Super-responders to cardiac resynchronization therapy remain at risk for ventricular arrhythmias and benefit from defibrillator treatment
Abstract
Aims
Mortality and ventricular arrhythmias are reduced in patients responding to cardiac resynchronization therapy (CRT). This response is accompanied by improvement in LVEF, and some patients even outgrow original eligibility criteria for implantable cardioverter-defibrillator (ICD) implantation. It is however unclear if these patients still benefit from ICD treatment. The current study aimed to evaluate if the incidence of ICD therapy is related to the extent of CRT response.
Methods and results
All patients who underwent primary prevention CRT-defibrillator implantation were included. They were divided into subgroups according to the reduction in LV end-systolic volume (LVESV) 6 months after implantation. Pre-defined subgroups were: negative responders (increased LVESV), non-responders (decreased LVESV 0–14%), responders (decreased LVESV 15–29%), and super-responders (decreased LVESV ≥30%). During a median follow-up of 57 months (25th–75th percentile 39–84), 512 patients were studied [101 (20%) negative responders, 101 (20%) non-responders, 149 (29%) responders, and 161 (31%) super-responders]. In the first year of follow-up super-responders received significantly less appropriate ICD therapy (3% vs. 12%; P < 0.001). The 5-year cumulative incidence of appropriate ICD therapy was 31% [95% confidence interval (CI) 19–43] in negative responders, 39% (95% CI 25–53) in non-responders, 34% (95% CI 25–43) in responders, and 27% (95% CI 18–35) in super-responders, respectively (p = 0.13).
Conclusions
The extent of CRT response was associated with a parallel reduction of appropriate device therapy during the first year of follow-up. Thereafter, no association was observed. Furthermore, 23% of super-responders were treated for potentially life-threatening arrhythmias and benefit from ICD treatment.
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment modality for patients with advanced stages of congestive heart failure. CRT is effective in relieving symptoms of heart failure, in decreasing the number of heart failure-related hospitalizations, and in reducing mortality.1-5 There is, however, a wide variability in the extent of response to CRT among patients. Of all CRT recipients, 9–47% experience major improvements with extensive LV reverse remodelling resulting in a significant improvement of LV function (super-responders), whereas ∼30% show unchanged or even increasing cardiac volumes and deteriorating LV function (non-responders and negative responders).6-13 In addition, a parallel association between the extent of response to CRT and survival has been described, in which a greater LV remodelling is associated with a favourable outcome and lower mortality.8, 14, 15 Therefore, a substantial part of the current research aims to predict response to CRT before implantation of the device. Patient characteristics predictive for super-response are a shorter period of symptomatic heart failure, non-ischaemic cardiomyopathy, prolonged QRS duration (especially due to LBBB), or echocardiographic mechanical dyssynchrony.6, 7 Nevertheless it is currently impossible to predict response accurately.
Furthermore, response to CRT has been associated with a lower rate of ventricular arrhythmias.16-19 It is often presumed that as for survival benefit, the occurrence of ventricular arrhythmias is also associated with the extent of response; subsequently it is questioned whether super-responders still benefit from implantable cardioverter-defibrillator (ICD) treatment. However, there are limited data supporting this assumption. Therefore, the purpose of this study was to evaluate the incidence of ICD therapy in CRT-defibrillator (CRT-D) negative responders, non-responders, responders, and super-responders. We aimed to clarify associations between extent of response and ICD therapy at 1- and 5-year follow-up and additionally assess whether super-responders, characterized by near normalization of LVEF, still benefit from ICD treatment.
Methods
Patients
All patients undergoing CRT-D implantation at the Leiden University Medical Centre, the Netherlands were registered in the departmental Cardiology Information System (EPD-vision®, Leiden University Medical Centre). Baseline characteristics and implantation reports were collected, patients were followed prospectively, and all visits were documented. All included CRT-D recipients met the following selection criteria for implantation: NYHA functional class ≥ II, reduced LVEF <35%, and QRS duration >120 ms; consequently, every CRT-D implantation was based on international guidelines.20 Additionally, patients with secondary prevention ICD implantation, patients with a CRT-pacemaker (CRT-P) device prior to CRT-D implantation, patients with congenital or monogenetic heart disease, patients with decompensated heart failure at the time of implant, patients with myocardial infarction <3 months prior to implantation, or patients after LV reconstructive surgery were excluded from this analysis.
Device implantation and programming
The CRT-D devices used were manufactured by Biotronik (Berlin, Germany), Boston Scientific [Natick, MA, USA, formerly CPI, Guidant (St. Paul, MN, USA)], Medtronic (Minneapolis, MN, USA), or St Jude Medical/Ventritex (St. Paul, MN, USA). The coronary sinus was visualized by venogram using a balloon catheter; thereafter the LV lead was inserted and positioned using an 8 F guiding catheter. LV lead positioning was preferred in a lateral or posterolateral vein. The right atrial and right ventricular leads were positioned conventionally. Additionally, sensing and pacing thresholds were tested and a defibrillation threshold test was performed. All devices were programmed with three different detection zones. The first zone (ventricular arrhythmia monitoring zone) was programmed to detect ventricular arrhythmias from 150 b.p.m. to 188–190 b.p.m (30–32 intervals were needed for detection or 8/10 with a 2.5 s initial delay depending on the manufacturer). The second ventricular arrhythmias zone was programmed to detect ventricular arrhythmias from 188–190 b.p.m to 220–231 b.p.m (22–30 intervals were needed for detection or 8/10 with a 2.5 s initial delay depending on the manufacturer); in this zone, with antitachycardia pacing (ATP), 2–4 separate bursts initially attempt to terminate the arrhythmia, followed by shock if the arrhythmia continues. The third zone was the fast ventricular tachycardia or ventricular fibrillation zone which was programmed to detect ventricular arrhythmias exceeding 220–231 b.p.m (12–30 intervals were needed for detection or 8/10 with a 1.0 s initial delay depending on the manufacturer); a defibrillator shock was the initial therapy. When clinically indicated, standard zone limits or detection intervals were adjusted. Moreover supraventricular tachycardia discriminators were enabled and atrial arrhythmia detection was set to >170 b.p.m.
Echocardiographic evaluation
All patients underwent extensive two-dimensional echocardiographic evaluation before and 6 months after implantation.
Echocardiographic images were obtained using commercially available systems (Vivid 7 and E9, GE-Vingmed Ultrasound, Milwaukee, WI, USA) using 3.5 MHz and M5S transducers. Two-dimensional and colour Doppler data were collected. The LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LVEF were calculated according to the Simpson's method using the apical two- and four-chamber views (EchoPac 112.0.1, GE Medical Systems, Horten, Norway).21 Volumes were traced from the two- and four-chamber views and then the LVEF is calculated.
Severe mitral regurgitation (MR) was defined as MR > grade 2. LV dyssynchrony was quantified using the systolic septal to lateral delay (S–L delay).22, 23 Based on the group median, patients were divided into S–L delay </>50 ms and LVESV </>130 mL.
Definition of response
For the current analysis, patients were divided into subgroups according to echocardiographic response at 6 months, as measured by the extent of LV reverse remodelling.7, 8 The pre-defined subgroups were: (i) negative responders (increased LVESV); (ii) non-responders (decreased LVESV 0–14%); (iii) responders (decreased LVESV 15–29%); and (iv) super-responders (decreased LVESV ≥30%).
Follow-up
Periodical follow-up visits were scheduled every 3–6 months, or more often when clinically indicated. During visits, patients were clinically assessed and devices were interrogated. Device printouts were checked, ICD treatment was registered, and intracardiac electrograms were classified by supervising electrophysiologists or device-cardiologists. Appropriate defibrillator therapy (ATP and shock) was defined as ICD therapy occurring in response to sustained ventricular tachycardia or ventricular fibrillation. All other triggers for ICD therapy were considered inappropriate. Patients were considered lost to follow-up if visits were not performed for more than 6 months. Survival status was based on municipal civil registries. Mode of death was retrieved from letters and follow-up reports in hospital or by general practitioners' records.
Endpoints
Primary endpoints were appropriate defibrillator therapy (ATP and shock) and appropriate defibrillator shock. All-cause mortality was defined as a secondary endpoint.
Statistical analysis
Statistical analyses were performed by using the statistical software program SPSS 20.0 (Chicago, IL, USA). Continuous variables are expressed as mean with standard deviation (SD) or, when indicated, median with 25th–75th percentile. Categorical variables are presented as numbers and percentages. Baseline differences were calculated using the one-way analysis of variance (ANOVA) test for continuous data, and χ2 test for categorical data. A value of P < 0.05 was considered significant. Cumulative event rates were calculated using Kaplan–Meier curves with log-rank statistics and, additionally, yearly cumulative incidences were compared using a local test. Finally, a prediction model of appropriate ICD therapy was constructed. Baseline variables and echocardiographic parameters were transformed to categorical variables and included in a univariate logistic regression model. Variables with a P < 0.10 were further analysed. Using Pearson's correlation test, we checked for correlation. If covariates had moderate to severe correlation (correlation coefficient significantly greater than 0.4), the covariate with the highest association with the primary endpoint was included. Subsequently a multivariate logistic regression model was constructed, using backward stepwise selection until all variables in the model reached a P < 0.05.
Results
Patients
Since 2000, 874 patients received a CRT-D system at the Leiden University Medical Centre. Subsequently, 204 (23%) secondary prevention ICD recipients, 71 (8%) patients with CRT-P prior to CRT-D, and 12 (1%) patients with congenital or monogenetic heart disease were excluded for this analysis. Within 6 months, 2 (0%) patients underwent a heart transplantation, 10 (2%) did not receive biventricular pacing, 31 (5%) died, and 32 (5%) had incomplete 6-month follow-up echocardiographic evaluations. These patients were also excluded.
The current study population therefore consisted of 512 patients with a median age of 68 years (25th–75th percentile 61–74 years), the majority had ischaemic heart disease (53%), advanced heart failure (77% NYHA class ≥ III), a reduced LVEF of 24 ± 6%, and a prolonged QRS duration 164 ± 23 ms (Table 1).
| All, n = 512 | Negative responders, n = 101 | Non-responders, n = 101 | Responders, n = 149 | Super-responders, n = 161 | P-value | |
|---|---|---|---|---|---|---|
| Clinical parameters | ||||||
| Median follow-up, months | 46 ± 40 | 37 (33) | 40 (28) | 50 (37) | 52 (45) | |
| Age, years | 68 ± 13 | 68 (15) | 69 (12) | 67 (15) | 67 (14) | |
| Male sex | 375 (73%) | 81 (80%) | 72 (71%) | 117 (79%) | 105 (65%) | 0.02 |
| Ischaemic heart disease | 270 (53%) | 61 (60%) | 64 (63%) | 81 (54%) | 64 (40%) | <0.001 |
| ICD upgraded to CRT-D | 11 (2%) | 2 (2%) | 3 (3%) | 1 (1%) | 5 (3%) | |
| NYHA functional class | ||||||
| II | 116 (23%) | 20 (20%) | 24 (24%) | 33 (22%) | 39 (24%) | |
| III | 366 (71%) | 72 (71%) | 72 (71%) | 110 (74%) | 112 (70%) | |
| IV | 30 (6%) | 9 (9%) | 5 (5%) | 6 (4%) | 10 (6%) | |
| QRS duration, ms | 164 (23) | 156 (23) | 165 (26) | 163 (23) | 169 (20) | <0.001 |
| History of atrial fibrillation/flutter | 159 (31%) | 30 (30%) | 34 (34%) | 45 (30%) | 50 (31%) | |
| Creatinine clearance, mL/min | 70 (31) | 67 (29) | 68 (29) | 71 (33) | 72 (31) | |
| Hypertension | 210 (41%) | 43 (42%) | 41 (44%) | 53 (38%) | 74 (48%) | |
| Diabetes | 115 (23%) | 31 (31%) | 19 (19%) | 32 (22%) | 33 (21%) | |
| Current smoking | 206 (43%) | 36 (38%) | 47 (50%) | 67(47%) | 56 (38%) | |
| Medication | ||||||
| ACE inhibitors/ARBs | 456 (89%) | 90 (90%) | 91 (90%) | 129 (87%) | 146 (91%) | |
| Aldactone | 248 (48%) | 50 (50%) | 44 (44%) | 75 (50%) | 79 (49%) | |
| Amiodarone | 80 (16%) | 23 (22%) | 15 (15%) | 22 (15%) | 20 (12%) | |
| Beta-blockers | 382 (75%) | 75 (74%) | 77 (76%) | 109 (68%) | 121 (75%) | |
| Sotalol | 40 (8%) | 8 (8%) | 11 (11%) | 5 (4%) | 16 (10%) | |
| Calcium antagonists | 34 (7%) | 4 (4%) | 7 (7%) | 12 (8%) | 11 (7%) | |
| Diuretics | 434 (85%) | 92 (91%) | 85 (84%) | 123 (83%) | 134 (83%) | |
| Statins | 272 (53%) | 58 (57%) | 51 (51) | 82 (55) | 81 (50%) |
- Categorical variables are expressed by n (%) and continuous variables are presented as mean (SD) and, when appropriate, median (interquartile range). Only P-values <0.05 are shown.
- ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization therapy-defibrillator.
Tables 1 and 2 show the clinical and echocardiographic characteristics for the specific subgroups with respect to CRT response; these include 101 (20%) negative responders, 101 (20%) non-responders, 149 (29%) responders, and 161 (31%) super-responders. The subgroups characterized by ≥15% LVESV reduction contained more frequently females (20% vs. 29% vs. 21% vs. 35%; P = 0.02), had less often ischaemic heart disease (60% vs. 63% vs. 54% vs. 40%; P < 0.001), and longer QRS duration (156 ms. vs. 165 ms. vs. 163 ms. vs. 169 ms; P < 0.001).
| Negative responders, n = 101 | Non-responders, n = 101 | Responders, n = 149 | Super-responders, n = 161 | P-value | |
|---|---|---|---|---|---|
| Baseline | |||||
| LVEDV, mL | 207 (73) | 233 (78) | 220 (76) | 227 (84) | |
| LVESV, mL | 156 (62) | 182 (68) | 168 (65) | 174 (72) | 0.04 |
| LVEF, % | 25 (7) | 23 (6) | 25 (6) | 24 (6) | 0.04 |
| S–L delay | 52 (38) | 48 (41) | 72 (46) | 79 (48) | <0.001 |
| Mitral regurgitation | 15 (15%) | 12 (12%) | 28 (19%) | 21 (13%) | |
| Six months after CRT implantation | |||||
| LVEDV, mL | 232 (78) | 224 (72) | 195 (66) | 157 (58) | <0.001 |
| LVESV, mL | 176 (68) | 168 (62) | 131 (52) | 101 (43) | <0.001 |
| LVEF, % | 25 (7) | 26 (7) | 33 (7) | 37 (9) | <0.001 |
| S–L delay | 41 (35) | 40 (37) | 33 (31) | 36 (32) | |
| Mitral regurgitation | 15 (15%) | 10 (10%) | 11 (7%) | 4 (3%) | <0.001 |
| ΔLVESV, % | 14 (14) | −7 (4) | −22 (5) | −41 (10) | <0.001 |
- Continuous variables are presented as mean (SD) and categorical variables as N (%); only P-values <0.05 are shown.
- LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end systolic volume; S–L delay, systolic septal to lateral delay.
Defibrillator therapy
During a median follow-up of 57 months (25th–75th percentile 39–84 months), 134 (26%) CRT-D recipients received appropriate ICD therapy; 25 (25%) negative responders, 27 (27%) non-responders, 45 (30%) responders, and 37 (23%) super-responders received appropriate ICD therapy (Table 3). As illustrated in Figure 1A, the 1-year cumulative incidences of ICD therapy were 15% [95% confidence interval (CI) 8–22%] in negative responders, 14% (95% CI 7–21%) in non-responders, 9% (95% CI 4–13%) in responders, and 3% (95% CI 0–6%) in super-responders (P < 0.001). The 5-year cumulative incidences of ICD therapy were 31% (95% CI 19–43%), 39% (95% CI 25–53%), 34% (95% CI 25–43%), and 27% (95% CI 18–35%) in negative responders, non-responders, responders, and super-responders, respectively (P = 0.13). At 5 years follow-up, the extent of response was not associated with a parallel reduction of ICD therapy (P = 0.34).
| Negative responders, n = 101 | Non-responders, n = 101 | Responders, n = 149 | Super-responders, n = 161 | P-value | |
|---|---|---|---|---|---|
| All-cause mortality | 41 (41%) | 39 (39%) | 47 (32%) | 35 (22%) | 0.004 |
| Cardiac mortality | 24 (24%) | 22 (22%) | 22 (15%) | 11 (6%) | 0.001 |
| Non-cardiac mortality | 9 (9%) | 7 (7%) | 16 (11%) | 18 (11%) | |
| Appropriate therapy | 25 (25%) | 27 (27%) | 45 (30%) | 37 (23%) | |
| Appropriate shock | 16 (16%) | 19 (19%) | 22 (15%) | 23 (14%) | |
| Inappropriate shock | 6 (6%) | 6 (6%) | 17 (11%) | 21 (13%) |
- Variables are presented as n (%); only P-values <0.05 are shown.
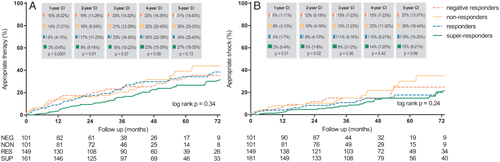
During follow-up, an appropriate shock was delivered in 16 (16%) negative responders, 19 (19%) non-responders, 22 (15%) responders, and 23 (14%) super-responders, respectively. The 1-year cumulative incidences of appropriate shock were 6% (95% CI 1–11%), 8% (95% CI 3–13%), 4% (95% CI 1–7%), and 2% (95% CI 0–4%) in negative responders, non-responders, responders, and super-responders, respectively (P = 0.01). The 5-year cumulative incidences of appropriate shock were 25% (95% CI 12–37%) in negative responders, 30% (95% CI 16–44%) in non-responders, 18% (95% CI 10–25%) in responders, and 15% (95% CI 8–21%) in super-responders, respectively (P = 0.09). The incidencce of appropriate shock was similar in all response groups (P = 0.24; Figure 1B).
Also in stratified analysis for patients with either ischaemic (n = 270) or non-ischaemic heart disease (n = 242) there was no difference observed in the incidence of ICD therapy between the different subgroups during long-term follow-up (P = 0.81 and P = 0.65, respectively).
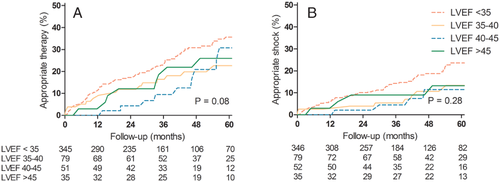
In addition, Figure 2 illustrates the incidence of appropriate ICD therapy and shock by LVEF 6 months after CRT implantation. In patients with an LVEF >45%, the 5-year cumulative incidences of ICD therapy and ICD shock were respectively 26% (95% CI 10 – 42%) and 13% (95% CI 1–25%). Cumulative incidences did not differ between patients with an LVEF above or below 45% (P = 0.08 and P = 0.28, respectively)
Prediction of defibrillator therapy
Comparison of patients who did and did not receive appropriate ICD therapy demonstrated that patients with appropriate ICD interventions more frequently had ischaemic heart disease (60% vs. 50%; P = 0.04). Echocardiographic characteristics at baseline were comparable. After 6 months follow-up, the CRT-D recipients with ICD interventions had more pronounced S–L delay (39 ms. vs. 31 ms; P = 0.02). The multivariate model for the prediction of defibrillator therapy contained the following variables: (i) age >65years; (ii) NYHA class IV; (iii) QRS duration >150 ms; (iv) S–L delay >50 ms; and (v) LVESV >130 mL. The strongest predictors were NYHA class IV [hazard ratio (HR) 2.51; 95% CI 1.41–4.47; P = 0.002] and LVESV >130 mL at 6 months follow-up (HR 1.80; 95% CI 1.28–2.54; P = 0.001; Table 4).
| Univariate, HR (95% CI) | P-value | Regression coefficient | Multivariate, HR (95% CI) | P-value | |
|---|---|---|---|---|---|
| Baseline | |||||
| Age >65 years | 1.46 (1.02–2.10) | 0.039 | 0.434 | 1.54 (1.08–2.22) | 0.019 |
| NYHA functional class > III | 2.23 (1.26–3.95) | 0.006 | 0.919 | 2.51 (1.41–4.47) | 0.002 |
| QRS >150 ms | 1.60 (1.12–2.28) | 0.010 | 0.476 | 1.61 (1.13–2.30) | 0.009 |
| Six months follow-up | |||||
| S–L delay >50 ms | 1.55 (1.05–2.26) | 0.026 | 0.417 | 1.52 (1.04–2.22) | 0.032 |
| LVESV >130 mL | 1.69 (1.20–2.38) | 0.003 | 0.588 | 1.80 (1.28–2.54) | 0.001 |
- CI, confidence interval; HR, hazard ratio; LVESV, left ventricular end-systolic volume; S–L delay, systolic septal to lateral delay.
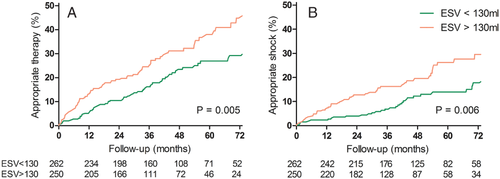
As demonstrated in Figure 3, 59 (23%) patients with and 75 (30%) patients without LVESV <130 mL at 6 months, respectively, received appropriate defibrillator therapy (P = 0.005).
Mortality
During follow-up, 162 (32%) patients died [41 (41%) negative responders; 39 (39%) non-responders; 47 (32%) responders; and 35 (22%) super-responders]. Annual mortality rates were 11.6, 10.4, 6.9, and 4.6%, respectively, for negative responders, non-responders, responders, and super-responders. Mortality decreases as the extent of response increases (P = 0.004). A similar association was observed for cardiac mortality (P = 0.001), while non-cardiac mortality was equal in all subgroups (Table 3).
Discussion
Key findings of this study are: (i) during long-term follow-up there is, with the exception of the first year of follow-up, no association between the extent of response to CRT and the incidence of defibrillator therapy; (ii) none of the baseline characteristics and echocardiographic parameters 6 months after implantation could accurately identify patients at risk for ventricular arrhythmias; and (iii) despite the (near) normalization of cardiac volumes and function, 23% of super-responders are treated for potentially life-threatening arrhythmias, and benefit from ICD therapy.
The current study provides insight into the long-term clinical outcome of CRT-D recipients stratified by extent of response to CRT. It focuses on ICD therapy and prediction of ICD therapy and therefore aims to contribute to the decision-making of future device replacement.
Occurrence of super-response
Previous studies have reported an incidence of super-response varying from 9% to 47%.6-13 This variation is mainly caused by the differences in definition of super-response used. Most frequently used definitions are, normalization of heart function (LVEF ≥45–50%) or a relative reduction of LVESV ≥30%. As a direct measure of (echocardiographic) LV reverse remodelling, in the current study relative LVESV reduction ≥30% was used to determine super-response. Previous studies using this definition reported an incidence of super-response varying from 22% to 40%, which is comparable with the 31% observed in the current study.7, 8, 10
Defibrillator therapy
The MADIT CRT investigators reported a 67% reduction of the risk of ventricular tachycardia in CRT recipients with an LVESV reduction ≥25% as compared with patients with an LVESV reduction <25%, after 2 years follow-up (P < 0.001).16 Previous clinical studies reported a lower incidence of ICD therapy comparing responders with non-responders to CRT at 1 year of follow-up.17, 19, 24
The current analysis confirms that the incidence of ICD therapy in super-responders is lower during the first years of follow-up. The results, however, emphasize the importance of accurate long-term follow-up; whereas the MADIT-CRT trial and clinical studies thus far only reported results of 1–2 years of follow-up, this study reports results after almost 5 years of follow-up. In contrast to short-term follow-up, this study did not establish an association between the extent of response to CRT and defibrillator therapy or defibrillator shock during long-term follow-up. These results are supported by Steffel et al. who also reported that even patients with profound LV reverse remodelling experienced appropriate ICD therapy during follow-up.10 An explanation may be that despite the fact that CRT results in electrical and mechanical ventricular synchrony, the underlying myocardial disease remains untreated. Thus, even in patients with (near) normalized LV dimensions and function, the underlying myocardial disease may trigger ventricular arrhythmias, as demonstrated by Fernandez-Armenta et al.25 The current study did not, however, assess myocardial scar characteristics.
The current study demonstrated that both LVESV and S–L delay measured 6 months after CRT-D implantation were independent predictors for defibrillator therapy. Absolute LVESV at 6 months was a more important prognostic factor for ICD therapy as compared with the change of the LVESV after 6 months CRT treatment. Persistent dilation of the left ventricle and persistent LV dyssynchrony as measured by LVESV and S–L delay 6 months after CRT implantation suggests that these patients suffer from persistent severe heart failure independent of the degree of LV reverse remodelling after device implantation, which is known to be associated with a higher risk of ICD intervention. Additionally, age, NYHA functional class, and QRS duration at baseline were also found to be predictive for ICD therapy. Several other baseline covariates have been demonstrated to be predictive for defibrillator therapy in primary prevention CRT-D recipients: sex,26 history of non-sustained ventriculoar tachycardia,26, 27 absence of beta-blocker,26 absence of ACE inhibitor or ARB,26 use of digoxin,27 NYHA functional class IV,26 LVEF,27 E/E′ ratio,27 and total size and heterogeneity of myocardial scar tissue.25 Nevertheless, none of these covariates or combinations of covariates can accurately predict defibrillator treatment.
Mortality
The present study confirms that the extent of response to CRT-D is associated with a parallel reduction of mortality.8 Super-response was associated with a 46% lower mortality as compared with responders. The survival gain is fully accounted for by the reduction of cardiac deaths, whereas there is no difference in non-cardiac mortality between the four subgroups.
Comparison with other studies describing mortality of super-responders must be carefully interpreted because of the different definitions of response used. Hsu et al. described similar results, after 15 months of follow-up, in the super-responders of the MADIT-CRT study population comparing them with all other positive responders to CRT-D (HR 0.44; P = 0.01).12 Super-response was defined by the highest quartile of LVEF change. Outside the setting of a clinical trial, a few small studies reported a similar beneficial effect on survival in super-responders.9, 10
Clinical implications
In view of both cost reduction and the potential side effects of ICD therapy (e.g. inappropriate ICD therapy), implantation of a CRT-P device, thus without defibrillator options, may be preferred when possible. Despite extensive research aiming to identify predictors of CRT response, it is currently difficult to predict whether patients will be responders to CRT. ICD treatment is often indicated in the primary implanted devices. However, after determination of response, a subgroup has outgrown eligibility for ICD according to international guidelines due to LV reverse remodelling. Yet, the current results suggest that in the case of replacement due to battery depletion, CRT-D implantation is preferred above CRT-P, since a high risk for potential life-threatening ventricular arrhythmias remains apparent even in super-responders to CRT (with either ischaemic or non-ischaemic heart disease).
Limitations
Since the focus was on ICD treatment, heart failure hospitalizations were not included in the results. Data were collected at a single centre and outside the setting of a clinical trial; however, the results may be representative for the ‘real-world’ CRT recipients. Patients were included since 2000; therefore, defibrillator treatment was not yet programmed according to the MADIT-RIT recommendations. Subsequently, incidences of ICD therapy and mortality may be lower in future CRT-D recipients; it is however unclear to what degree.
Patients were stratified according to echocardiographic measurements at 6 months follow-up; thus, patients who died or were too sick to attend their follow-up visit were excluded. These patients were probably more likely to be negative responders, which could have led to an underestimation of the measured differences of both mortality and ICD therapy. Finally, in this analysis, long-term echocardiographic outcome was not evaluated.
Conclusion
In routine clinical practice, the extent of response to CRT is not associated with a parallel reduction of defibrillator therapy during long-term follow-up (with the exception of the first year of follow-up). This study demonstrates that even CRT recipients with marked LV reverse remodelling and (near) normalization of cardiac function receive appropriate defibrillator therapy; as a consequence, ICD treatment remains indicated in these patients.
Funding
This work was supported by the Department of Cardiology, Leiden University Medical Centre, the Netherlands. M.J.S., A.C.v.d.H., and U.H. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: the Department of Cardiology receives grants from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and St. Jude Medical. The authors report no conflicts of interest.




