Presence of perforin in endomyocardial biopsies of patients with inflammatory cardiomyopathy predicts poor outcome
Abstract
Aims
Intramyocardial inflammation is considered an adverse prognostic factor in inflammatory cardiomyopathy (CMi). However, the precise nature of immune system factors relevant for the prediction of long-term course remains elusive. The aim of this study was to analyse the prognostic relevance of perforin in a large cohort of patients with CMi.
Methods and results
We investigated 495 consecutive patients with suspected CMi, undergoing endomyocardial biopsies (EMBs), and examined haemodynamic measurements after a long follow-up period (interquartile range 10.2–37.1 months). In EMBs, myocardial inflammation was assessed by histology and immunohistology. At follow-up, 388 patients (Group I) showed stable mild dysfunction or significant improvement, with LVEF rising from 46.2 ± 14.8% to 64.3 ± 12.3% (P < 0.0001). Lack of improvement of LV function or significant deterioration of LVEF from 42.1 ± 14.2% to 32.3 ± 11.6% (P < 0.0001) was observed in 107 patients (Group II). Multivariable statistical analysis of LVEF and immunohistochemical parameters in all patients revealed that the single most important predictor of LVEF development was detection of perforin in EMBs, with an odds ratio (OR) of 7.922 [95% confidence interval (CI) 4.380–14.326; P < 0.001] for deteriorating LVEF. Importantly, baseline LVEF (OR 0.962), LV end-diastolic diameter (OR 1.847), and other immmunohistochemical parameters (CD3, Mac-1, CD45R0, LFA-1, HLA-1, and ICAM-1) made minor or insignificant contributions to LVEF course in these 495 patients.
Conclusions
In this EMB-based analysis of the long-term course of CMi we identified, for the first time, that detection of perforin in the myocardium is a key predictor of LVEF course.
Introduction
Inflammatory cardiomyopathy (CMi) represents a major cause of heart failure with potential for transition to the clinical picture of dilated cardiomyopathy.1-3 The pathogenesis of CMi encompasses a chronic myocardial process with cardiotropic virus-induced immune responses as well as autoimmune reactions involving autoantigen-specific T cells. Myocardial inflammation is reflected by infiltration of lymphocytes, macrophages, and cell adhesion molecules (CAMs).4-10 Several studies have shown that cytotoxic cells expressing cytotoxic effector molecules are also increased in this cardiac inflammatory process.11, 12 However, nothing is known about the prognostic impact of perforin for the outcome of CMi patients. One key mediator of cytotoxicity is perforin,13 a molecule released by T cells and natural killer (NK) cells. Its expression upon T-cell maturation is strongly regulated after activation, and effector T cells have been shown to express the highest levels of intracellular perforin.14-17 In the present endomyocardial biopsy (EMB)-based analysis, we investigated whether intramyocardial detection of perforin-positive cells has predictive value regarding the long-term functional outcome of patients suffering from CMi.
Methods
Patients
Between January 2002 and November 2008 we screened all patients admitted to our clinic for further evaluation of suspected CMi by the complete spectrum of clinical and EMB-based diagnostics. These patients complained about symptoms of heart failure with fatigue, reduced physical capacity, or dyspnoea on exertion, and cardiac dysfunction. CAD and other possible causes of myocardial dysfunction (hypertension and valvular heart disease) had been excluded by angiography prior to EMB in all patients. Up to eight EMBs from the right ventricular (RV) septum were obtained using a flexible biotome (Fa. Westmed, Germany). The LVEF was determined by echocardiography. Patients presenting with signs of acute myocarditis with very recent onset of symptoms (e.g. mimicking acute myocardial infarction with elevated serum markers of troponin T and creatine kinase/creatine kinase-MB) were excluded, as were those with proof of intramyocardial genomes of enterovirus (EV), adenovirus (ADV), Epstein–Barr virus (EBV), and human herpes virus 6 (HHV6).
Low copy numbers of erythrovirus (B19V) genomes (<500/µg genome equivalents) were present in n = 268 (54.1%) of the myocardial tissues, but in all these cases there was no evidence of transcriptional activity from the virus (i.e. detection of VP1-VP2-mRNA), indicating latent virus replication without pathogenic relevance.18, 19 Other exclusion criteria were antiviral, immunomodulatory, or immunosuppressive therapy within the past 6 months, clinical or biochemical evidence for concomitant chronic inflammatory disease, chronic renal insufficiency (creatinine ≥1.8 mmol/L), inability to understand the consent form, participation or consent to participate in another study, or malignant disease. In the current study we included only those patients with myocardial inflammation. The demographic, clinical, and EMB characteristics of these 495 patients (321 males, 174 females) are summarized in Table 1. For LV function details see Table 2.
| n | 495 |
|---|---|
| Age (years) | 54.3 ± 15.4 |
| Gender (male/female) | 321/174 |
| LVEF (%) | 47.3 ± 18.6 |
| NYHA classification, n | |
| I | 171 |
| II | 224 |
| III | 85 |
| IV | 9 |
| Angina on rest, n | 86 |
| Angina on exercise, n | 105 |
| Dyspnoea on exercise, n | 224 |
| Arrhythmias, n | 56 |
| Supraventricular | 39 |
| Ventricular | 17 |
| History of hypertension, n | 142 |
| History of diabetes, n | 30 |
| Medication, n | |
| Beta-blockers | 187 |
| ACE inhibitors | 287 |
| Diuretic agents | 209 |
| Aldosterone antagonists | 81 |
| Cardiac glycosides | 20 |
| Warfarin | 31 |
| Group I | Group II | |
|---|---|---|
| Number | 388 | 107 |
| Gender, male/female | 239/149 | 82/25 |
| Follow-up period, months | 28.7–33.0 | 27.9–34.9 |
| LVEF, %, baseline | 46.2 ± 14.8 | 42.1 ± 14.2 |
| LVEF, %, follow-up | 64.3 ± 12.3a | 32.3 ± 11.6 |
| Median LVEDD, mm | 52.4 ± 8.5 | 57.5 ± 10.4b |
| Laboratory parameters | ||
| Leucocytes, per nL | 7.2 ± 2.3 | 6.8 ± 1.8 |
| hs-CRP, mg/dL | 1.9 ± 5.9 | 1.9 ± 5.2 |
| Immunohistochemistry | ||
| CD3+ cells/mm2 | 8.0 ± 8.9 | 8.6 ± 11.3 |
| Mac-1+ cells/mm2 | 40.1 ± 32.9 | 40.6 ± 35.6 |
| LFA-1+ cells/mm2 | 20.5 ± 23.4 | 21.0 ± 22.3 |
| HLA class I/AF | 7.5 ± 3.3 | 7.7 ± 3.1b |
| ICAM-1/AF | 2.2 ± 1.3 | 2.1 ± 1.2 |
| Perforin+ cells/mm2 | 0.8 ± 1.0 | 3.9 ± 3.6b |
| Medication, n (%) | ||
| Beta-blockers | 93 (23.9%) | 93 (86.9%)b |
| ACE inhibitors | 186 (47.9%) | 101 (94.3%)b |
| Diuretic agents | 106 (27.3%) | 103 (96.2%)b |
| Aldosterone antagonists | 23 (5.9%) | 58 (54.2%)b |
| Cardiac glycosides | 4 (1.0%) | 16 (14.9%)b |
| Warfarin | 10 (2.5%) | 21 (19.6%)b |
- The data are presented as mean ± standard deviation or range 75–95%.
- AF, area fraction; CD3, T lymphocytes; hs-CRP: high sensitivity C-reactive protein; HLA, human leuocyte antigen; ICAM-I, intercellular adhesion molecule 1; LFA-1, lymphocyte function-associated antigen-1; LVEDD, left ventricular end-diastolic diameter; Mac-1, macrophage-1 antigen.
- a Significantly different; baseline vs. follow up.
- b Significantly different; Group I vs. Group II.
Ethics approval
The study was performed within the CRC Transregio 19 and was approved by the local ethics committees of the participating clinical centre as well as by the committees of the respective federal states. An informed written consent is obtained from each study patient.
Detection of viral genomes by nested polymerase chain reaction and reverse transcription–polymerase chain reaction
Four EMBs were subjected to molecular biological investigation of cardiotropic viral genomes according to published techniques.20 In brief, polymerase chain reaction (PCR) was performed on RNA extracted from EMBs for EV and ADV, and on DNA for EBV, erythrovirus genomes, and HHV6. As a control for the successful extraction of DNA and RNA from heart muscle tissue, oligonucleotide sequences were chosen from the DNA sequence of the glyceraldehyde 3-phosphate dehydrogenase gene. The specificity of all amplification products was confirmed by automatic DNA sequencing.
Histological and immunohistochemical staining for assessment of inflammation
Endomyocardial biopsies were obtained from the RV septum. Histology was developed by haematoxylin–eosin staining in light microscopy. For immunohistological evaluation, specimens were embedded in Tissue Tec (SLEE, Mainz, Germany) and immediately snap-frozen in methyl butane which had been cooled in liquid nitrogen, and then stored at –80 °C until processing. Embedded specimens were cut serially into cryosections of 5 mm thickness and placed on 10% poly-l-lysine-pre-coated slides. Immunohistochemistry was used for the characterization of inflammatory infiltrates, and myocardial inflammation was diagnosed by >7.0 CD3+ lymphocytes/mm2 and/or >35.0 CD11b+/Mac-1+ macrophages/mm2. Antibodies used: CD3+ lymphocytes (Dako, Glostrup, Denmark, dilution 1:25), CD11a+/LFA-1+ lymphocytes (Immuno Tools, Friesoythe, Germany, dilution 1:250), CD11b+/Mac-1+ macrophages (ImmunoTools, dilution 1:500), CD8+ T lymphocytes (Dako, dilution 1:50), and granzyme B (Abcam, Cambridge, UK, dilution 1:100). There was association between cellular infiltrates and enhanced expression of CAMs: human leucocyte antigen-1 (HLA-1; Dako, dilution 1:2000) and intercellular adhesion molecule 1 (ICAM-1; ImmunoTools, dilution 1:800). Perforin-positive cellular infiltrates were also defined by immunohistochemistry (clone δG9, BD Bioscience, San Jose, CA, USA, dilution 1:150). As secondary antibody we used enhancing EnVision™ peroxidase-conjugated antimouse antibody (DakoCytomation, Hamburg, Germany). Immunohistological staining was visualized using 3-amino-9-ethylcarbazole (Merck, Darmstadt, Germany) as chromogenic substrate. Finally, slides were counterstained in haematoxylin and mounted with Kaiser's gelatinR (Merck). The staining and peroxidase reactions in all samples were carried out identically and in parallel for all samples.21 Specimens with perforin cellular infiltrates were classified as perforin positive (mean cell count 1.5 ± 2.3/mm2).22, 23 Immunoreactivity was quantified by digital image analysis (DIA). Basically, the DIA macros used consisted of three steps: (i) grabbing of the image; (ii) recognition of artefacts (areas not covered by cardiac tissue) for the calculation of the net myocardial area [heart area (HA) = area of the measurement frame – lit area/mm2]; and (iii) recognition of the peroxidase-converted chromogen 3-amino-9-ethylcarbazole. The percentage ratio between the heart and frame area was expressed as a percentage of HA. Colour-coded thresholds for steps (ii) and (iii) were set by choosing multiple representative points within the grabbed images from 10 randomly selected immunohistochemically stained EMB sections. The images for the quantification of infiltrates were grabbed at ×200 magnification. The calculated objects were related to the unit HA (mm2).24 The presence of focally clustered infiltrates was assessed visually. Foci were considered to be present when more than three infiltrates were clustered focally and encircled definite cardiomyocytes, suggestive of incipient myocytolysis. Involvement of certain phenotypes of infiltrates in these foci was observed in serial sections of the same myocardial sample.
Statistical analyses
Data are shown as mean values and standard deviation. After having established that no data were distributed normally, the non-parametric Mann–Whitney U-test for group comparisons, Wilcoxon's signed rank test for comparisons between baseline and follow-up, and Spearman's correlation coefficient for correlation analyses were used. Adjustment regarding potential confounders such as baseline LVEF and parameters differing significantly between the groups ‘improvement/stable disease’ and ‘deterioration’ in the univariable analysis has been performed using logistic regression analysis with backward and forward selection and with perforin, baseline LVEF, HLA-1, and LV end-diastolic diameter (LVEDD) as the independent variables. In the multiple logistic regression model, all variables which differed significantly between the two study groups were included in the model prior to forward and backward selection. In order to allow comparison of the magnitude of association across predictor variables with different scaling, odds ratios (ORs) emerging from the logistic regression analysis are additionally presented in standardized form based upon the z-transformed original measurements. These estimates are identified as standardized estimates in the results and tables. A probability value of <0.05 was considered statistically significant. Comparisons between different AUCs (areas under the curve) in ROC (receiver operating characteristic) analysis were performed using the DeLong test. The optimal cut-off was calculated through maximization of the Youden index. All statistical analyses have been performed with SPSS.21 or STATA.13.
Results
The demographic and LVEF data of the CMi patients are summarized in Table 1. No patient died within the observation period. We classified the patients clinically regarding development of LVEF from baseline to follow-up as follows: in Group I patients (n = 388), a significant improvement of LVEF, rising from 46.2 ± 14.8% to 64.3 ± 12.3% (P < 0.001), was observed. This was accompanied by a significant decrease of LVEDD from 56.3 ± 15.6 mm to 52.4 ± 8.5 mm (P < 0.5). Lack of improvement of LV function or further LVEF deterioration was observed in Group II of n = 107 patients (from 42.1 ± 14.2% to 32.3 ± 11.6%, P < 0.001). This was paralleled by an increase of LVEDD from 54.0 ± 8.8 mm to 57.5 ± 10.4 mm (P < 0.5). There were no significant differences between patients with improvement of LVEF and deterioration of LVEF with respect to age or gender.
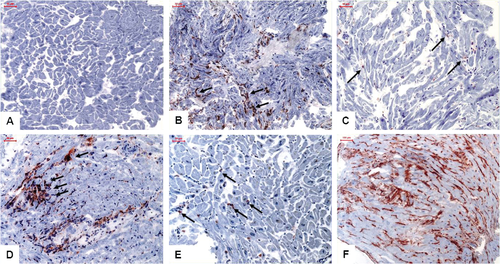
Perforin-positive cells were present in n = 332 (67.0%, mean cell count 1.5 ± 2.3/mm2) of the myocardial tissues. Focally clustered infiltrates were observed in n = 39 (7.8%) of the cases. Histological analysis did not detect active myocarditis in any of the analysed samples.25 Representative aspects of immunohistologically detected infiltrates and CAM expression are shown in Figure 1. Concurrent abundance of HLA-1 and perforin was confirmed in n = 339 (68.4%) of myocardial samples. Although in cases with focally clustered cellular infiltrates a focally accentuated abundance of CAMs was also observed, CAM expression was extended over the entire sections with local enhancements. In a subgroup analysis of 213 patients we have investigated granzyme B in EMBs and found a direct correlation with perforin-positive cells (P < 0.0001, r = 0.3). There was no significant association of low copy number B19V genome detection with either intramyocardial inflammation or perforin-positive cells as evaluated by the above diagnostic approaches (P > 0.05).
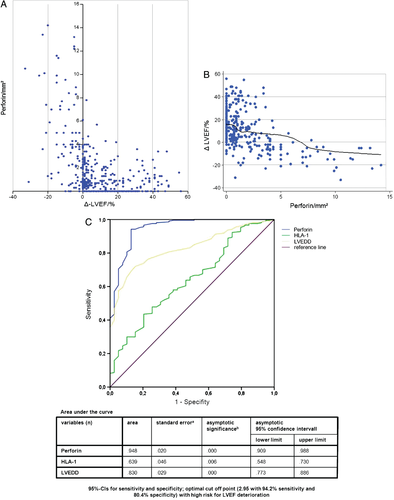
The statistical analyses of all possible determinants (immunohistochemical, functional, and morphological) of LVEF course were performed by both univariable (Figure 2) and multivariable methods (Table 3). Univariable analysis showed close correlation between Δ-LVEF, i.e. the difference between follow-up and baseline LVEF, and perforin expression levels across the clinically defined groups (Figure 2A and B). Therefore, significantly increased perforin-positive infiltrates in patients with a lack of improvement of LV function were seen at follow-up, in contrast to those who improved (Table 2). Multiple logistic regression analysis as described under ‘Statistical analyses’ showed that perforin was the leading predictor for failure to improve (i.e. at least 5% increase in LVEF), with an OR of 7.922 [95% confidence interval (CI) 4.380–14.326; P < 0.001] for deterioration (Table 3). The multivariable analysis furthermore demonstrated that baseline LVEF (OR 0.962, 95% CI 0.962–0.962) and baseline LVEDD (OR 1.847, 95% CI 1.252–2.725) were minor determinants of LVEF course (Δ-LVEF) as compared with perforin-positive infiltrates, and that the other immmunohistochemical parameters (CD3, Mac-1, CD45R0, LFA-1, HLA-1, CD54, and CD106) made minor or insignificant contributions to LVEF course. In Table 3, the values in parentheses indicate the ORs and 95% CIs after analysis based on standardized measurements.
| Groupa | Factor | Odds ratiob | 95% CI for odds ratiob | P-value |
|---|---|---|---|---|
| Deterioration of LVEF | Perforin | 2.138 (7.922) | 1.720–2.658 (4.380–14.326) | <0.001 |
| HLA-1 | 1.150 (1.520) | 1.024–1.292 (1.074–2.151) | 0.018 | |
| LVEDD | 1.064 (1.847) | 1.023–1.107 (1.252–2.725) | 0.002 |
- CI, confidence interval; HLA-1, human leuocyte antigen-1; LVEDD, left ventricular end-diastolic diameter.
- a Reference category = ‘Improvement’.
- b Values in parentheses indicate the odds ratios and 95% confidence intervals after analysis based on standardized measurements.
We present 95% CIs for sensitivity and specificity and calculated the optimal cut-off point (2.95 with 94.2% sensitivity and 80.4% specificity) according to the maximal Youden index with a high risk for LVEF deterioration (Figure 2C).
Discussion
The present study has demonstrated that the presence of perforin-positive myocardium-infiltrating cells predicts an adverse LVEF course over a long follow-up period (interquartile range 10.2–37.1 months) in 495 CMi patients. To our knowledge, this is the first report elucidating the prognostic impact of perforin-positive cells for the outcome of CMi patients and indicates that exact analysis and quantification of intramyocardial infiltrates has clinical value for the assessment of long-term LVEF prognosis in CMi. Furthermore, these data strongly suggest routinely including perforin staining of EMBs in the differential diagnostic work-up in all CMi patients.26-28
During the follow-up of our CMi patients, grossly different LVEF developments were observed, ranging from ‘spontaneous’ normalization of LVEF to progression of ventricular dysfunction despite extended heart failure medication. In conjunction with the fact that a marker of myocardial inflammation, i.e. perforin, emerged as a key predictor of Δ-LVEF, this indicates that conventional heart failure treatment does not adequately and optimally control the underlying pathogenetic inflammatory process in these patients. The ROC analyses showed with 95% CIs for sensitivity and specificity that the optimal cut-off point (2.95 with 94.2% sensitivity and 80.4% specificity) is the prognostic cut-off value of perforin for LVEF deterioration (Figure 2C).
The indication for immunosuppressive therapy is commonly based on a standard set of myocardial inflammation markers.25 The data reported here show that perforin merits inclusion in this set. Whatever therapeutic regime is considered beyond standard heart failure medication (e.g. immunosuppressive treatment), earlier instalment of such upgraded therapy will logically reduce the extent of irreversible cardiac injury. Our data should be helpful in starting immunosuppressive therapy earlier in high risk patients (i.e. with high perforin levels in EMBs). Future controlled prospective immunosuppressive or immunomodulating clincal trials will show whether perforin is also a marker of response to therapy, in addition to being an important marker of spontaneous disease course as shown here. In our study, CMi patients deteriorating in LV function had significantly higher perforin-positive infiltrates than those with primary LVEF improvement (3.9 ± 3.6 cells/mm2 vs. 0.8 ± 1.0 cells/mm2). In a previous study by Kindermann et al.,29 the prognostic role of standard immunohistochemical markers was demonstrated in regard to cardiovascular death and need for heart transplantation, and Corsten et al.30 stated that cardiac immune response directly contributes to irreversible damage; however, perforin staining was not employed in these studies.
From the perspective of pathogenesis, chronic and insidiously progressive inflammation-mediated tissue injury will result if an inflammatory response of the myocardium triggered by infectious agents or other tissue injuries is not adequately controlled. The primary purpose of the inflammatory response aiming at adequate tissue repair or reparative remodelling has then failed, and the need for immunosuppression or immunomodulation arises. These therapies need to be installed, however, at a time when no irreversible myocardial damage has yet occurred, since they are only able to influence active pathogenic processes when cell migration and tissue remodelling are still taking place. For this reason, timely prediction of the probable disease course is of particular importance with regard to clinical CMi therapy.
Importantly, in our study, the prevalence of routinely used immunohistological markers of intramyocardial inflammation (lymphocytes, macrophages, and CAMs) was not substantially different from that in previous studies.30 Regarding the molecular pathogenic role of perforin, there are several mechanisms of immune-mediated myocyte injury which may be involved in regard to the poor LVEF outcome of patients who are positive for this protein. According to the most widely supported granule exocytosis model, cytotoxic effector cells degranulate on target cell recognition and release cytolytic mediators such as perforin. Released perforin monomers are then inserted into the membrane of target cells, and membrane-bound perforin monomers polymerize and form pores. The pores resemble that of the complement membrane attack complex; the latter are made by several complement components (C5b, C6, C7, C8, and C9). Death of the target cell via perforin is mediated either by uncontrolled influx of small molecules such as Ca2+ from the extracellular fluid or by induction of osmotic stress, which results in colloid osmotic lysis. Further, perforin pores may serve as a conduit for other cytotoxic cell-derived killing proteins such as granzymes. Granules that store perforin and granzymes can be seen in CD8+ cytotoxic effector T cells and in NK cells in infected tissue. In a post-hoc subgroup analysis of 213/495 patients, there was no significant correlation between CD8-positive cells and perforin expression (P = 0.25, r = 0.09). These subgroup data suggest that differences in NK cells rather that CD8-positive cells underly the differential perforin staining in our two cohorts with improvement vs. deterioration of LVEF.
In the setting of experimental myocarditis, perforin is known to mediate myocytolysis, thereby contributing to continuous loss of contractile units,22, 23, 31, 32 and ultimately leading to the observed failure of recovery. In murine myocarditis models, perforin-positive infiltrates cause extensive myocarditis, severely damaging the host, and are associated with dramatically increased mortality upon CVB3 infection.11, 12, 33, 34 In the present clinical study, a relatively high rate of focally clustered perforin-positive cellular infiltrates suggestive of incipient myocytolysis was also observed. This is consistent with the hypothesis that low level myocytolysis in areas of activated and clustered infiltrates may contribute to an insidious but progressive myocyte loss in CMi and thus to the deterioration of cardiac contractility. In addition, the higher rate of focally clustered perforin-positive infiltrates may contribute to increased levels of proinflammatory cytokines in these patients.4, 30, 35 It should be emphasized, however, that our study has shown prognostic association of perforin with clinical course, but not documented a causal relationship.
We conclude that perforin-positive infiltrates have high clinical predictive value in CMi. Using long-term follow-up in a large cohort of 495 patients with EMB-proven myocardial inflammation, we were able to show that lack of perforin-positive infiltrates in the first EMB is associated with spontaneous LVEF improvement. In contrast, LV function deteriorates in patients with detection of perforin-positive infiltration and progresses towards substantial cardiac dysfunction over the follow-up period of 30.0 ± 35.0 months despite continued heart failure medication. Perforin-positive infiltrates may constitute a major cause of progressing LV dysfunction in CMi and should be routinely measured to improve the assessment of prognosis in these patients. Since the likelihood of LVEF deterioration is significantly higher in patients with myocardial perforin persistence, this EMB-based finding should prompt the clinician to continue clinical surveillance at narrow intervals. In this sense, we believe that our results should be integrated into the clinical practice of cardiologists in the future.
Acknowledgements
We thank Ms K. Winter, S. Ochmann, and C. Seifert for expert technical assistance.
Funding
This study was supported by the Deutsche Forschungsgesellschaft (DFG; SFB/TR-19); the Deutsches Zentrum für Herz-Kreislauf-Forschung (German Centre for Cardiovascular Research).
Conflict of interest: none declared.




