Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction
Abstract
Background
Increases in serum creatinine with renin–angiotensin–aldosterone system (RAAS) inhibitors can lead to unnecessary discontinuation of these agents. The dual-acting angiotensin receptor neprilysin inhibitor LCZ696 improves clinical outcome patients with heart failure with reduced ejection fraction, and pilot data suggest potential benefit in heart failure with preserved ejection fraction (HFpEF). The effects of LCZ696 on renal function have not been assessed.
Methods and results
A total of 301 HFpEF patients were randomly assigned to LCZ696 or valsartan in the PARAMOUNT trial. We studied renal function [creatinine, estimated glomerular filtration rate (eGFR), cystatin C, and urinary albumin to creatinine ratio (UACR)] at baseline, 12 weeks, and after 36 weeks of treatment. Worsening renal function (WRF) was determined as an serum creatinine increase of >0.3 mg/dL and/or >25% between two time-points. Mean eGFR at baseline was 65.4 ± 20.4 mL/min per 1.73 m2. The eGFR declined less in the LCZ696 group than in the valsartan group (–1.5 vs. –5.2 mL/min per 1.73 m2; P = 0.002). The incidence of WRF was lower in the LCZ696 group (12%) than in the valsartan group (18%) at any time-point, but this difference was not statistically significant (P = 0.18). Over 36 weeks, the geometric mean of UACR increased in the LCZ696 group (2.4–2.9 mg/mmol), whereas it remained stable in the valsartan group (2.1–2.0 mg/mmol; P for difference between groups = 0.016).
Conclusion
In patients with HFpEF, therapy with LCZ696 for 36 weeks was associated with preservation of eGFR compared with valsartan therapy, but an increase in UACR.
Introduction
Treatment of heart failure with renin–angiotensin–aldosterone system (RAAS) inhibitors is generally accompanied by increases in serum creatinine,1 and concern over worsening renal function can lead to unnecessary discontinuation of appropriate medication.
LCZ696 is a first in class angiotensin receptor neprilysin inhibitor (ARNi) and compromises the molecular moieties of the angiotensin II AT1 receptor antagonist valsartan and the neprilysin inhibitor AHU377.2 A large outcomes trial in heart failure with reduced ejection fraction (HFrEF) recently showed that LCZ696 was superior to enalapril in reducing the risks of death and of hospitalization for heart failure,3 and in patients with heart failure with preserved ejection fraction (HFpEF), LCZ696 reduced N-terminal prohormone brain natriuretic peptide (NT-proBNP) levels, and was associated with left atrial reverse remodelling and improvement in symptoms.4 However, although the renal effects of the compounds have been defined separately, the effects of LCZ696 on renal function in patients with heart failure have not been described.
Antagonism of the AT1 receptor inhibits the RAAS and blocks the effects of angiotensin II on mainly the efferent glomerular arteriole, resulting in efferent vasodilatation and, as a result of reduced filtration pressure, a decline in glomerular filtration rate (GFR).5 Inhibition of neprilysin results in persistently elevated levels of natriuretic peptides, bradykinin, and adrenomedullin.2, 6-10 It has been hypothesized that natriuretic peptides are involved in several glomerular hemodynamic changes.11-13 Natriuretic peptides increase natriuresis and decrease circulating blood flow, thereby reducing renal blood flow, perfusion, and glomerular filtration. In addition, natriuretic peptides inhibit renin and angiotensin II-stimulated aldosterone release.14 Several nephroprotective properties of bradykinin have been described, such as inhibition of renal inflammation, apoptosis, fibrosis, and glomerulosclerosis. Finally, adrenomedullin inhibits glomerular sclerosis, interstitial fibrosis, and renal arteriosclerosis. AS LCZ696 both blocks the action of angiotensin II and elevates biologically active natriuretic peptides, bradykinin, and adrenomedullin, the impact on measures of renal function might be expected to be different from treatment with RAAS blockers alone. In addition, increases in serum creatinine after initiation of RAAS-inhibiting drugs does not necessarily portend a worse outcome in patients with HFrEF, while an increase in serum creatinine after initiation of RAAS-inhibiting drugs in patients with HFpEF is associated with an excessive risk.15-17 These contrasting results suggest a possible different pathophysiological mechanism in patients with HFpEF. The aim of the present study was to compare the effects of LCZ696 with valsartan on renal function in patients with HFpEF.
Methods
Patient population
Patients with HFpEF were enrolled in the Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) study, a multicentre, randomized, double-blind, parallel-group, active controlled trial.4 The main results have been published.4 Eligible patients were ≥40 years old, with a left ventricular ejection fraction (LVEF) of ≥45%, and had a documented history of heart failure with associated signs or symptoms (dyspnoea on exertion, orthopnoea, paroxysmal dyspnoea, and peripheral oedema). Additional inclusion criteria included NT-proBNP levels >400 pg/mL at screening, being on diuretic therapy, a systolic blood pressure of ≤140 mm Hg, or ≤160 mm Hg if there was a drug administration of three or more antihypertensive drugs at randomization, an estimated GFR (eGFR) of ≥30 mL/min.1.73 m2 at baseline, and a potassium concentration of ≤5.2 mmol/L. Exclusion criteria included documentation of previous LVEF ≤45% at any time, isolated right heart failure owing to pulmonary disease, dyspnoea from non-cardiac causes, primary valvular or myocardial diseases, or coronary or cerebrovascular diseases needing revascularization within 3 months of screening or during the trial. The incidence of atrial fibrillation in the study population was limited to approximately 25%. The trial was approved by the Ethics Committee of each participating site. All patients provided written informed consent. The PARAMOUNT trial was registered at https://Clinicaltrials.gov, number NCT00887588.
Study procedures
After a single-blind, placebo run-in period of 1–2 weeks, patients were randomized to one of the two intervention groups receiving either LCZ696 or valsartan. Patients started with LCZ696 50 mg twice daily or valsartan 40 mg twice daily. Over a period of 2–4 weeks, patients were titrated to their final dose of LCZ696 200 mg twice daily or valsartan 160 mg twice daily. Systemic exposure to valsartan and AT1 blockade of 200 mg LCZ696 is similar to 160 mg valsartan.18, 19 The duration of the trial was 36 weeks, encompassing a 12 week main study period and 24 week extension period. Blood samples for laboratory analyses were collected at screening, randomization, week 4, week 12, and week 36, or at the end of study or early termination visits. As in previous analyses, 12 week outcomes were analysed using last observation carried forward among those randomized with at least one post-baseline assessment, while 36 week analyses included completers only. Echocardiography was performed at the same time, accept for week 4. Blood pressure and NT-proBNP levels were measured at screening, randomization, week 4, week 12, and week 36, or at end of study or at early termination visits. Screening of NT-proBNP was established by table-top device at point of care, local laboratory, or central laboratory. Assessment of NT-proBNP for efficacy was measured at a central laboratory (Quest Diagnostics, Valencia, CA, USA) with the Elecsys NT-proBNP immunoassay (Roche Diagnostics, Indianapolis, IN, USA).
Renal function
Renal function was studied by serum creatinine, eGFR, cystatin C, urinary albumin to creatinine ratio (UACR) and worsening renal function (WRF). The eGFR was calculated using the Modification of Diet in Renal Disease formula.20 WRF was determined by change in serum creatinine, and was defined as >0.3 mg/dL increase in creatinine in combination with an increase of more than 25% in serum creatinine between two time points.21 One physiologically implausible baseline cystatin C value (0.05 mg/L) was excluded from these analyses.
Statistical analysis
Results are summarized using mean and standard deviation for normally distributed variables and geometric mean and/or median [interquartile range (IQR)] if non-normally distributed. Categorical variables are presented as percentages of observations. To compare groups, two-sample t-tests and rank-sum tests were used for continuous variables, and χ2 or Fisher's exact tests were used for categorical variables, as appropriate. Log transformations were applied to skewed variables (UACR) for regression models. Post-randomization changes from baseline were compared using linear, logistic, and quantile (median) regression, controlling for stratification factors [previous therapy with angiotensin-converting enzyme (ACE) inhibitors, region, treatment allocation], and baseline value of the outcome of interest as independent variables. Predictors of WRF, including baseline characteristics, were assessed via univariate and multivariate logistic regression models using a stepwise forward selection procedure (baseline characteristic variables as well as change in systolic and diastolic blood pressure were considered in the forward selection step) with P-value threshold of 0.05, and treatment assignment and stratification factors included in all multivariate models considered. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata, version 13.0 (Stata Corp., College Station, TX, USA).
Results
For the present analyses, we included all 301 patients randomized in the PARAMOUNT trial. Availability of measurements of creatinine/eGFR, cystatin C, UACR, and systolic blood pressure throughout the study are presented in supplementary Table 1. Characteristics at baseline according to the different intervention groups are presented in the main study paper.4 Briefly, patient characteristics did not differ between treatment groups, mean age was 71 ± 9.1 years and mean LVEF was 58 ± 7.7%. The majority (93%) used an ACE-inhibitor or an angiotensin receptor blocker (ARB), and 21% used a mineralocorticoid receptor antagonist (MRA). Mean serum creatinine was 1.05 ± 0.32 mg/dL, and mean eGFR was 65.4 ± 20.4 mL/min.1.73 m2, while 42% had chronic kidney disease defined as a reduction in eGFR to less than 60 mL/min.1.73 m2. The geometric means of UACR and cystatin C were within normal reference ranges [2.2 mg/mmol (IQR = 1.9–2.7) and 1.17 mg/L (IQR = 1.14–1.21), respectively]. Characteristics at baseline according to the development of worsening renal function are presented in Table 1. Patients who developed WRF tended to have lower eGFR and diastolic blood pressure, and higher cystatin C and NT-proBNP levels at baseline.
| No WRF, n = 260 | WRF, n = 41 | P-value | |
|---|---|---|---|
| Randomized to LCZ696 | 133 (51.2%) | 16 (39.0%) | 0.15 |
| Mean age (years) | 71 ± 9 | 69 ± 9 | 0.15 |
| Female gender (%) | 150 (57.7%) | 20 (48.8%) | 0.28 |
| BMI (kg/m2) | 29.8 ± 5.7 | 30.9 ± 6.0 | 0.26 |
| Heart Rate (bpm) | 69.4 ± 12.9 | 68.7 ± 13.8 | 0.75 |
| NYHA class | 0.65* | ||
| Class II (%) | 208 (80.0%) | 31 (75.6%) | |
| Class III (%) | 50 (19.2%) | 10 (24.4%) | |
| History of heart failure hospitalization (%) | 110 (42.3%) | 17 (41.5%) | 0.92 |
| Atrial fibrillation at screening (%) | 69 (26.5%) | 16 (39.0%) | 0.10 |
| History of hypertension (%) | 245 (94.2%) | 37 (90.2%) | 0.33 |
| History of diabetes (%) | 99 (38.1%) | 15 (36.6%) | 0.85 |
| eGFR (mL/min.1.73 m2) | 66.2 ± 20.4 | 60.0 ± 19.5 | 0.07 |
| eGFR <60 mL/min.1.73 m2 (%) | 104 (40.6%) | 21 (51.2%) | 0.20 |
| Haemoglobin (g/L) | 13.5 ± 1.7 | 13.7 ± 1.5 | 0.46 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.3 ± 0.5 | 0.31 |
| Sodium (mmol/L) | 140 ± 3 | 140 ± 3 | 0.80 |
| Chloride (mmol/L) | 103 ± 4 | 103 ± 3 | 0.41 |
| Cystatin C (mg/L) | |||
| Median [IQR] | 1.1 [1.0, 1.4] | 1.3 [1.1, 1.5] | 0.005 |
| Geometric mean (95% CI) | 1.15 (1.11, 1.19) | 1.33 (1.21, 1.45) | 0.003 |
| UACR (mg/mmol) | |||
| Median [IQR] | 1.6 [0.7, 4.2] | 1.9 [1.0, 10.5] | 0.09 |
| Geometric mean (95% CI) | 2.1 (1.7, 2.6) | 3.2 (1.9, 5.2) | 0.13 |
| Systolic blood pressure (mmHg), | 135 [128, 144] | 133 [125, 139] | 0.13 |
| Diastolic blood pressure (mmHg) | 79 [72, 83] | 75 [66, 80] | 0.01 |
| NT-proBNP (pg/mL) | |||
| Median [IQR] | 805 (482, 1300) | 1144 (673, 1876) | 0.01 |
| Geometric mean (95% CI) | 783 (693, 884) | 1170 (927, 1476) | 0.01 |
| ACE inhibitors (%) | 139 (53.5%) | 24 (58.5%) | 0.54 |
| ARBs (%) | 106 (40.8%) | 13 (31.7%) | 0.27 |
| ACE inhibitors or ARBs (%) | 244 (93.8%) | 36 (87.8%) | 0.16 |
| Diuretics (%) | 260 (100%) | 41 (100%) | 1.00 |
| Beta-blockers (%) | 204 (78.5%) | 34 (82.9%) | 0.51 |
| Aldosterone antagonists (%) | 51 (19.6%) | 12 (29.3%) | 0.16 |
- BMI, body mass index; NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate; UACR, urine albumin to creatinine ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker
- * P-value for comparison of NYHA class distribution (including two patients with NYHA class I).
Changes in blood pressure
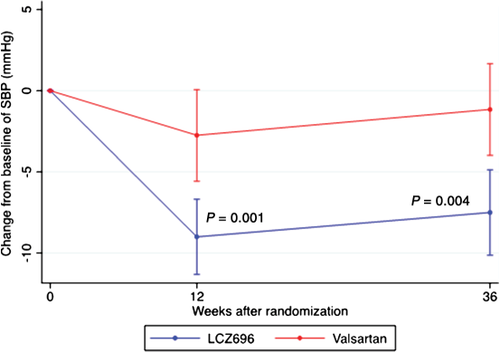
After 12 weeks of treatment, LCZ696 reduced mean blood pressure (BP) by –9.0 ± 13.8/–4.8 ± 10.0 mmHg, whereas valsartan reduced mean BP by –2.8 ± 16.9/–1.9 ± 10.9 mmHg (adjusted P for difference between groups = 0.001 for systolic BP, P for difference between groups = 0.08 for diastolic BP; Figure 1). After 36 weeks, mean BP was reduced by –7.5 ± 15.1/–5.1 ± 10.8 mmHg in the LCZ696 group and by –1.2 ± 16.1/–0.2 ± 11.6 mmHg in the valsartan group (P for difference between groups = 0.004 for systolic BP, P for difference between groups = 0.001 for diastolic BP; Figure 1).
Change in renal function
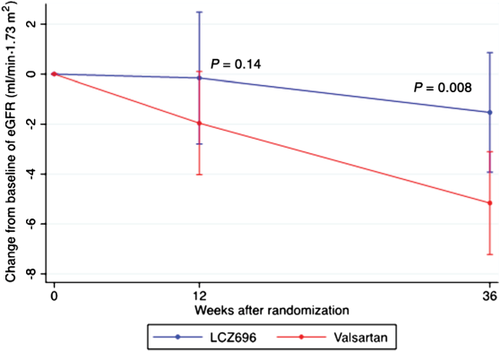
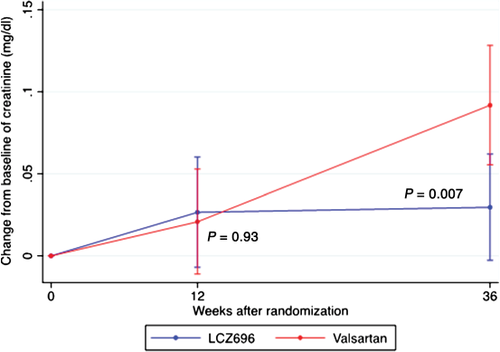
Baseline eGFR was similar between both groups. Mean change in eGFR between baseline and 12 weeks of treatment was not significantly different between groups (P = 0.14; Table 2 and Figure 2). However, between baseline and 36 weeks, LCZ696 patients experienced a smaller decline in eGFR compared with patients treated with valsartan (–1.5 ± 13.1 vs. –5.2 ± 11.4 mL/min.1.73 m2, between-group P = 0.008; Table 2 and Figure 2). Corresponding to the changes in eGFR, mean serum creatinine increased by +0.03 ± 0.18 mg/dL in the LCZ696 group and by +0.09 ± 0.20 mg/dL in the valsartan group at 36 weeks (between group P = 0.007; Figure 3). Baseline cystatin C was lower in the LCZ696 than in the valsartan group [1.1 mg/L (IQR 1.0–1.3) vs. 1.3 mg/L (IQR 1.0–1.5), between group P < 0.001; Table 3]. Mean change between baseline and 12 weeks (between-group P = 0.44) and between baseline and 36 weeks (between-group P = 1.0) was similar between both groups (Table 3).
| eGFR (ml/min per 1.73 m2) | LCZ696 | Valsartan | P-value | Adjusted P-value |
|---|---|---|---|---|
| Baseline | 66.5 ± 19.4 | 64.3 ± 21.3 | 0.34 | 0.35a |
| 12 weeks: change from baseline | –0.2 ± 14.9 | –2.0 ± 11.9 | 0.29 | 0.14b |
| 36 weeks: change from baseline | –1.5 ± 13.1 | –5.2 ± 11.4 | 0.025 | 0.008b |
- Data are presented as mean ± SD.
- a Adjusted for stratification criteria.
- b Adjusted for stratification criteria and baseline value of the outcome of interest (eGFR).
| Cystatin C | LCZ696 | Valsartan | P- value | Adjusted P-value |
|---|---|---|---|---|
| Baseline | 1.1 [1.0–1.3] | 1.3 [1.0–1.5] | 0.003 | <0.001 |
| 12 weeks: change from baseline | 0 [−0.1–+0.1] | 0 [−0.1–+0.1] | 1.00 | 0.44 |
| 36 weeks: change from baseline | 0.1 [−0.1–+0.2] | 0.1 [0.0–0.3] | 1.00 | 1.00 |
- Data are presented as median and interquartile range.
- a Adjusted for stratification criteria.
- b Adjusted for stratification criteria and baseline value of the outcome of interest (cystatin C).
Change in urinary albumin excretion
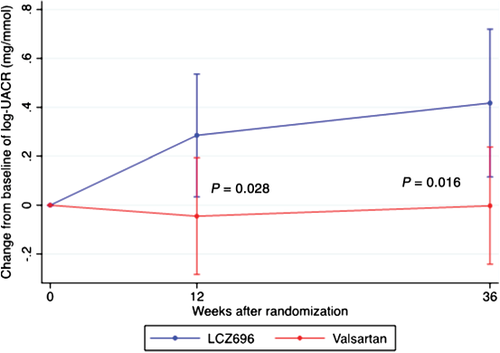
Changes in UACR during treatment observed at 12 weeks and 36 weeks are presented in Table 4 and Figure 4. As quantified by geometric means, UACR increased rapidly after starting treatment in the LCZ696 group, while UACR remained stable in the valsartan group, resulting in a significant difference in UACR between the two intervention groups. Over 36 weeks, the geometric mean of UACR increased in the LCZ696 group (2.4–2.9 mg/mmol), whereas it remained stable in the valsartan group (2.1–2.0 mg/mmol; P for difference at 36 weeks = 0.016). A sensitivity analysis of median UACR values did not detect a significant difference (LCZ696 1.9–1.9 mg/mmol; valsartan 1.4–1.3 mg/mmol; P = 0.23 for difference).
| UACR (mg/mmol) | LCZ696 | Valsartan | P- value | Adjusted P-value |
|---|---|---|---|---|
| Baseline | 2.4 (1.8–3.2) | 2.1 (1.6–2.6) | 0.40 | 0.44a |
| 12 weeks: change from baseline | 3.0 (2.2–4.0): +33.0% | 2.1 (1.6–2.7): –4.4% | 0.06 | 0.028b |
| 36 weeks: change from baseline | 2.9 (2.1–4.0): +51.8% | 2.0 (1.5–2.6): –0.2% | 0.034 | 0.016b |
- Baseline data are presented as geometric mean (95% confidence interval) and % change from baseline.
- a Adjusted for stratification criteria.
- b Adjusted for stratification criteria and baseline value of the outcome of interest (log-UACR).
Worsening renal function and independent predictors
During treatment, 5% and 6% of patients developed WRF at 12 weeks and 36 weeks, respectively, in the LCZ696 group. In the valsartan group, WRF occurred in 7% and 13% of patients at 12 weeks and 36 weeks, respectively (adjusted P for stratification criteria and baseline creatinine = 0.68 and 0.08, respectively; Table 5). In addition in the overall group, 15% of patients developed WRF at any time-point, of which 18% in the valsartan group vs. 12% in the LCZ696 group (adjusted P = 0.28).
| WRF | LCZ696 | Valsartan | P-value | Adjusted P-valuea |
|---|---|---|---|---|
| Baseline | ||||
| 12 weeks | 6 (5%) | 9 (7%) | 0.60* | 0.68 |
| 36 weeks | 7 (6%) | 16 (13%) | 0.08* | 0.08 |
| At anytime | 16 (12%) | 25 (18%) | 0.18* | 0.28 |
- Worsening renal function (WRF) defined as increase of serum creatinine >0.3 g/dL and >25%.;WRF (compared with baseline) at 12, 36 weeks, and at anytime in both groups.
- Data are presented as n (%).
- a Adjusted for stratification criteria and baseline value of the outcome of interest (creatinine).
- * Fisher exact test.
Safety analysis
Adverse events were coded using the Medical Dictionary for Regulatory Activities.22, 23 A total of 15 adverse events were associated with the high-level group term ‘renal disorders (excluding nephropathies)’. These occurred in three patients in the LCZ969 group (2.0%) and nine patients in the valsartan group (5.9%) (Fisher exact P = 0.14).
Discussion
In the first study reporting on the renal effects of the first-in-class ARNi LCZ696 in patients with HFpEF, we found that treatment with LCZ696 resulted in lower serum creatinine and higher eGFR after 36 weeks of treatment, compared with valsartan. However, cystatin C levels remained similar and UACR was significantly higher in the LCZ696 group, compared with the valsartan group.
As presented in the main study report,4 blood pressure dropped more in the LCZ696 group compared to the valsartan group. The greater effect of LCZ696 on blood pressure reduction compared with valsartan alone confirms previous findings.19 In normal kidneys, GFR can be kept constant, despite changes in blood pressure, by autoregulation.24 However, heart failure is often accompanied by renal failure, which blunts the capacity of the pre-glomerular circulation to either constrict or dilate in response to changes in the renal perfusion pressure and thereby narrows the range of blood pressure changes to which GFR can remain stable.24 Furthermore, RAAS inhibiting agents, which are often prescribed to heart failure patients, may partly block the autoregulatory function of the kidney.25 Therefore, in patients with heart failure, changes in blood pressure often correspond to direct changes in GFR. The present study has shown that although blood pressure was reduced more in the LCZ696 group, eGFR was better preserved in this group of patients compared with the valsartan only patients. In addition, safety analysis of the exciting breakthrough PARADIGM trial comparing LCZ696 with enalapril in 8442 patients with HFrEF revealed that elevated creatinine levels of 2.5 mg/dL or more were less frequent in the LCZ696 group (P < 0.05). despite higher incidence of hypotension (P < 0.001). Furthermore, LCZ696-treated patients experienced less renal impairment that required discontinuation of the study drug compared with enalapril treated patients (P = 0.002).3 These findings corroborate our results suggesting that LCZ696 may have direct renal effects on top of the effects of RAAS blockade, such as by valsartan alone. The renal effects of AT1 receptor antagonists and neprilysin inhibitors have been studied separately. AT1 receptor antagonism inhibits the RAAS and often reduces GFR.5 Neprilysin inhibition augments biologically active natriuretic peptides, including atrial natriuretic peptide (ANP), brain natriuretic peptide and C-type natriuretic peptide, as well as other bradykinin and adrenomedullin.26-31 Interestingly, the biological effects of neprilysin inhibition are similar to the reported effects of intravenously administered ANP,26 raising the possibility that the observed biological effects of neprilysin inhibition may be the result of elevated biologically active ANP levels. Several experimental and animal studies have demonstrated that ANP acts directly on the kidney by dilating and constricting the afferent and efferent renal arterioles, respectively, resulting in increased intraglomerular capillary pressure and enhanced GFR.32-36 A study in healthy humans revealed that infusion of ANP was associated with a rise in GFR.13 Thus, evidence suggests that the observed decline in GFR with valsartan in our study may be attributable to AT1 antagonism and/or an expected decline over time, but that the neprilysin inhibiting component of LCZ696 may be capable of neutralizing this decline in GFR, thereby preserving renal function in HFpEF patients treated with LCZ696.
In the present study, worsening renal function occurred in 12% of patients allocated to the LCZ696 group, while the incidence of worsening renal function was 18% in the valsartan group at any time-point. Ain increase in creatinine (and decrease in eGFR) is often seen after initiation of RAAS-inhibiting drugs in both HFrEF and HFpEF. In HFrEF patients, this development of WRF is not associated with worse outcomes.37-40 In contrast, it has recently been demonstrated that in patients with HFpEF, WRF after initiation of irbesartan was associated with an increased risk of hospitalizations because of heart failure and mortality.17 It is conceivable that the lack of increase in creatinine levels seen with LCZ696 compared with a RAAS inhibitor alone would not only reduce the likelihood that practitioners discontinue or underdose beneficial therapies in heart failure, but may also have a beneficial effect on prognosis in HFpEF. However, whether the lesser increase in serum creatinine after initiation of LCZ696 is associated with clinical outcomes in patients with HFpEF should be further investigated.
Interestingly, while we demonstrated a significant difference in serum creatinine (and eGFR) between the two intervention groups, we found no differences in cystatin C levels. Cystatin C is considered to be a better marker of renal function as it is unaffected by increases in age, diet, or muscle mass.41-45 Therefore, one would expect to observe results/trends comparable with creatinine. However, cystatin C has been associated with atherosclerosis and inflammation,46-48 and higher cystatin C levels are observed in hypertensive patients.49-51 In addition, cystatin C has been related to diastolic function.46 These factors may have biased our results as our study population included patients with HFpEF.
In addition to preservation of renal function, treatment with LCZ696 was accompanied by slightly higher UACR compared with valsartan.. Increases in albuminuria have been observed after administration of neprilysin inhibitors and intravenously administered ANP in previous studies.27, 52-57 The possible albuminuric effect of LCZ696 may not simply be the result of changes in renal haemodynamic arising from changes in tonus of the renal arterioles. Few in vitro and in vivo studies reported increased vascular endothelial permeability and capillary hydraulic conductivity58-61, an effect which also may occur in the vascular wall of glomerular capillaries.62 Further, natriuretic peptides may also have a direct relaxing and proliferation inhibitory effects on mesangial cells of the kidney, with the potential of albuminuria progression.63-67 While the majority of patients did not demonstrate substantive changes in UACR with LCZ696, that several patients may have increased UACR with the drug raises the possibility that some individuals may be more susceptible to this effect. In previous studies of LCZ696 in hypertensive patients, increases in UACR were not observed and whether increases in UACR are indeed induced by LCZ696 in patients with heart failure needs to be confirmed in the PARADIGM trial.3, 19 In addition, the prognostic significance of a potential effect of LCZ696 on UACR remains unclear. The rise in UACR in patients treated with LCZ696 may not be a marker of disease progression, but may be an intrinsic effect of the drug.
The present study has several limitations in the addition to the well-known expected limitations of such post hoc analysis. In the PARAMOUNT study, only patients with an estimated glomerular filtration rate of at least 30 mL/min.1.73 m2 were included, and, therefore, we could not assess the renal effects of LCZ696 in patients with severely impaired renal function. In addition, the renal variables of interest were not available for all patients for unknown reasons, and may thus have biased our results even though the numbers of missing were small. Furthermore, we included all patients enrolled in the PARAMOUNT study for the present analyses, but PARAMOUNT was not originally designed to evaluate the renal effects of LCZ696 and because of the low number of patients, this study may have been underpowered.
In summary, in patients with HFpEF, LCZ696 better preserved renal function compared with valsartan after 36 weeks of therapy, as was shown by lower levels of serum creatinine and higher eGFR. These results suggest that LCZ696 may attenuate decline in renal function in patients with HFpEF.
Funding
This work was supported by Novartis.
Conflicts of interest: A.A.V., M.Z., B.P., J.J.V.M., M.P., and S.D.S were members of the executive committee of PARAMOUNT and received consultancy fees from Novartis. V.S. and M.L. are employees of Novartis.




