Impact of worsening renal function related to medication in heart failure
Abstract
Aims
Renal failure is a major challenge in treating heart failure (HF) patients. HF medication may deteriorate renal function, but the impact thereof on outcome is unknown. We investigated the effects of HF medication on worsening renal function (WRF) and the relationship to outcome.
Methods and results
This post-hoc analysis of TIME-CHF (NT-proBNP-guided vs. symptom-guided management in chronic HF) included patients with LVEF ≤45% and ≥1 follow-up visit (n = 462). WRF III was defined as a rise in serum creatinine ≥0.5 mg/dL (i.e. 44.2 µmol/L) at any time during the first 6 months. Four classes of medication were considered: loop diuretics, beta-blockers, renin–angiotensin system (RAS)-blockers, and spironolactone. Functional principal component analysis of daily doses was used to comprehend medication over time. All-cause mortality after 18 months was the primary outcome. Interactions between WRF, medication, and outcome were tested. Patients with WRF III received on average higher loop diuretic doses (P = 0.0002) and more spironolactone (P = 0.02), whereas beta-blockers (P = 0.69) did not differ and lower doses of RAS-blockers were given (P = 0.09). There were significant interactions between WRF III, medicationn and outcome. Thus, WRF III was associated with poor prognosis if high loop diuretic doses were given (P = 0.001), but not with low doses (P = 0.29). The opposite was found for spironolactone (poor prognosis in the case of WRF III with no spironolactone, P <0.0001; but not with spironolactone, P = 0.31). Beta-blockers were protective in all patients (P <0.001), but most in those with WRF III (P <0.05 for interaction). RAS-blockade was associated with improved outcome (P = 0.006), irrespective of WRF III.
Conclusion
Based on this analysis, it may be hypothesized that high doses of loop diuretics might have detrimental effects, particularly in combination with significant WRF, whereas spironolactone and beta-blockers might be protective in patients with WRF.
Introduction
Diuretics, inhibitors of the renin–angiotensin system (RAS), and mineralocorticoid receptor antagonists (MRAs) are known to improve symptoms and/or prognosis of heart failure (HF). However, they can also be associated with worsening renal function (WRF), which in turn may be associated with poor outcome.1 There are some data indicating that the beneficial effects of RAS inhibitors and MRAs may be present even if renal function deteriorates,2-4 as nicely summarized in a recent meta-analysis of five large trials.5 This suggests that there are mitigating factors that can change the prognostic importance of WRF and that the renin–angiotensin–aldosterone system plays an important role herein. However, the differential effects of loop diuretics and agents blocking the renin–angiotensin–aldosterone system on renal function and prognosis remain unknown, and the effects of beta-blockers in this regard have not yet been properly addressed. Moreover, these studies were performed in relatively young, selected patients, have not addressed the potential influence of exposure to medication longitudinally over time, and did not consider doses of medication. In clinical practice, doses of agents known to improve prognosis of HF patients are often reduced or even withheld if renal function deteriorates.6 Whether this practice is justified remains to be determined. We therefore investigated the effects of longitudinal use and dosage of medical HF therapy on outcome in relation to the occurrence or absence of significant WRF in an elderly HF cohort. We modelled dose of medication and its changes on an individual patient level to address the importance of adjusting medication in patients developing WRF.
Methods
This is a post-hoc analysis of the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF; isrctn.org identifier: ISRCTN43596477). The design7 and main results8 of the trial have been published previously. In brief, TIME-CHF was a randomized, controlled multicentre trial comparing an NT-proBNP-guided vs. a symptom-guided management in patients with chronic HF, age ≥60 years, symptoms corresponding to NYHA ≥ II, HF-related hospitalization within 12 months prior to inclusion, and an age-adjusted elevated NT-proBNP level (>400 ng/L in those <75 years, >800 ng/L in those ≥75 years). Patients with both reduced (n = 499) and preserved LVEF (n = 123) were included between January 2003 and December 2006 and followed-up for at least 18 months. The study was approved by the local ethics committees, and all participants provided written informed consent.
The present analysis includes patients with reduced LVEF of ≤45% as only in such patients is medical treatment evidence based.9 Moreover, patients with only one visit (n = 37, 7%; 16 died, 21 withdrew consent) are excluded as in these patients WRF cannot be determined, leaving 462 patients in the study. Patients were followed up in the outpatient clinics after 1, 3, 6, 12, and 18 months, with every visit including history, focused clinical examination, and laboratory analyses including serum creatinine measurement. There were no interim visits in TIME-CHF. Adjustment of medication followed pre-defined escalation rules as previously defined.7
Significant WRF is defined as previously described (i.e. any rise in serum creatinine of ≤0.5 mg/dL as compared with baseline within the first 6 months).10 As we did not find any prognostic impact on slight to moderate WRF, we investigated the interaction between WRF III [(i.e. rise in serum creatinine ≥0.5 mg/dL (44.2 µmol/L)] and medical treatment only.10 The history of various disorders including ‘a history of renal failure’ was not strictly defined, but was based on the medical history mentioned in the patients' records.
Outcome events
All-cause mortality was the primary outcome event for this study, with death or any hospitalization as well as death or HF hospitalization as secondary outcomes, each after 18 months.
Medication
The following four classes of medication were considered for this analysis: loop diuretics, beta-blockers, RAS-blockers (i.e. ACE inhibitors and ARBs), and MRAs. Dosages of RAS-blockers (combination of ACE inhibitors and ARBs) and beta-blockers are expressed as a percentage of recommended target dose as previously described in detail.11 Dosage of loop diuretics is expressed as equivalent dosages of furosemide (40 mg = 10 mg torasemide = 1 mg bumetanide). Spironolactone was the only MRA used and the dosage is given in milligrams.
Patients were clinically followed and the exact medication including dosages was recorded on a day-to-day basis. Patients used a log to record all changes in medication, relatives were involved as required, and in the case of uncertainty the treating physicians including general practitioners (GPs) were contacted and medical records of hospital stays were used. Thus, the medication including doses was known for each patient on a daily basis (Figure 1).
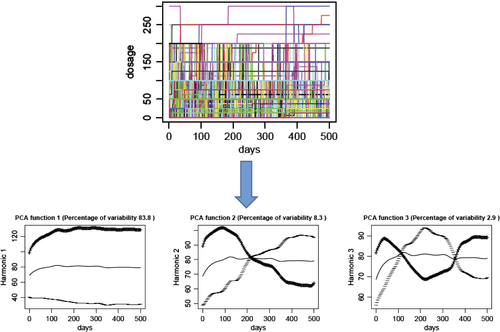
Statistical analysis
Functional principal component analysis (fPCA) was used to comprehend the variability of dosage of medication. fPCA is an extension of principal component analysis to functional data where the principal components are replaced by time-dependent basis functions.12 The goal of fPCA is to represent the variation of the data in the most parsimonious way as a representation of a fixed number of basis functions. In this case, b-splines of degree 19 were used as basis functions.
Variability of dosage of medication was modelled over the first 500 days of the study. The variability of each of the four classes of medication was reduced to three functions, explained in Figure 1. In fact, fPCA allows depiction of the variability of daily medication doses in three simple functions. The fPCA function 1 mainly explained the overall dosage level (low vs. high) and contributed most to explaining the variability (Figure 1, left), function 2 explained any increase or decrease in dosage over time (Figure 1, centre), and function 3 explained any decrease followed by an increase and vice versa (Figure 1, right). Figure S1 of the Supplementary material online, including its legend, depicts four examples for better understanding. As function 3 contributed least and relatively little to the explanation of the daily variability of dosages, only functions 1 and 2 were used for further analysis. The three functions explained >90% (MRA and loop diuretics) to ≥95% (RAS-blockers and beta-blockers) of the daily dose variation. Table 1 depicts the percentage of variability explained by each of these three functions for each of the four classes of drugs.
| fPCA function 1 | fPCA function 2 | fPCA function 3 | Total | |
|---|---|---|---|---|
| RAS-blockade | 83.8% | 8.3% | 2.9% | 95.0% |
| Beta-blockade | 86.5% | 7.1% | 2.3% | 95.9% |
| MRA (spironolactone) | 78.4% | 9.0% | 4.1% | 91.5% |
| Loop diuretics | 82.9% | 7.9% | 3.6% | 94.4% |
- fPCA, functional principal component analysis; MRA, mineralocorticoid receptor antagonist; RAS, renin–angiotensin system.
The results are presented as frequencies (%), mean (SD), or median [interquartile range (IQR)], as appropriate. Parameters of the components of fPCA are presented as quintiles or split by median, but not mentioned numerically, because they cannot be directly expressed in meaningful dosage. Still, mean dosages above and below the median of the first component are presented. Between-group comparisons were performed using the t-test, Mann–Whitney test, or Pearson χ2. Survival and event-free survival were analysed by the Kaplan–Meier method using the log-rank test to compare groups. The main effects and the interaction between WRF and medication were tested using Cox proportional hazard regression models correcting for baseline characteristics age, gender, cause of HF, kidney function, anaemia, NYHA class, systolic blood pressure, presence of AF, NT-proBNP levels, and group allocation. A two-sided P-value of 0.05 was considered to be statistically significant. Calculations were performed with the use of the SPSS statistical package version 21.0 (SPSS Inc., Chicago, IL, USA). Calculations of the fPCA were done in R version 2.13.213 using the package ‘fda’.14
Results
Baseline characteristics of patients with and without worsening renal function III
Baseline characteristics of patients with and without WRF III during the first 6 months are shown in Table 2. Interestingly, most characteristics did not differ significantly between the two groups. Still, patients with WRF III more often had previously known renal dysfunction and peripheral oedema, had higher NT-proBNP levels, and were treated with higher doses of loop diuretics.
| No WRF III (n = 365) | WRF III (n = 97) | P-value | |
|---|---|---|---|
| Age (years) | 76 ± 7 | 75 ± 8 | 0.31 |
| Male gender | 232 (64%) | 70 (72%) | 0.12 |
| Body mass index (kg/m2) | 25.3 ± 4.1 | 26.0 ± 4.5 | 0.15 |
| CAD as main cause of HF | 200 (55%) | 58 (60%) | 0.42 |
| Hypertension | 257 (70%) | 73 (75%) | 0.38 |
| Diabetes | 116 (32%) | 37 (38%) | 0.28 |
| Stroke/TIA | 56 (15%) | 15 (16%) | 1.00 |
| COPD | 71 (19%) | 26 (27%) | 0.12 |
| PAOD | 66 (18%) | 19 (20%) | 0.77 |
| Renal failure | 182 (50%) | 66 (68%) | 0.002 |
| Charlson score | 3 (2–4) | 3 (2–4) | 0.24 |
| Systolic BP (mmHg) | 118 ± 18 | 119 ± 18 | 0.78 |
| Heart rate (b.p.m.) | 76 ± 15 | 75 ± 14 | 0.87 |
| Sinus rhythm | 228 (77%) | 67 (70%) | 0.34 |
| Atrial fibrillation | 117 (32%) | 4 (25%) | 0.17 |
| NYHA class ≥ III | 330 (75%) | 92 (74%) | 0.91 |
| History of oedema | 191 (44%) | 65 (53%) | 0.08 |
| Oedema at inclusion | 170 (39%) | 60 (49%) | 0.05 |
| Rales at inclusion | 184 (42%) | 60 (49%) | 0.22 |
| NT-proBNP (ng/L) | 3805 (2080–6665) | 5083 (2741–8610) | 0.009 |
| Creatinine (µmol/L) | 115 ± 37 | 120 ± 40 | 0.23 |
| Potassium (mmol/L) | 4.1 ± 0.5 | 4.1 ± 0.6 | 0.95 |
| BUN (mmol/L) | 10.8 ± 4.6 | 11.8 ± 6.5 | 0.18 |
| Haemoglobin (g/L) | 134 ± 17 | 131 ± 19 | 0.20 |
| LVEF (%) | 30 ± 8 | 30 ± 8 | 0.78 |
| QRS width (ms) | 125 ± 36 | 126 ± 35 | 0.74 |
| ACE inhibitor/ARB (% target dose) | 50 (25–66.7) | 50 (25–66.7) | 0.54 |
| Beta-blocker (% target dose) | 25 (12.5–50) | 18.8 (5–50) | 0.16 |
| Spironolactone (mg) | 0 (0–25) | 0 (0–25) | 0.13 |
| Loop diuretic (mg furosemide equivalent) | 40 (40–80) | 80 (40–120) | <0.001 |
- BP, blood pressure; BUN, blood urea nitrogen; HF, heart failure; PAOD, peripheral arterial obstructive disease; TIA, transient ischaemic attack; WRF, worsening renal function.
Medication according the first function of the functional principal component analysis
Average doses of medication above and below the median of the first function were by definition significantly different (all P < 0.0001). Thus, the median (IQR) dose was 49 (27–59) vs. 100 (89–117)% of the target dose for RAS-blockers, 13 (9–25) vs. 50 (38–72)% of the target dose for beta-blockers, 37 (20–45) vs. 97 (79–157) mg of furosemide equivalent for loop diuretics, and 0 (0–0.3) vs. 24 (16–25) mg for spironolactone.
Medication related to worsening renal function III
Overall levels of medication during the course of the study in patients with and without WRF III differed mainly for diuretics and spironolactone and to a limited extent for ACE inhibitors, whereas beta-blockers were not different between the two groups (Figure 2). Thus, overall dosage of ACE inhibitors tended to be lower in patients with WRF III. Significantly more patients with WRF III received the highest overall dosages of loop diuretics and fewer dosages in the lowest two quintiles. Regarding spironolactone, patients who experienced WRF III less often received any spironolactone, but there was no clear dose–response relationship in those receiving spironolactone (Figure 2). These associations were independent of clinical characteristics in multivariable analysis (data not shown). In particular, history of renal dysfunction, signs of congestion, and NT-proBNP levels did not influence these associations, but were independently related to WRF III.
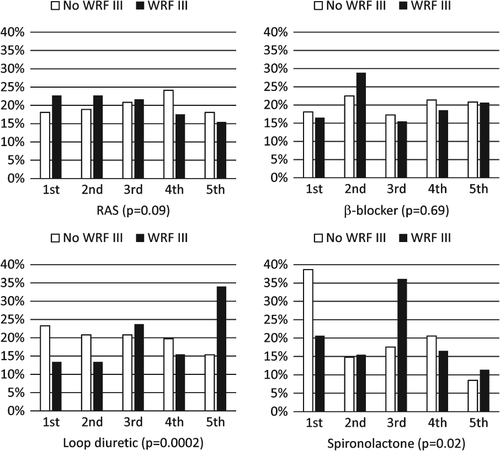
The second function (increase in dose over time as compared with a decrease) differed significantly regarding spironolactone (P = 0.03). Thus, down-titration as compared with up-titration was more often seen in patients with WRF III. For the other drugs, no significant effect was seen (RAS-blockers, P = 0.11; beta-blockers, P = 0.38; loop diuretics, P = 0.83).
Effects of worsening renal function III on outcome related to therapy
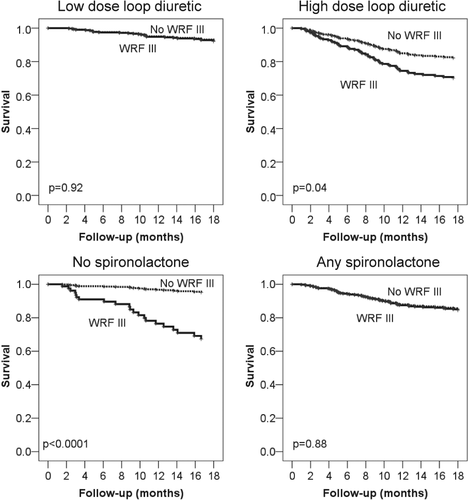
There was a significant interaction between the effect of WRF III on outcome and medication (Table 3), particularly for both loop diuretics and spironolactone, as depicted in Figure 3. Thus, in patients receiving low dose loop diuretics (based on the first function of fPCA), WRF III was not significantly related to poor outcome, whereas this was the case in those with high doses of loop diuretics (interactions: survival, P = 0.04; survival free of HF hospitalization, P = 0.1; survival free of any hospitalization, P = 0.01). The opposite was seen with respect to spironolactone. In patients receiving no spironolactone during the first 6 months or minimal doses (based on the first function of fPCA), WRF III was associated with poor outcome. In contrast, in patients receiving spironolactone, WRF III did not result in worse outcome as compared with patients not having WRF III (any spironolactone during the first 6 months interactions: survival, P = 0.001; survival free of HF hospitalization, P = 0.05; survival free of any hospitalization, P = 0.14; dose of spironolactone interactions: survival, P = 0.04; survival free of HF hospitalization, P = 0.002; survival free of any hospitalization, P = 0.02). No significant interactions were seen regarding beta-blockers and RAS-blockers in this regard (all interactions P > 0.1, apart for beta-blockade with survival free of HF hospitalization, P = 0.04; and survival free of any hospitalization, P = 0.003).
| Loop diuretic dose below median | Loop diuretic dose above median | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 1.05 | 0.40–2.81 | 0.92 | 1.81 | 1.03–3.18 | 0.04 |
| Survival free of HF hospitalization | 0.98 | 0.47–2.06 | 0.97 | 1.84 | 1.20–2.84 | 0.006 |
| Survival free of any hospitalization | 0.85 | 0.51–1.40 | 0.52 | 1.58 | 1.10–2.29 | 0.01 |
| No spironolactone during first 6 months | Any spironolactone during first 6 months | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 8.05 | 3.00–21.53 | <0.0001 | 1.04 | 0.59–1.86 | 0.88 |
| Survival free of HF hospitalization | 2.66 | 1.26–5.61 | 0.01 | 1.28 | 0.84–1.97 | 0.25 |
| Survival free of any hospitalization | 1.66 | 0.94–2.94 | 0.08 | 1.06 | 0.76–1.49 | 0.73 |
| Spironolactone dose below median | Spironolactone dose above median | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 2.96 | 1.34–6.58 | 0.007 | 1.11 | 0.58–2.14 | 0.75 |
| Survival free of HF hospitalization | 2.56 | 1.38–4.72 | 0.003 | 0.89 | 0.54–1.47 | 0.65 |
| Survival free of any hospitalization | 1.62 | 1.02–2.56 | 0.04 | 0.94 | 0.63–1.39 | 0.94 |
| RAS-blocker dose below median | RAS-blocker dose above median | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 1.36 | 0.73–2.52 | 0.33 | 1.76 | 0.74–4.18 | 0.20 |
| Survival free of HF hospitalization | 1.62 | 1.01–2.56 | 0.04 | 1.34 | 0.70–2.58 | 0.37 |
| Survival free of any hospitalization | 1.20 | 0.86–1.76 | 0.35 | 1.17 | 0.74–1.85 | 0.51 |
| Beta-blocker dose below median | Beta-blocker dose above median | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 1.96 | 1.10–3.48 | 0.02 | 1.44 | 0.60–3.47 | 0.42 |
| Survival free of HF hospitalization | 2.16 | 1.34–3.47 | 0.002 | 0.97 | 0.53–1.78 | 0.91 |
| Survival free of any hospitalization | 1.78 | 1.21–2.61 | 0.003 | 0.77 | 0.49–1.21 | 0.26 |
- CI, confidence interval; HF, heart failure; HR, hazard ratio; RAS, renin–angiotensin system.
Results on the prognostic effect of WRF III related to the second function of medication in fPCA are depicted in Table 4. Thus, WRF III was associated with poor outcome in patients with increasing doses of loop diuretics over time, but not when loop diuretics were reduced over time. The same was true for spironolactone, but it is important to note that in patients newly receiving spironolactone during the course of the trial, WRF III had no negative effect on outcome [survival, hazard ratio (HR) 0.69, 95% confidence interval (CI) 0.20–2.45, P = 0.57; survival free from HF hospitalization, HR 1.40, 95% CI 0.71–2.75, P = 0.33; survival free from any hospitalization, HR 1.11, 95% CI 0.67–1.85, P = 0.68]. Effects of changes in dose of RAS-blockade and beta-blockade were less distinct, but WRF III tended to be more related to poor outcome in patients with decreasing RAS-blockade dose.
| Loop diuretic dose ‘increase’ | Loop diuretic dose ‘decrease’ | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 2.97 | 1.39–6.35 | 0.005 | 1.37 | 0.69–2.70 | 0.37 |
| Survival free of HF hospitalization | 2.25 | 1.33–3.82 | 0.003 | 1.07 | 0.61–1.89 | 0.81 |
| Survival free of any hospitalization | 1.38 | 0.90–2.12 | 0.14 | 1.02 | 0.68–1.55 | 0.91 |
| Spironolactone dose ‘increase’ | Spironolactone dose ‘decrease’ | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 3.63 | 1.66–7.91 | 0.001 | 1.06 | 0.57–1.97 | 0.87 |
| Survival free of HF hospitalization | 2.53 | 1.44–4.44 | 0.001 | 1.05 | 0.64–1.73 | 0.85 |
| Survival free of any hospitalization | 1.63 | 1.05–2.51 | 0.03 | 0.98 | 0.66–1.46 | 0.93 |
| RAS-blocker dose ‘increase’ | RAS-blocker dose ‘decrease’ | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 1.18 | 0.53–2.60 | 0.69 | 2.49 | 1.30–4.76 | 0.006 |
| Survival free of HF hospitalization | 1.55 | 0.87–2.76 | 0.13 | 1.91 | 1.15–3.16 | 0.01 |
| Survival free of any hospitalization | 1.17 | 0.75–1.82 | 0.49 | 1.43 | 0.95–2.15 | 0.09 |
| Beta-blocker dose ‘increase’ | Beta-blocker dose ‘decrease’ | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Survival | 1.47 | 0.69–3.13 | 0.33 | 1.60 | 0.85–3.02 | 0.14 |
| Survival free of HF hospitalization | 1.60 | 0.94–2.73 | 0.08 | 1.38 | 0.81–2.34 | 0.23 |
| Survival free of any hospitalization | 1.27 | 0.84–1.93 | 0.26 | 1.18 | 0.78–1.79 | 0.43 |
- CI, confidence interval; HF, heart failure; HR, hazard ratio; RAS, renin–angiotensin system.
Effects of medication on outcome related to worsening renal function III
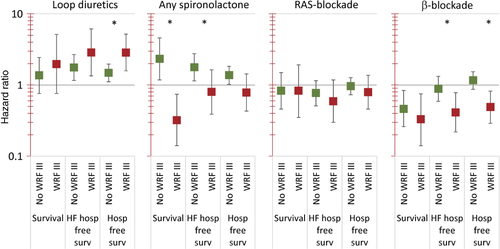
The effects of higher doses of medication on outcome differed to some extent depending on whether patients without and with WRF III were considered. Thus, results on the effects of high vs. low doses after adjustment of multiple covariates known to be related to prognosis in patients with and without WRF III are depicted in Figure 4. High doses of loop diuretics were accompanied by poor outcome, but this effect was seen most in patients with WRF III. In contrast, spironolactone was associated with poor outcome even after adjustment of covariates if WRF III was absent, but with improved prognosis in the case of WRF III (interactions for all endpoints P <0.01). The effects of overall dose of RAS-blockade were not statistically significant apart from a positive effect on survival free of HF hospitalization with higher overall RAS-blockade dosage. The use of higher doses of beta-blocker was associated with better outcome even after adjustment for multiple factors. This was the case in both patients with and without WRF III. Still, this effect was larger in patients with WRF III. If the absolute value of the first function of fPCA was used instead of dichotomized values, similar effects were seen (data not shown). This was also true for spironolactone, when considering the first function of fPCA (corresponding to dose over time) instead of the presence or absence of spironolactone during the first 6 months as used in Figure 4.
Discussion
The data of this study provide intriguing new insights into the cardiorenal syndrome that could have an important impact on the treatment of HF patients with RF. Diverging effects of the most important classes of HF medications in relation to significant WRF and its effect on prognosis were found. The negative effects of high doses of loop diuretics on outcome were documented predominantly in patients with significant WRF. For spironolactone, the opposite was seen, i.e. it was associated with most beneficial effects in patients with significant WRF. Similar results were found with beta-blockers. Also for RAS-blockade, significant WRF was not associated with negative effects. Therefore, it may be hypothesized that the often encountered clinical practice of reducing or even stopping spironolactone, RAS-blockade, and sometimes also beta-blockers and increasing doses of loop diuretics to overcome renal resistance to diuretic therapy15 might not be appropriate. However, prospective studies are required to test this hypothesis before these results are implemented in clinical practice.
Use of diuretics in patients with HF has not widely been studied. There are no large randomized trials that have investigated the long-term effects of diuretics on prognosis, as diuretics are used to treat symptoms of congestion and to maintain euvolaemia.9 Attempts to withhold diuretics after the introduction of ACE inhibitors in the therapy of HF failed.16 However, patients with HF treated with diuretics usually have worse prognosis even after propensity score matching,17 as seen in this study. This is, at least in part, related to the fact that patients requiring (high doses of) diuretics usually have more advanced HF, but overuse of diuretics may also be an underestimated problem in HF.18 To what extent this negative effect is related to WRF is difficult to determine. Our data suggest that the interaction between loop diuretics and renal dysfunction is of importance as significant WRF was not associated with poor outcome if low doses of loop diuretics were used. Importantly, treatment by drugs that have been shown to improve outcome, i.e. ACE inhibitors, MRAs, and beta-blockers, may also lead to reduced filling pressures in the long term.19 In line with this is the finding that (NT-pro)BNP-guided studies that resulted in intensifying therapy with such drugs in the intervention group showed more positive effects on outcome compared with those that mainly focused on the increase in diuretic therapy.20
Such negative association between high doses, WRF, and poor outcome was not seen for spironolactone, RAS-blockade, and beta-blockade. In fact, the opposite was the case for beta-blockers and spironolactone. In patients with significant WRF III, spironolactone and beta-blockers in higher doses were associated with better outcome. The latter is in line with post-hoc analyses of two of the large beta-blocker trials, where beta-blockers were found to be equally21 or even more effective22 in patients with renal dysfunction as compared with those with normal or only slightly reduced renal function. In addition, beta-blockers were shown to reduce risk in patients with chronic kidney disease, incident HF, and systolic LV dysfunction.23 However, to the best of our knowledge, there is no information available on the association of beta-blocker treatment in HF patients related to WRF. The underlying reason for the positive finding in our study is not clear, but improvement in cardiac function by beta-blockade is most probably an important part of it. Thus, beta-blockers in patients with HF and reduced LVEF resulted in the most prominent improvement in prognosis,24 but also showed improvement in cardiac function as measured by increased LVEF,25 which seems to be larger than with other HF drugs.26, 27 However, positive effects on the kidneys themselves are also possible. Thus, in an experimental model on daunorubicin toxicity, carvedilol was able to reduce both nephrotoxicity and cardiotoxicity.28 It might be speculated that the positive effects are most prominent in the most susceptible patients, but obviously prospective intervention trials are required to investigate this hypothesis further.
Similarly, spironolactone was associated with better outcome in those patients with WRF III, which is in line with post-hoc analyses of RALES and EPHESUS.2-5 No overall beneficial effect of spironolactone was found in our study even after adjustment for baseline characteristics, which is in line with results of trials not randomizing for MRAs or population-based studies;29-31 but results have not been uniform.32 It is obvious that one has to be very cautious about the interpretation of such post-hoc analyses because it is impossible to adjust for all confounding factors. This is particularly true given the evidence of using MRAs in HF with reduced LVEF based on large randomized controlled trials.33, 34 Thus, it is likely that imbalance between patients with and without use of spironolactone contributed to our finding. Therefore, our data must not be interpreted to indicate that MRAs should not be used in patients without significant WRF, but rather that significant WRF must not be a reason for not using MRAs, obviously with the required precaution of regularly measuring potassium and renal function9 and possibly with some reduction in dose if required, as suggested by our findings on the association between decreasing doses of spironolactone and better outcome in WRF patients. Thus, it may be speculated that adding spironolactone even in the case of significant WRF may be safe, but that an increase in dose might be harmful, in contrast to further increasing doses of RAS-blockade and beta-blockade. Obviously, this remains speculative until properly tested in a prospective manner.
This study has important limitations. The most important one is the retrospective analysis on effects of medication related to outcome that is not based on randomized allocation to any specific medication. Moreover, the group with WRF III was rather small. Therefore, the data presented here must be considered as hypothesis generating only, and prospective testing is required. Still, the results are appealing for more properly treating a challenging, clinically important group of HF patients in the future as well as better understanding and addressing the pathophysiology of cardiorenal syndrome. Various definitions of WRF has been used,1 and the definition of WRF may influence the results. In fact, when using a less strict absolute change of 0.3 mg/dL or a relative change of 25%, effects of WRF on outcome related to medication were less, but trends were similar and some still statistically significant (data not shown). However, as we did not find any influence of less severe WRF on outcome,10 it might be argued that relatively small changes over a longer period of up to 6 months as used in this study are not relevant and fewer or no interactions could be expected. In addition, we did not test the effects of changing medication on short-term clinical effects. Therefore, the data cannot be interpreted in a way that short-term use of diuretics may be harmful and that other drugs such as MRAs, RAS-blockers, and beta-blockers should be used in decompensated HF. This is particularly true since renal venous congestion is an important factor of WRF.15 The results reinforce the long-term strategies, which are to a large extent in line with the current guidelines.9 but which are often not applied in clinical practice, particularly in elderly patients and those with significant WRF. Finally, we cannot guarantee that every single dose adjustment has been recorded and that the patients were always compliant. However, we put a lot of effort into recording medication and recording compliance. Therefore, it is unlikely that incorrect recording significantly influenced the results. In fact, an important strength of our analysis is the consideration of the dose of individual medication and longitudinal changes in it.
Conclusion
Based on this analysis, it may be hypothesized that HF medication may have different effects on outcome in patients with significant WRF. Whereas long-term use of high doses of loop diuretics might be particularly deleterious, long-term use of low doses of spironolactone and high doses of beta-blockers may be of particular benefit in HF patients with WRF III. In addition, RAS-blockers also in higher doses do not seem to have any negative effects in such patients.5 These retrospective findings are hypothesis generating only, but may help to better understand cardiorenal syndrome and to set up trials for more appropriate treatment of this difficult patient population.
Funding
The TIME-CHF study was sponsored by the Horten Research Foundation (Lugano, Switzerland; 55% of the study's budget), as well as by smaller unrestricted grants from AstraZeneca Pharma, Novartis Pharma, Menarini Pharma, Pfizer Pharma, Servier, Roche Diagnostics, Roche Pharma, and Merck Pharma (all Switzerland). This analysis was additionally supported by Roche Diagnostics, Germany.
Conflict of interest: none declared.




