhibitors are not recommended in patients with HF, as they increase the risk of HF worsening and HF hospitalization. | III | B |
| Recommendation for treatment of patients with HF and cancer | ||
| It is recommended that cancer patients at increased risk for cardiotoxicity, defined by a history or risk factors of CV disease, previous cardiotoxicity or exposure to cardiotoxic agents, undergo CV evaluation before scheduled anticancer therapy, preferably by a cardiologist with experience/interest in Cardio-Oncology. | I | C |
| Recommendations for treatment of patients with HF and amyloidosis | ||
| Tafamidis is recommended in patients with genetic testing proven hTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality. | I | B |
| Tafamidis is recommended in patients with wtTTR-CA and NYHA class I or II symptoms to reduce symptoms, CV hospitalization and mortality. | I | B |
- ACE-I = angiotensin-converting enzyme inhibitor; AF = atrial fibrillation; AHF = acute heart failure; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; AV = atrio-ventricular; BNP = B-type natriuretic peptide; CHA2DS2-VASc = congestive heart failure or left ventricular dysfunction, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled)-Vascular disease, Age 65–74, Sex category (female) (score); CMP = cardiomyopathy; CMR = cardiac magnetic resonance; CRT = cardiac resynchronization therapy; CSA = central sleep apnoea; CV = cardiovascular; DOAC = direct-acting oral anticoagulant; DPP-4 = dipeptidyl peptidase-4; ECG = electrocardiogram; ECV = electrical cardioversion; HbA1c = glycated haemoglobin; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; hTTR = hereditary transthyretin; IABP = intra-aortic balloon pump; ICD = implantable cardioverter-defibrillator; LBBB = left bundle branch block; LMWH = low-molecular-weight heparin; LV = left ventricular/ventricle; LVEF = left ventricular ejection fraction; MCS = mechanical circulatory support; MI = myocardial infarction; MRA = mineralocorticoid receptor antagonist; NSAID = non-steroidal anti-inflammatory drug; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; OMT = optimal medical therapy; PaO2 = partial pressure of oxygen; QOL = quality of life; QRS = Q, R, and S waves (on an ECG); RV = right ventricular; SAVR = surgical aortic valve replacement; SGLT2 = sodium-glucose co-transporter 2; SpO2 = transcutaneous oxygen saturation; SR = sinus rhythm; T2DM = type 2 diabetes mellitus; TAVI = transcatheter aortic valve implantation; TSAT = transferrin saturation; VAD = ventricular assist device; VKA = vitamin K antagonist; wtTTR-CA = wild-type transthyretin cardiac amyloidosis.
- a Class of recommendation.
- b Level of evidence.
- c References are listed in section 4.2 for this item.
- d In those who are able to adhere to the exercise programme.
18 Quality indicators
QIs are tools that may be used to evaluate care quality, including that of processes of care and clinical outcomes.996 They may also serve as a mechanism for enhancing adherence to guideline recommendations, through quality assurance endeavours and benchmarking of care providers.997 As such, the role of QIs in driving quality improvement is increasingly recognized and attracts interest from healthcare authorities, professional organizations, payers, and the public.998
The ESC recognizes the need for measuring and reporting quality and outcomes of CV care. The methodology by which the ESC QIs are developed has been published998 and, to date, a suite of QIs for an initial tranche of CV conditions has been produced.999,1000 To facilitate quality improvement initiatives, the disease-specific ESC QIs are included in corresponding ESC Clinical Practice Guidelines.7,1001 This is further enhanced by way of their integration in the ESC registries, such as the EURObservational Research Programme (EORP) and the European Unified Registries On Heart Care Evaluation and Randomized Trials (EuroHeart) project.1002
For patients with HF, QIs may help healthcare providers to simultaneously operationalize discrete guideline recommendations and enable the discrimination between missed opportunities and appropriate care. Furthermore, QIs allow the capture of patients’ experience. As such, and in parallel with the writing of these guidelines, a suite of QIs for the evaluation of care and outcomes for patients with HF was developed. These QIs, alongside their specifications and development process are published separately with a short summary shown in Table 37.
| Domain 1. Structural QIsa |
| Main (1): Centre should have a dedicated multidisciplinary team to manage patients with HF |
| Numerator: Availability of a dedicated multidisciplinary team to manage patients with HF. |
| Domain 2. Patient assessmentb |
| Main (1): Proportion of patients with HF who have a documentation of their HF clinical type (HFrEF, HFmrEF, HFpEF) |
|
| Main (2): Proportion of patients with HF who have a documentation of their ECG findings |
|
| Main (3): Proportion of patients with HF who have their NPs measured |
|
| Domain 3. Initial treatment |
| Main (1). Proportion of patients with HFrEF who are prescribed the beta-blocker bisoprolol, carvedilol, sustained-release metoprolol succinate, or nebivolol in the absence of any contraindications |
|
| Main (2). Proportion of patients with HFrEF who are prescribed ACE inhibitor, ARB or ARNI in the absence of any contraindications |
|
| Main (3). Proportion of patients with HF who are prescribed diuretic therapy if they have evidence of fluid retention |
|
| Main (4): Proportion of patients with HFrEF who are prescribed an MRA in the absence of any contraindications |
|
| Main (5): Proportion of patients with HFrEF who are prescribed a SGLT2 inhibitor in the absence of any contraindications |
|
- ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; ICD = implantable cardioverter-defibrillator; IHD = ischaemic heart disease; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; NP = natriuretic peptide; NYHA = New York Heart Association; OMT = optimal medical therapy; QI = quality indicator; SGLT2 = sodium-glucose co-transporter 2.
- a Structural QIs are binary measurements (Yes/No), and thus, have only numerator definitions.
- b Blood tests include urea, creatinine, electrolytes, full blood count, glucose, glycated haemoglobin, thyroid-stimulating hormone, liver function test, lipids, and iron profile.
19 Supplementary data
Supplementary data with additional Supplementary Figures, Tables, and text complementing the full text are available on the European Heart Journal website and via the ESC website at www.escardio.org/guidelines.
20 Author information
Author/Task Force Member Affiliations: Marianna Adamo, Cardiology and Cardiac Catheterization laboratory, ASST Spedali Civili di Brescia and Department of Medical and Surgical Specialties, Radiological Sciences and Public, ASST Spedali Civili di Brescia, Brescia, Italy; Andreas Baumbach, Barts Heart Centre, Queen Mary University of London, London, United Kingdom; Michael Böhm, Klinik für Innere Medizin III, Saarland University, Homburg/Saar, Saarland, Germany; Haran Burri, Cardiology, University Hospital of Geneva, Geneva, Switzerland; Jelena Čelutkienė, Clinic of Cardiac and Vascular Diseases, Vilnius University, Faculty of Medicine, Vilnius, Lithuania; Ovidiu Chioncel, Emergency Institute for Cardiovascular Diseases ‘Prof. Dr.C.C.Iliescu’, University of Medicine Carol Davila, Bucuresti, Romania; John G.F. Cleland, Robertson Centre for Biostatistics and Clinical Trials, Institute of Health & Wellbeing, Glasgow, Lanarkshire, United Kingdom; Andrew J.S. Coats, University of Warwick, Coventry, United Kingdom; Maria G. Crespo-Leiro, Cardiology, Complexo Hospitalario Universitario A Coruña (CHUAC), CIBERCV, Universidade da Coruña (UDC), Instituto de Investigación Biomedica de A Coruña (INIBIC), La Coruña, Spain; Dimitrios Farmakis, University of Cyprus Medical School, Nicosia, Cyprus; Roy S. Gardner, Scottish National Advanced Heart Failure Service, Golden Jubilee National Hospital, Clyderbank, Glasgow, Scotland, United Kingdom; Martine Gilard, Cardiology, Brest University, Brest, France; Stephane Heymans, Department of Cardiology, Maastricht University, CARIM School for Cardiovascular Diseases, Maastricht, Netherlands; Arno W. Hoes, University Medical Center Utrecht, Utrecht, Netherlands; Tiny Jaarsma, Department of Health, Medicine and Caring Science, Linköping University, Linköping, Sweden; Ewa A. Jankowska, Department of Heart Diseases, Wroclaw Medical University, Wroclaw, Poland; Mitja Lainscak, Division of Cardiology, General Hospital Murska Sobota, Murska Sobota, Slovenia; Carolyn S.P. Lam, National Heart Centre Singapore and Duke-National University of Singapore, Singapore; Alexander R. Lyon, Department of Cardiology, Royal Brompton Hospital, London, United Kingdom; John J.V. McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, Scotland, United Kingdom; Alexandre Mebazaa, Anesthesiology and Critical Care, Université de Paris - Hôpital Lariboisière, Paris, France; Richard Mindham, United Kingdom, ESC Patient Forum, Sophia Antipolis, France; Claudio Muneretto, Cardiothoracic Surgery, ASST Spedali Civili University of Brescia, Brescia, Italy; Massimo Francesco Piepoli, Cardiology, Guglielmo da Saliceto Hospital, AUSL Piacenza, Piacenza, Italy; Susanna Price, Cardiology & Adult Intensive Care Unit, Royal Brompton Hospital, London, United Kingdom; Giuseppe M.C. Rosano, IRCCS San Raffaele, Roma, Italy; Frank Ruschitzka, Department of Cardiology, University Hospital Zurich, Zurich, Switzerland; Anne Kathrine Skibelund, Denmark, ESC Patient Forum, Sophia Antipolis, France.
21 Appendix
ESC Scientific Document Group
Includes Document Reviewers and ESC National Cardiac Societies.
Document Reviewers: Rudolf A. de Boer (CPG Review Coordinator) (Netherlands), P. Christian Schulze (CPG Review Coordinator) (Germany), Magdy Abdelhamid (Egypt), Victor Aboyans (France), Stamatis Adamopoulos (Greece), Stefan D. Anker (Germany), Elena Arbelo (Spain), Riccardo Asteggiano (Italy), Johann Bauersachs (Germany), Antoni Bayes-Genis (Spain), Michael A. Borger (Germany), Werner Budts (Belgium), Maja Cikes (Croatia), Kevin Damman (Netherlands), Victoria Delgado (Netherlands), Paul Dendale (Belgium), Polychronis Dilaveris (Greece), Heinz Drexel (Austria), Justin Ezekowitz (Canada), Volkmar Falk (Germany), Laurent Fauchier (France), Gerasimos Filippatos (Greece), Alan Fraser (United Kingdom), Norbert Frey (Germany), Chris P. Gale (United Kingdom), Finn Gustafsson (Denmark), Julie Harris (United Kingdom), Bernard Iung (France), Stefan Janssens (Belgium), Mariell Jessup (United States of America), Aleksandra Konradi (Russia), Dipak Kotecha (United Kingdom), Ekatirini Lambrinou (Cyprus), Patrizio Lancellotti (Belgium), Ulf Landmesser (Germany), Christophe Leclercq (France), Basil S. Lewis (Israel), Francisco Leyva (United Kingdom), Aleš Linhart (Czech Republic), Maja-Lisa Løchen (Norway), Lars H. Lund (Sweden), Donna Mancini (United States of America), Josep Masip (Spain), Davor Milicic (Croatia), Christian Mueller (Switzerland), Holger Nef (Germany), Jens-Cosedis Nielsen (Denmark), Lis Neubeck (United Kingdom), Michel Noutsias (Germany), Steffen E. Petersen (United Kingdom), Anna Sonia Petronio (Italy), Piotr Ponikowski (Poland), Eva Prescott (Denmark), Amina Rakisheva (Kazakhstan), Dimitrios Richter (Greece), Evgeny Schlyakhto (Russia), Petar Seferovic (Serbia), Michele Senni (Italy), Marta Sitges (Spain), Miguel Sousa-Uva (Portugal), Carlo Gabriele Tocchetti (Italy), Rhian Touyz (United Kingdom), Carsten Tschoepe (Germany), Johannes Waltenberger (Germany).
ESC National Cardiac Societies actively involved in the review process of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure:
Algeria: Algerian Society of Cardiology, Messaad Krim; Armenia: Armenian Cardiologists Association, Hamlet Hayrapetyan; Austria: Austrian Society of Cardiology, Deddo Moertl; Azerbaijan: Azerbaijan Society of Cardiology, Isakh Mustafayev; Belarus: Belorussian Scientific Society of Cardiologists, Alena Kurlianskaya; Belgium: Belgian Society of Cardiology, Michel Depauw; Bosnia and Herzegovina: Association of Cardiologists of Bosnia and Herzegovina, Zumreta Kušljugić; Bulgaria: Bulgarian Society of Cardiology, Plamen Gatzov; Croatia: Croatian Cardiac Society, Davor Milicic; Cyprus: Cyprus Society of Cardiology, Petros Agathangelou; Czech Republic: Czech Society of Cardiology, Vojtěch Melenovský; Denmark: Danish Society of Cardiology, Brian Bridal Løgstrup; Egypt: Egyptian Society of Cardiology, Ahmed Magdy Mostafa; Estonia: Estonian Society of Cardiology, Tiina Uuetoa; Finland: Finnish Cardiac Society, Johan Lassus; France: French Society of Cardiology, Damien Logeart; Georgia: Georgian Society of Cardiology, Zviad Kipiani; Germany: German Cardiac Society, Johann Bauersachs; Greece: Hellenic Society of Cardiology, Christina Chrysohoou; Hungary: Hungarian Society of Cardiology, Róbert Sepp; Iceland: Icelandic Society of Cardiology, Inga Jóna Ingimarsdóttir; Ireland: Irish Cardiac Society, Jim O'Neill; Israel: Israel Heart Society, Israel Gotsman; Italy: Italian Federation of Cardiology, Massimo Iacoviello; Kazakhstan: Association of Cardiologists of Kazakhstan, Amina Rakisheva; Kosovo (Republic of): Kosovo Society of Cardiology, Gani Bajraktari; Kyrgyzstan: Kyrgyz Society of Cardiology, Olga Lunegova, Latvia: Latvian Society of Cardiology, Ginta Kamzola; Lebanon: Lebanese Society of Cardiology, Tony Abdel Massih; Libya: Libyan Cardiac Society, Hisham Benlamin; Lithuania: Lithuanian Society of Cardiology, DianaŽaliaduonytė; Luxembourg: Luxembourg Society of Cardiology, Stephanie Noppe; Malta: Maltese Cardiac Society, Alice Moore; Moldova (Republic of): Moldavian Society of Cardiology, Eleonora Vataman; Montenegro: Montenegro Society of Cardiology, Aneta Boskovic; Morocco: Moroccan Society of Cardiology, Ahmed Bennis; Netherlands: Netherlands Society of Cardiology, Olivier C. Manintveld; North Macedonia: North Macedonian Society of Cardiology, Elizabeta Srbinovska Kostovska; Norway: Norwegian Society of Cardiology, Geeta Gulati; Poland: Polish Cardiac Society, Ewa Straburzyńska-Migaj; Portugal: Portuguese Society of Cardiology, José Silva-Cardoso; Romania: Romanian Society of Cardiology, Roxana Cristina Rimbaş; Russian Federation: Russian Society of Cardiology, Yury Lopatin; San Marino: San Marino Society of Cardiology, Marina Foscoli; Serbia: Cardiology Society of Serbia, Sinisa Stojkovic; Slovakia: Slovak Society of Cardiology, Eva Goncalvesova; Slovenia: Slovenian Society of Cardiology, Zlatko Fras; Spain: Spanish Society of Cardiology, Javier Segovia; Sweden: Swedish Society of Cardiology, Krister Lindmark; Switzerland: Swiss Society of Cardiology, Micha T. Maeder; Syrian Arab Republic: Syrian Cardiovascular Association, Walid Bsata; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Leila Abid; Turkey: Turkish Society of Cardiology, Hakan Altay; Ukraine: Ukrainian Association of Cardiology, Leonid Voronkov; United Kingdom of Great Britain and Northern Ireland: British Cardiovascular Society, Ceri Davies, Uzbekistan: Association of Cardiologists of Uzbekistan, Timur Abdullaev.
ESC Clinical Practice Guidelines Committee (CPG): Colin N. Baigent (Chairperson) (United Kingdom), Magdy Abdelhamid (Egypt), Victor Aboyans (France), Sotiris Antoniou (United Kingdom), Elena Arbelo (Spain), Riccardo Asteggiano (Italy), Andreas Baumbach (United Kingdom), Michael A. Borger (Germany), Jelena Čelutkienė (Lithuania), Maja Cikes (Croatia), Jean-Philippe Collet (France), Volkmar Falk (Germany), Laurent Fauchier (France), Chris P. Gale (United Kingdom), Sigrun Halvorsen (Norway), Bernard Iung (France), Tiny Jaarsma (Sweden), Aleksandra Konradi (Russia), Konstantinos C. Koskinas (Switzerland), Dipak Kotecha (United Kingdom), Ulf Landmesser (Germany), Basil S. Lewis (Israel), Ales Linhart (Czech Republic), Maja-Lisa Løchen (Norway), Jens-Cosedis Nielsen (Denmark), Steffen E. Petersen (United Kingdom), Eva Prescott (Denmark), Lis Neubeck (United Kingdom), Amina Rakisheva (Kazakhstan), Marta Sitges (Spain), Rhian M. Touyz (United Kingdom).




