Impaired left atrial function in heart failure with preserved ejection fraction
Abstract
Aims
Left atrial (LA) enlargement is present in the majority of heart failure with preserved ejection fraction (HFpEF) patients and is a marker of risk. However, the importance of LA function in HFpEF is less well understood.
Methods and results
The PARAMOUNT trial enrolled HFpEF patients (LVEF ≥45%, NT-proBNP >400 pg/mL). We assessed LA reservoir, conduit, and pump function using two-dimensional volume indices and speckle tracking echocardiography in 135 HFpEF patients in sinus rhythm at the time of echocardiography and 40 healthy controls of similar age and gender. Systolic LA strain was related to clinical characteristics and measures of cardiac structure and function. Compared with controls, HFpEF patients had worse LA reservoir, conduit, and pump function. The differences in systolic LA strain (controls 39.2 ± 6.6% vs. HFpEF 24.6 ± 7.3%) between groups remained significant after adjustments and even in the subsets of HFpEF patients with normal LA size or without a history of AF. Among HFpEF patients, lower systolic LA strain was associated with higher prevalence of prior HF hospitalization and history of AF, as well as worse LV systolic function, and higher LV mass and LA volume. However, NT-proBNP and E/E′ were similar across the quartiles of LA function.
Conclusions
In this HFpEF cohort, we observed impairment in all phases of LA function, and systolic LA strain was decreased independent of LA size or history of AF. LA dysfunction may be a marker of severity and play a pathophysiological role in HFpEF.
Trial registration: NCT00887588.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is common,1, 2 particularly among elderly, female, and hypertensive patients, and is frequently associated with AF.3, 4 This condition is also associated with increased mortality and hospital readmission.5, 6 The pathophysiological mechanisms underlying HFpEF are heterogeneous and incompletely understood. Traditionally, HFpEF has been attributed to abnormal LV diastolic function, including abnormalities in active relaxation and passive stiffness.7, 8 Left atrial (LA) enlargement is a recognized marker for LV diastolic function and is independently associated with an increased risk for morbidity and mortality.9-11 While increased LA size is present in the majority of HFpEF patients, approximately one-third do not have LA enlargement.10, 12 The role of all three phases of LA function in HFpEF patients is less well understood,13, 14 particularly in those without a history of AF and with normal LA size.
We used baseline data from the The Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) trial, a large well-phenotyped cohort of HFpEF patients, to test the hypothesis that LA function is abnormal in HFpEF patients, even among patients without LA enlargement or history of AF. We also sought to determine the clinical and echocardiographic correlates of reduced systolic LA strain in patients with HFpEF.
Methods
Study population
The PARAMOUNT trial (Clinicaltrials.gov NCT00887588) recruited patients between November 2009 and March 2011, and was undertaken in 65 centres and 13 countries. The trial enrolled men and women over 40 years of age, with LVEF ≥45%, documented history of heart failure with NYHA class II–IV symptoms, and NT-proBNP levels >400 pg/mL at the baseline visit.15 Patients were excluded if they had a previous LVEF <45% at any time, isolated right heart failure due to pulmonary diseases, dyspnoea due to non-cardiac causes such as pulmonary diseases, anaemia, or severe obesity, or primary valvular, coronary, or cerebrovascular disease. The number of patients enrolled with AF was limited to ∼25% of the total sample, checked by ECG at screening. Of the 301 patients enrolled in the PARAMOUNT trial, 135 patients were in sinus rhythm (SR) at the time of echocardiography and had image quality sufficient for LA speckle tracking analysis [excluded patients: 75 in AF at the time of echocardiography; 47 non-DICOM images; and 44 missing view(s) and/or unsuitable images for LA speckle tracking analysis]. Among the 135 included patients, 32 self-reported a history of AF and/or were in AF according to the screening ECG (performed 1 week before the echocardiogram), but were in SR at the time of echocardiography.
A group of 40 healthy controls was retrospectively identified from the medical records of the Brigham and Women's Hospital (BWH). The search strategy targeted patients >55 years of age who had an echocardiogram, and no International Classification of Diseases 9th Revision (ICD-9) code in their record for any of the following conditions: hypertension, ischaemic heart disease, cardiac arrhythmia, hypercholesterolaemia, COPD, diabetes mellitus, cerebrovascular disease, arterial vascular disease, and cancer. This group was further selected to have normal LVEF, no LV regional motion abnormalities, normally sized cardiac chambers, no significant valvular disease, and suitable echocardiogram image quality. Controls were of a similar age and gender distribution to the HFpEF group. Our final control sample was achieved from an initial search including 2000 patients. The study protocol was approved by the BWH Institutional Review Board.
Echocardiographic analyses
Standard echocardiographic and Doppler parameters were analysed using an offline analysis workstation at the Cardiovascular Imaging Core Lab at BWH, Boston, MA, USA. All pre-specified measurements in the PARAMOUNT trial were made in triplicate in accordance with the recommendations of the American Society of Echocardiography16, 17 and included LA and LV diameters and volumes, LV wall thickness, LV mass, LVEF, mitral inflow propagation, and lateral mitral annular relaxation velocities. LV stroke work (SW) was calculated as follows: SW = systolic blood pressure × stroke volume × 0.014.18
Left atrial and LV function indices were measured using B-mode speckle tracking vendor-independent software with algorithms designed for the LV (TomTec Imaging Systems, Unterschleissheim, Germany) that is angle independent and identifies cardiac motion by tracking multiple reference points over time.19, 20 The LA and LV endocardial borders were traced at the end-diastolic frame of 2D images acquired from the 12 segments using apical two- and four-chamber views.19 The PARAMOUNT echocardiography protocol required the proper alignment of apical views, in order to capture the LA in full, avoiding foreshortening of the chamber and to maintain a frame rate of 50–80 frames s−1 during the acquisition. End-diastole was defined by the QRS complex or as the frame after mitral valve closure. Speckles were tracked frame by frame throughout the LA and LV myocardium over the course of one cardiac cycle; basal, mid, and apical regions of interest were then created. Semi-quantitative segment tracking was carefully inspected for each image and manually adjusted as needed. If more than two segments could not be tracked or if there was a lack of a full cardiac cycle or significant foreshortening of the cavity, the measurements were considered unreliable and the patient was excluded from the analysis. For LV function analysis, global longitudinal strain was calculated as the average LV longitudinal strain across the apical four- and two-chamber views.21 From LA speckle tracking analysis, LA phasic function was estimated using volumes and strain indices calculated as the average across the apical four- and two-chamber views. LA volumes vs. time curves were generated by calculating LA volume at each phase of the cardiac cycle (LA maximal, LA pre-A, and LA minimum volumes) using the biplane Simpson's method (Figure 1, left panel). From LA volumes, LA phasic function was estimated as:
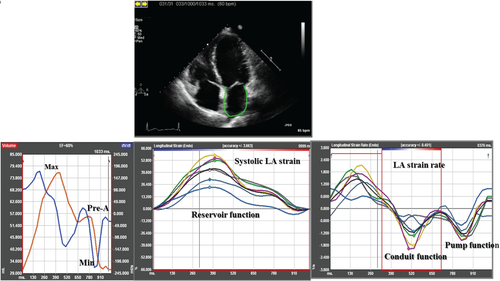
LA emptying fraction (reservoir function) = [(LA maximum volume – LA minimal volume)/LA maximum volume] × 100
LA passive emptying fraction (conduit function) = [(LA maximum volume – LA pre-A volume)/LA maximum volume] × 100
LA active emptying fraction (pump function) = [(LA pre-A volume – LA minimal volume)/LA pre-A volume] × 100
Also LA reservoir function was estimated as [LA expansion index (LA maximum volume – LA minimal volume)/LA minimal volume] × 100. From LA strain analysis, LA phasic function was estimated using: peak strain during systole (systolic LA strain) to assess reservoir function, early peak strain rate during diastole (LA passive strain rate) to assess conduit function, and late peak strain rate during diastole (LA active strain rate) to assess pump function (Figure 1, middle and right panel). All strain analyses on HFpEF patients and normal controls were performed by a single investigator.
Intraobserver variability for systolic LA strain was assessed in 20 randomly selected PARAMOUNT studies which included 12 (60%) participants with LA enlargement [LA volume index (LAVi) >29 mL/m2]. The overall coefficient of variation was 6.3% and the intraclass correlation coefficient was 0.86 [95% confidence interval (CI) 0.75–0.98] for systolic LA strain. Among HFpEF patients, we did not observe a significant difference in mean systolic LA strain from apical four-chamber and two-chamber views (systolic LA strain from apical four-chamber, 24.6% ± 8.1 vs. Systolic LA strain from apical two-chamber, 23.6% ± 7.7, P = 0.23).
Statistical analysis
All normally distributed data were presented as mean and standard deviation (continuous data) or as count and proportion (categorical data). Since NT-proBNP distribution was skewed, it was displayed as median and interquartile range and was log-transformed for analysis. Comparisons between groups were assessed using two-sample t-test with unequal variance or analysis of variance (ANOVA; followed by Bonferroni correction) and χ2 test. After univariate screening, multivariable linear regression models were used to adjust systolic LA strain for selected clinically and statistically significant covariates (age, gender, heart rate, systolic blood pressure, body mass index, LAVi, LV global longitudinal strain, LV end-diastolic volume, LV SW, E/A, E′, and E/E′).
Additionally, we categorized the HFpEF patients according to quartiles of systolic LA strain, and applied trend tests across ordered groups to assess the association between LA dysfunction and demographic characteristics and echocardiographic measures of cardiac structure and function. All statistical analyses were performed with STATA 12.0 (Stata Corp, College Station, TX, USA). All tests were two-sided, and P-values of <0.05 were considered statistically significant.
Results
Clinical characteristics
Patients with HFpEF were elderly, and more frequently Caucasian (81%), female, and overweight. Most (92%) had arterial hypertension, but blood pressure was well controlled (Table 1). All patients were using diuretics (inclusion criteria) and the majority of those patients were using an ACE inhibitor or ARB (93%) and a beta-blocker (81%). As compared with the excluded HFpEF patients, the HFpEF patients included in this analysis had higher systolic blood pressure (139 ± 15 mmHg vs. 133 ± 14 mmHg, P < 0.001), and slightly higher LVEF (59 ± 7% vs. 57 ± 8%, P = 0.04) and filling pressure (E/E′: 13.7 ± 8.6 vs. 11.7 ± 6.0, P = 0.04). Also, fewer of the patients included had a history of AF (23% vs. 56%, P < 0.001), and they had a lower heart rate (66 ± 13 vs. 72 ± 12, P < 0.001) and smaller LAVi (33.4 ± 11.5 vs. 38.1 ± 14.8, P = 0.004) than patients not included, probably due to the exclusion of patients with AF at the time of echocardiography.
| Controls (n = 40) | HFpEF (n = 135) | P-value | |
|---|---|---|---|
| Age (years) | 68 ± 6 | 70 ± 9 | 0.051 |
| Women, n (%) | 27 (68) | 83 (61) | 0.49 |
| NYHA class II, n (%) | – | 108 (81) | |
| NYHA class III, n (%) | – | 26 (19) | |
| Previous hospitalization for HF, n (%) | 0 (0) | 66 (50) | |
| History of atrial fibrillation, n (%) | 0 (0) | 31 (23) | |
| History of hypertension, n (%) | 0 (0) | 123 (92) | |
| History of diabetes, n (%) | 0 (0) | 47 (35) | |
| History of myocardial infarction, n (%) | 0 (0) | 30 (22) | |
| Heart rate (b.p.m.) | 71 ± 14 | 66 ± 13 | 0.04 |
| Systolic blood pressure (mmHg) | 127 ± 15 | 139 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 74 ± 11 | 78 ± 11 | 0.04 |
| Body mass index (kg/m2) | 25.2 ± 3.7 | 29.6 ± 5.7 | <0.001 |
| NT-proBNP (pg/mL) | – | 809 (446–1300) | |
| Baseline treatments | |||
| ACE inhibitors or ARBs, n (%) | 0 (0) | 125 (93) | |
| Diuretic, n (%) | 0 (0) | 135 (100) | |
| Beta-blockers, n (%) | 0 (0) | 109 (81) | |
| Aldosterone antagonists, n (%) | 0 (0) | 24 (18) | |
| Echocardiographic measures | |||
| LVEF (%) | 60 ± 3 | 59 ± 7 | 0.22 |
| LV Global longitudinal strain (%) | −19.9 ± 2.2 | −15.0 ± 3.4 | <0.001 |
| LV end-diastolic volume (mL) | 85.2 ± 24.5 | 114.1 ± 28.1 | <0.001 |
| LV end-diastolic volume/BSA (mL/m2) | 48.4 ± 11.0 | 61.8 ± 14.3 | <0.001 |
| LV end-systolic volume (mL) | 34.9 ± 13.6 | 47.3 ± 16.4 | <0.001 |
| LV end-systolic volume/BSA (mL/m2) | 19.6 ± 6.5 | 25.5 ± 8.5 | <0.001 |
| Relative wall thickness | 0.42 ± 0.07 | 0.38 ± 0.09 | 0.004 |
| LV mass index (g/m2) | 77.5 ± 17.0 | 79.4 ± 21.8 | 0.57 |
| LV mass/height2.7 (g/m2.7) | 35.7 ± 7.6 | 38.5 ± 11.3 | 0.09 |
| LV stroke work (g-m) | 92.4 ± 21.8 | 130.3 ± 37.4 | <0.001 |
| E′ (cm/s) | 9.4 ± 2.1 | 6.6 ± 2.4 | <0.001 |
| A' (cm/s) | 10.8 ± 2.8 | 7.2 ± 2.5 | <0.001 |
| E/E′ | 7.5 ± 2.5 | 13.7 ± 8.6 | <0.001 |
| E (cm/s) | 66.7 ± 15.6 | 78.0 ± 27.7 | 0.002 |
| A (cm/s) | 72.2 ± 18.0 | 74.0 ± 27.6 | 0.63 |
| E/A | 0.95 ± 0.23 | 1.20 ± 0.67 | <0.001 |
| Deceleration time (ms) | 203.8 ± 41.0 | 214.3 ± 39.0 | 0.18 |
| Left atrial volume index (mL/m2) | 21.1 ± 5.3 | 33.4 ± 11.5 | <0.001 |
- Data are presented as n (%) and mean ± SD.
- P-values were calculated by t-test or χ2.
- A, late mitral inflow velocity; A', late lateral mitral relaxation velocity; BSA, body surface area; E, early mitral inflow velocity; E′, early lateral mitral relaxation velocity; E/A, early to late mitral inflow velocity ratio; E/E′, mitral inflow to mitral relaxation velocity ratio; HF, heart failure; HFpEF, heart failure with preserved ejection fraction.
Compared with controls, patients with HFpEF had similar LVEF, but lower LV global longitudinal strain. HFpEF patients also had higher LV and LA volumes, lower mitral annular relaxation velocities (E′ and A'), and higher E/E′ compared with controls, who presented diastolic function parameters consistent with their age.22, 23 There was no difference in LV mass between groups, even after adjusting for height2.7. The relative wall thickness was higher in controls than in HFpEF patients, which was driven by a larger LV end-diastolic diameter in the HFpEF group (Table 1). The elevated NT-proBNP, as an inclusion criterion in the PARAMOUNT trial, can favour patients with larger left ventricles. Indeed, in our study, LV end-diastolic diameter was significantly associated with NT-proBNP levels (P = 0.04).
Left atrial function
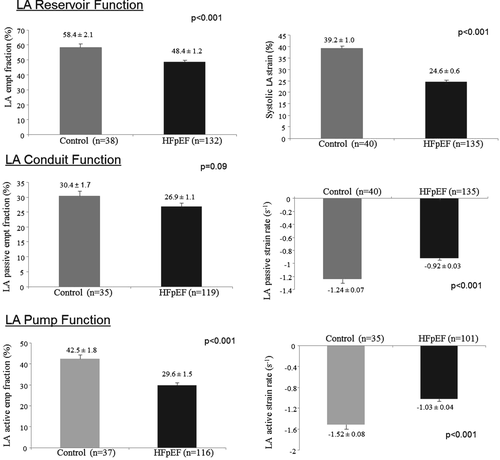
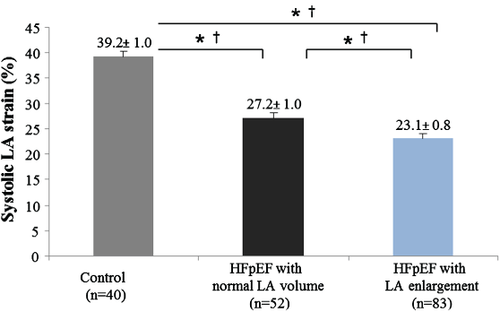
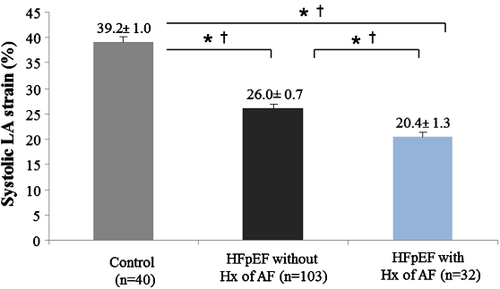
Left atrial reservoir (systolic LA strain and LA emptying fraction), LA conduit (LA passive strain rate), and LA pump (LA active strain rate and LA active emptying fraction) function were significantly lower in HFpEF patients than in controls (Figure 2). Also, the LA expansion index (another measurement of LA reservoir function) was significantly lower in our HFpEF patients than in controls (114.4 ± 7.6 in HFpEF vs. 158.8 ± 11.1 in controls, P = 0.002). The difference in LA reservoir function (measured by systolic LA strain) between groups remained significant even after adjustment for age, gender, heart rate, systolic blood pressure, body mass index, LAVi, LV global longitudinal strain, LV end-diastolic volume, LV SW, E/A, E′, and E/E′ (P < 0.001) (Table 2). As compared with controls, systolic LA strain was lower even in the subset of HFpEF patients with normal LAVi (n = 52) (≤29 mL/m2)16 (Figure 3) and in the subset of HFpEF patients without known history of AF (n = 103) (Figure 4).
| Unadjusted | Adjusted* | |||
|---|---|---|---|---|
| B | P | B | P | |
| HFpEF | −14.6 | <0.001 | −8.41 | <0.001 |
| Age (years) | −0.21 | 0.013 | −0.15 | 0.02 |
| Male | 0.31 | 0.837 | −1.8 | 0.12 |
| Heart rate (b.p.m) | 0.12 | 0.022 | 0.05 | 0.23 |
| Systolic blood pressure (mmHg) | −0.09 | 0.039 | −0.05 | 0.25 |
| Body mass index (kg/m2) | −0.37 | 0.002 | −0.11 | 0.35 |
| LV Global longitudinal strain (%) | −1.35 | <0.001 | −0.7 | <0.001 |
| LV end-diastolic volume/BSA (mL/m2) | −0.11 | 0.021 | −0.01 | 0.92 |
| LV stroke work (g-m) | −0.03 | 0.168 | 0.08 | 0.01 |
| E/E′ | −0.037 | <0.001 | −0.06 | 0.47 |
| E′ (cm/s) | 1.02 | <0.001 | −0.08 | 0.79 |
| E/A | −5.35 | <0.001 | −2.7 | 0.01 |
| Left atrial volume index (mL/m2) | −0.41 | <0.001 | −0.2 | 0.001 |
- * Adjusted for all variables presented in this table.
- BSA, body surface area; E, early mitral inflow velocity; E′, early lateral mitral relaxation velocity; E/A, early to late mitral inflow velocity ratio; HFpEF, heart failure with preserved ejection fraction.
Among patients with HFpEF (n = 135), those with lower systolic LA strain had a higher prevalence of prior heart failure hospitalization and history of AF, as well as worse LV systolic function (measured by LVEF and LV global longitudinal strain), and higher LV mass and LAVi, when compared with patients with higher systolic LA strain. However, NT-proBNP levels and E/E′ were similar across the quartiles of LA function (Table 3).
| Quartiles of systolic LA strain | P-for trend | ||||
|---|---|---|---|---|---|
| Worse | Better | ||||
| 15.7 ± 2.9% (n = 34) | 22.2 ± 1.3% (n = 34) | 26.9 ± 1.5% (n = 34) | 34.1 ± 4.3% (n = 33) | ||
| Age (years) | 70 ± 9 | 72 ± 8 | 72 ± 11 | 67 ± 8 | 0.18 |
| Female, n (%) | 19 (56) | 27 (79) | 18 (53) | 19 (58) | 0.57 |
| BMI (kg/m2) | 30.0 ± 5.3 | 29.5 ± 6.5 | 29.6 ± 5.6 | 29.3 ± 5.4 | 0.67 |
| HR (b.p.m.) | 63 ± 9 | 67 ± 12 | 67 ± 18 | 67 ± 13 | 0.17 |
| SBP (mmHg) | 141 ± 17 | 139 ± 15 | 135 ± 19 | 140 ± 13 | 0.66 |
| Previous HF hospitalization, n (%) | 23 (68) | 19 (56) | 16 (47) | 8 (26) | 0.001 |
| History of AF, n (%) | 15 (45) | 5 (15) | 7 (21) | 4 (12) | 0.004 |
| History of HTN, n (%) | 29 (88) | 32 (94) | 31 (91) | 31 (94) | 0.48 |
| History of DM, n (%) | 11 (33) | 11 (32) | 13 (38) | 12 (36) | 0.68 |
| History of MI, n (%) | 9 (27) | 3 (9) | 10 (29) | 8 (24) | 0.71 |
| LVEF (%) | 56.5 ± 6.2 | 59.8 ± 7.6 | 58.8 ± 8.3 | 61.2 ± 6.2 | 0.02 |
| LV GLS (%) | −13.5 ± 3.0 | −14.7 ± 3.6 | −15.3 ± 3.1 | −16.7 ± 3.1 | <0.001 |
| LV end-diastolic volume/BSA (mL/m2) | 63.5 ± 13.3 | 59.1 ± 12.8 | 63.7 ± 14.8 | 60.9 ± 16.1 | 0.78 |
| LV end-systolic volume/BSA (mL/m2) | 27.8 ± 8.1 | 24.1 ± 9.0 | 26.4 ± 8.5 | 23.8 ± 8.1 | 0.14 |
| RWT | 0.37 ± 0.11 | 0.38 ± 0.08 | 0.39 ± 0.08 | 0.38 ± 0.08 | 0.55 |
| LV mass index (g/m2) | 84.5 ± 27.8 | 78.3 ± 19.4 | 80.7 ± 16.7 | 74.1 ± 21.1 | 0.09 |
| LV mass/height2.7 (g/m2.7) | 41.9 ± 15.2 | 37.9 ± 9.4 | 38.8 ± 8.0 | 35.2 ± 10.6 | 0.03 |
| LAVi (mL/m2) | 40.3 ± 14.3 | 32.8 ± 9.0 | 32.7 ± 9.8 | 27.5 ± 8.5 | <0.001 |
| Deceleration time (ms) | 207.5 ± 34.3 | 212.6 ± 34.2 | 211.0 ± 37.6 | 226.3 ± 47.3 | 0.07 |
| E′ (cm/s) | 6.7 ± 3.0 | 6.3 ± 1.7 | 6.9 ± 2.6 | 6.3 ± 2.0 | 0.82 |
| E/E′ | 15.6 ± 14.3 | 14.0 ± 6.5 | 12.5 ± 5.5 | 12.7 ± 4.8 | 0.14 |
| NT-proBNP (pg/mL) | 948 (601–1914) | 727 (499–1697) | 620 (399–1004) | 830 (374–1198) | 0.07 |
- Data are shown as mean ± SD, median (interquartile range) or n (%).
- BMI, body mass index; BSA, body surface area; DM, diabetes mellitus; E′, early lateral mitral relaxation velocity; E/E′, mitral inflow to mitral relaxation velocity ratio; GLS, left ventricular global longitudinal strain; HF, heart failure; HR, heart rate; HTN, hypertension; LA, left atrial; LAVi, left atrial volume index; MI, myocardial infarcion; RWT, relative wall thickness; SBP, systolic blood pressure.
Discussion
We found that HFpEF patients had lower LA reservoir, conduit, and pump function than healthy controls. LA reservoir function (measured by systolic LA strain) remained significantly lower in the HFpEF group, even after adjustment for potential confounders, despite normal LA size and among those without a known history of AF. In HFpEF patients, lower systolic LA strain was associated with higher prevalence of prior heart failure hospitalization and history of AF, as well as lower LV systolic function, and higher LV mass and LAVi. These findings suggest that LA dysfunction is prevalent in HFpEF and may contribute to its pathophysiology.
Left atrial dysfunction has previously been described in HFpEF patients.14, 24, 25 In previous 2D speckle tracking studies, lower LA reservoir and pump function were demonstrated in HFpEF patients as compared with healthy controls26 or with asymptomatic patients with diastolic dysfunction.13 Strain analysis using speckle tracking is a direct measurement of intrinsic LA myocardial deformation, relatively independent of loading conditions and geometric assumptions27, 28 and with high feasibility and reproducibility.19 Our results in a relatively large well-defined HFpEF group corroborate these prior studies and extend the findings of LA dysfunction to all three phases of LA function that may reflect an advanced stage of this syndrome. Further, we found that systolic LA strain was the more robust measure of LA dysfunction in HFpEF patients in that it remained significantly different from controls even after multivariable adjustments and in the subsets with normal LA volume or without prior AF. These findings suggest that LA dysfunction may occur in HFpEF patients independent of LA dilation or remodelling caused by AF. However, due to the cross-sectional nature of this study, we cannot conclusively discern whether early LA dysfunction is a consequence of HFpEF, or if LA dysfunction is a mechanism that contributes to an increased susceptibility to HFpEF.
We also found that lower systolic LA strain was associated with a higher prevalence of prior heart failure hospitalization and history of AF. Previous studies showed that impaired LA function was a predictor of HF hospitalization in patients with HF with reduced EF29 and among patients with coronary disease and preserved EF.30 Moreover, LA dysfunction has been described in AF patients,31 which may be attributable to LA wall fibrosis32 and may also contribute to increased incidence of AF in HFpEF patients.4 We also observed that lower systolic LA strain was related to worse LV systolic function, and greater LV hypertrophy (LVH) and LA structural remodelling. Impaired LV longitudinal strain has been associated with worse systolic LA strain due to the influence of downward motion of the mitral plane in the diastolic phase of LA.33-35 LVH may also contribute to LA dysfunction through pressure overload and increased LA wall tension; and worse systolic LA strain has been shown to differentiate pathological from physiological LVH.36, 37 Thus, these pathophysiological mechanisms may play a pathophysiological role in LA dysfunction associated with HFpEF. Higher LV filling pressures may lead to deterioration of LA function as a result of haemodynamic overload and mechanical stretch of the LA wall.38, 39 We did not find an independent association between E/E′ or NT-proBNP and systolic LA strain in our HFpEF group, which may be secondary to the fact that all patients enrolled in PARAMOUNT were required to have an elevated NT-proBNP.
Several limitations of our analysis should be noted. We analysed a subset of the patients enrolled in the PARAMOUNT trial due to technical and quality requirements for LA speckle tracking analysis and high prevalence of AF at the time of echocardiography, with some notable differences between the included and excluded patients. Although the analyses of 3D images may be a more accurate measurement, the protocol of the PARAMOUNT trial required only 2D images.40 Also, invasive methods to measure LV filling pressure were not available. In addition, the generalizability of these findings to HFpEF patients in the community may be limited because of the inclusion/exclusion criteria of the overall PARAMOUNT trial.
In summary, LA dysfunction was present among HFpEF patients, and impaired LA reservoir function occurred regardless of LA size or history of AF. In HFpEF patients, lower systolic LA strain was associated with higher prevalence of prior heart failure hospitalization and history of AF, as well as worse LV systolic function, LV hypertrophy, and LA remodelling, suggesting that LA dysfunction may be a marker of severity in HFpEF and may further play a pathophysiological role in HFpEF. The additional clinical and prognostic relevance of LA function in HFpEF remains to be determined.
Acknowledgements
A.B.S.S. acknowledges grant support (0281-12-3) from CAPES (Brazil).
Funding
The PARAMOUNT trial was sponsored by Novartis.
Conflict of interest: M.R.Z., B.P., A.A.V., M.P., J.J.V.M., A.M.S., and S.D.S. have received research support and have consulted for Novartis. M.L., T.B., and V.S. are employees of Novartis. The remaining authors have no conflicts to declare.




