Computed tomography or chest X-ray to assess pulmonary congestion in dyspnoeic patients with acute heart failure
Abstract
Aims
While computed tomography (CT) is widely acknowledged as superior to chest radiographs for acute diagnostics, its efficacy in diagnosing acute heart failure (AHF) remains unexplored. This prospective study included consecutive patients with dyspnoea undergoing simultaneous low-dose chest CT (LDCT) and chest radiographs. Here, we aimed to determine if LDCT is superior to chest radiographs to confirm pulmonary congestion in dyspnoeic patients with suspected AHF.
Methods and results
An observational, prospective study, including dyspnoeic patients from the emergency department. All patients underwent concurrent clinical examination, laboratory tests, echocardiogram, chest radiographs, and LDCT. The primary efficacy measure to compare the two radiological methods was conditional odds ratio (cOR). The primary outcome was adjudicated AHF, ascertained by comprehensive expert consensus. The secondary outcome, echo-bnp AHF, was an objective AHF diagnosis based on echocardiographic cardiac dysfunction, elevated cardiac filling pressure, loop diuretic administration, and NT-pro brain natriuretic peptide > 300 pg/mL. Of 228 dyspnoeic patients, 64 patients (28%) had adjudicated AHF, and 79 patients (35%) had echo-bnp AHF. Patients with AHF were older (78 years vs. 73 years), had lower left ventricular ejection fraction (36% vs. 55%), had higher elevated left ventricular filling pressures (98% vs. 18%), and had higher NT-pro brain natriuretic peptide levels (3628 pg/mL vs. 470 pg/mL). The odds to diagnose adjudicated AHF and echo-bnp AHF were up to four times greater using LDCT (cOR: 3.89 [2.15, 7.06] and cOR: 2.52 [1.45, 4.38], respectively). For each radiologic sign of pulmonary congestion, the LDCT provided superior or equivalent results as the chest radiographs, and the interrater agreement was higher using LDCT (kappa 0.88 [95% CI: 0.81, 0.95] vs. 0.73 [95% CI: 0.63, 0.82]). As first-line imaging modality, LDCT will find one additional adjudicated AHF in 12.5 patients and prevent one false-positive in 20 patients. Similar results were demonstrated for echo-bnp AHF.
Conclusions
In consecutive dyspnoeic patients admitted to the emergency department, LDCT is significantly better than chest radiographs in detecting pulmonary congestion.
Introduction
Dyspnoea is one of the most common symptoms in elderly patients admitted to the emergency department and is the key symptom of most respiratory and cardiac diseases. Differentiating cardiogenic pulmonary congestion from respiratory causes is difficult because the diseases frequently coexist and often have atypical clinical presentations with symptoms masked by co-morbidities.1-3
Current guidelines highlight the importance of early recognition of heart failure in the acute setting to facilitate key investigations, appropriate treatment and access to specialist care to improve patient outcome.2, 4 However, patients are often managed by non-cardiologists in the emergency department, and there is an unmet need to improve objective diagnostic tests to diagnose acute heart failure (AHF) and cardiogenic pulmonary congestion.
Pulmonary congestion in heart failure can be described as two phenotypes, intravascular and tissue congestion, although the majority of patients have a combination.5 With echocardiography, the degree of intravascular congestion and elevated cardiac filling pressures can be evaluated.6 Pulmonary tissue congestion can be detected on the chest radiographs or lung ultrasound, both of which may be used to confirm the AHF diagnosis.4, 7 However, approximately 20% of patients with AHF have a nearly normal chest radiograph.8
The chest computed tomography (CT) is considered more sensitive than chest radiographs for early phase cardiogenic pulmonary congestion,9 and high-resolution pulmonary CT scans have been suggested as the gold standard for the assessment of pulmonary interstitial oedema.5 However, there are no prospective, observational, or randomized studies to examine if a low-dose, non-contrast chest CT (LDCT) provides more diagnostic information for AHF than chest radiographs in consecutive dyspnoeic patients in the emergency department.
Methods
Design
We conducted a prospective, observational study including consecutive adult dyspnoeic patients admitted to the emergency department at Copenhagen University Hospital, Bispebjerg, Denmark. The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the National Ethics Committee on Health Research Ethics in Copenhagen, Denmark (H-17000869), and all patients had to provide initial informed written consent.
Population
We screened medical records of all patients ≥50 years of age admitted at the Accident and Emergency Department, Emergency Department and Department of Cardiology, between 2 am until 3 pm on 216 randomly selected weekdays from 1 November 2017 to 8 August 2019 (Figure 1). We chose the age limit of 50 years because this represents the elderly population with concomitant co-morbidities, because AHF is not as common in younger patients,10 and because there is an unnecessary increased risk of radiation in subjects below 50 years.11, 12
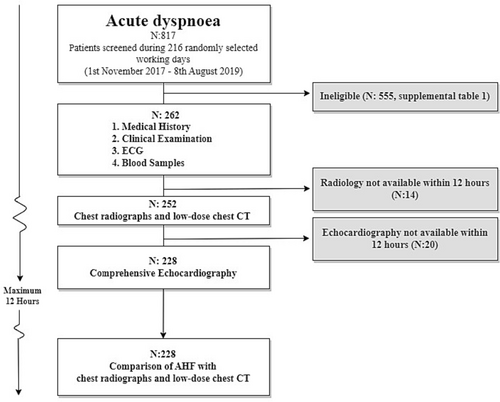
The main inclusion criterion was acute dyspnoea as primary or co-primary symptom, supported by at least one abnormal respiratory parameter. An abnormal respiratory parameter was defined as: a respiratory rate > 20/min, the need for oxygen to maintain an oxygen saturation > 94% (or 92% in case of chronic obstructive pulmonary disease), rhonchi or prolonged respiration on auscultation or any objective sign of heart failure (bilateral rales on auscultation, jugular vein distention, orthopnoea, or bilateral pedal oedemas). Patients were excluded if the expected time from emergency department admission until LDCT or echocardiography was above 12 h. Patients' ineligible for the study are presented in Table S1.
Clinical examination and blood samples
At admission, and following informed written consent, all patients underwent immediate blood samples including NT-pro brain natriuretic peptide (NT-proBNP), clinical examination, comprehensive echocardiography, a chest radiograph, and a LDCT. The clinical examination was performed according to current clinical guidelines by the first emergency physician to attend the patient.
The New York Heart Association Functional Classification (NYHA) was used as a measure of dyspnoea in all patients disregard of heart disease. The ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2), PaO2/FiO2 ratio, normally used to classify severity of acute respiratory distress syndrome, was used as an objective tool to identify hypoxemia when supplemental oxygen had been administered. An abnormal ratio <400 defined hypoxia.13
Advanced echocardiogram
All patients underwent a comprehensive echocardiogram within a few hours performed by experienced cardiologists with a comprehensive examination according to the 2016 ESC guidelines,8 including a systematic evaluation of left ventricular (LV) filling pressure and diastolic dysfunction.14 For full details, see Miger et al.15
Chest radiographs
If possible, all chest radiographs were performed standing posteroanterior and lateral projections, using direct radiography equipment acquired as a digital radiograph. If the patient was unable to stand/sit, the examination was performed supine as anteroposterior projection using portable radiography device. Radiation dose was 0.1 mSV.
Low-dose non-contrast chest computed tomography
The LDCT was performed in continuation of the chest radiographs using a multislice CT scanner (Somatom Definitions Flash, Siemens Medical Solutions, Forschheim, Germany) with a low-dose protocol (<2 mSV), and without contrast. Full details of CT parameters have previously been published.15
Interpretation of the chest radiographs and computed tomography
The chest radiographs and LDCT were performed concurrently but analysed separately by two independent radiologists specialized in thoracic radiology. The radiologists, working at two different university hospitals, were blinded to all other data except the radiology images of the current study.
The radiology evaluation of pulmonary congestion was performed according to the Fleischner Society most commonly observed radiology patterns for pulmonary and cardiac pathology16, 17 with emphasis on radiologic features for pulmonary congestion. The categorization of pulmonary congestion included evaluation of ground-glass opacities, interlobular thickening, interlobar effusion, consolidation, crazy paving, atelectasis, peribronchial cuffing, enlarged heart, pleural effusion, and vascular redistribution (defined as increased vascular diameter/distension of the pulmonary veins).15-17
The classification of pulmonary congestion was supported by the evaluation of all radiologic features and was adjudicated on a Likert scale from 1–5 on both the chest radiographs and LDCT (1. very unlikely, 2. somewhat unlikely, 3. neutral, 4. somewhat probable, 5. definite). Imaging based pulmonary congestion on both chest radiographs and LDCT required agreement of the radiologists on Likert item 4–5.
Acute heart failure as reference diagnosis
To increase validity of planned tests for the association between the radiology diagnosis of pulmonary congestion and the clinical diagnosis of AHF, we used two diagnostic approaches: (i) adjudicated AHF and (ii) echo-bnp AHF.
Adjudicated acute heart failure
The primary outcome was an AHF diagnosis adjudicated by two cardiologists according to the 2017 cardiovascular and stroke endpoint definitions for clinical trials consensus report18 (Appendix S1). Disagreements were settled by a third cardiologist. The adjudicated AHF diagnosis was decided into somewhat probable (AHF with concomitant acute pulmonary disease) or definite AHF (AHF without significant acute pulmonary diseases). In the current study, only definite AHF was termed adjudicated AHF.
To mitigate any bias from radiology findings to the primary outcome diagnosis, the adjudicating cardiologists used elevated LV filling pressures (grade II + III) by echocardiography as support of pulmonary congestion.8, 19 Grade II-III LV filling pressures was determined according to current recommendations by Nagueh et al.14
Echo-bnp acute heart failure
We predefined an observer-independent secondary outcome, echo-bnp AHF, to avoid any bias from medical record review that the radiologic images may have indirectly influenced. The secondary AHF diagnosis was based on four criteria: echocardiographic signs of abnormal structure/function, echocardiographic signs of elevated LV filling pressure, elevated NT-proBNP and loop diuretic treatment. More specifically we required the presence of (I) echocardiographic phenotype according to abnormal structure or function: left ventricular ejection fraction (LVEF) ≤ 40%, LVEF 41–49%, LVEF ≥ 50% with diastolic dysfunction or severe valve disease4; and (II) NT-proBNP >300 pg/mL4; and (III) echocardiographic signs of elevated LV filling pressure (grade II + III)14; and (IV) administration of loop diuretics at admission, during hospitalization or at discharge.
Statistics
R version 4.2.220 was used for all statistical analyses. Continuous variables are presented with mean (±1 standard deviation) or median [interquartile range] as appropriate and compared with Student's t-test or Wilcoxon. Categorical variables are presented as absolute numbers (percentages) and compared using the χ2-test or Fischer test, as suitable. All tests are two-sided and a P-value of < 0.05 was considered significant.
We calculated the conditional odds ratio (cOR) as the primary efficacy measure to directly compare the association between AHF and pulmonary congestion determined by the two radiological methods. For this, we used a conditional logistic regression analysis and stratified the data according to each patient-ID. The conditional odds ratio method has the advantage of providing less biased results by controlling for potential confounding variables, including reader variability.21
Performance measurement was also demonstrated by sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). We used univariate logistic regression models to calculate odds ratio (OR) for each radiologic feature and the diagnosis of overall radiologic congestion.
To assess the clinical implication of using chest LDCT instead of chest radiographs, we calculated the number needed to undergo LDCT to prevent one incorrect AHF diagnosis (e.g. similar to numbers needed to treat if a drug was examined) (Table S2).
Results
Patients
We included 228 consecutive dyspnoeic patients (Figure 1). AHF criteria for the primary outcome, adjudicated AHF, was met in 64 (28%), and 79 (35%) for the secondary outcome, echo-bnp AHF. Patients with AHF were older, had lower LVEF, had more elevated LV filling pressures, had higher NT-proBNP concentrations, and had fewer concomitant acute pulmonary diseases than patients without AHF (Table 1).
| Adjudicated AHF | Echo-bnp AHF | |||||
|---|---|---|---|---|---|---|
| No AHF | AHF | No AHF | AHF | |||
| N = 164 | N = 64 | N = 149 | N = 79 | |||
| Age, mean (SD) | 73.0 (9.8) | 77.8 (10.1) | 0.002 | 72.3 (9.6) | 78.3 (9.8) | <0.001 |
| Male, N (%) | 92 (56.0) | 38 (59.0) | 0.764 | 84 (56.0) | 46 (58.0) | 0.898 |
| History of chronic heart failure, N (%) | 26 (15.9) | 31 (48.4) | <0.001 | 28 (18.8) | 29 (36.7) | 0.005 |
| Signs and symptoms at admission | ||||||
| Systolic blood pressure (mmHg), mean (SD) | 140 (25.5) | 153 (32.1) | 0.007 | 140 (24.7) | 151 (32.1) | 0.005 |
| Orthopnoea, N (%) | 66 (40.2) | 46 (71.9) | <0.001 | 59 (39.6) | 53 (67.1) | <0.001 |
| Paroxysmal nocturnal dyspnoea, N (%) | 80 (48.8) | 26 (40.6) | 0.336 | 70 (47.0) | 36 (45.6) | 0.949 |
| Bilateral pedal oedemas, N (%) | 41 (25.0) | 29 (45.3) | 0.005 | 30 (20.1) | 40 (50.6) | <0.001 |
| Bilateral rales on auscultation, N (%) | 51 (31.1) | 37 (57.8) | <0.001 | 44 (29.5) | 44 (55.7) | <0.001 |
| NYHA, N (%) | 0.845 | |||||
| I | 1 (0.61) | 0 (0.0) | 1 (0.67) | 0 (0.0) | 0.715 | |
| II | 54 (32.9) | 18 (28.1) | 50 (33.6) | 22 (27.8) | ||
| III | 72 (43.9) | 30 (46.9) | 66 (44.3) | 36 (45.6) | ||
| IV | 37 (22.6) | 16 (25.0) | 32 (21.5) | 21(26.6) | ||
| Concomitant pulmonary disease | ||||||
| Pneumonia/COPD in exacerbation, N (%) | ||||||
| Without hypoxia | 45 (27.4) | 2 (3.1) | <0.001 | 10 (26.8) | 7 (8.9) | 0.003 |
| With hypoxia | 82 (50.0) | 12 (18.8) | <0.001 | 72 (48.3) | 22 (27.8) | 0.004 |
| Laboratory data | ||||||
| NT-proBNP (pg/mL), median [IQR] | 470 [134; 1886] | 3628 [2055; 7876] | <0.001 | 354 [121; 1000] | 3450 [2110; 7053] | <0.001 |
| Echocardiographic data | ||||||
| LVEF, mean (SD) | 55.4 (9.1) | 36.4 (15.5) | <0.001 | 54.9 (10.1) | 40.9 (16.0) | <0.001 |
| vTricuspid velocity (cm/s), mean (SD) | 262 (78.8) | 311 (50.3) | <0.001 | 252 (77.3) | 319 (46.8) | <0.001 |
| E/é, median [IQR] | 8.8 [7.2; 11.4] | 17.6 [13.9; 23.1] | <0.001 | 8.7 [7.2; 11.4] | 15.4 [11.2; 21.6] | <0.001 |
| Indexed left atrial volume (mL), mean (SD) | 30.0 (14.1) | 46.3 (13.4) | <0.001 | 27.9 (11.7) | 47.3 (14.3) | <0.001 |
| Elevated filling pressure (grade II + III), N (%) | 29 (17.7) | 63 (98.4) | <0.001 | 13 (8.7) | 79 (100) | <0.001 |
| Echocardiographic phenotype, N (%) | ||||||
| Severe valve disease | 3 (1.8) | 6 (9.38) | 0.016 | 2 (1.3) | 7 (8.9) | 0.009 |
| Reduced LVEF ≤ 40% | 15 (9.2) | 37 (57.8) | <0.001 | 15 (10.1) | 37 (46.8) | <0.001 |
| Mildly reduced LVEF from 41% to 49% | 12 (7.3) | 10 (15.6) | 0.097 | 13 (8.7) | 9 (11.4) | 0.679 |
| LVEF ≥ 50% with diastolic dysfunction | 31 (18.9) | 16 (25.0) | 0.401 | 15 (10.1) | 32 (40.5) | <0.001 |
- AHF, acute heart failure; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; NT-proBNP, NT-pro brain natriuretic peptide; NYHA, New York Heart Association Functional Classification; SD, standard deviation.
Performance of the low-dose chest computed tomography to diagnose pulmonary congestion
Pulmonary congestion on LDCT, as compared with chest radiographs, had a significantly stronger association with AHF for both the primary outcome adjudicated AHF (cOR: 3.89, 95% CI: 2.15–7.96) and the secondary outcome echo-bnp AHF (cOR: 2.52, 95% CI: 1.08, 3.16) (Figure 2). In addition, similar associations were found for specific radiologic features of congestion, notably an enlarged heart, vascular redistribution, and any pleural effusion (Figure S1).
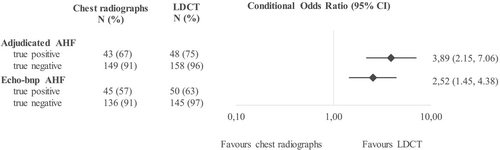
For adjudicated AHF, congestion on LDCT also resulted in higher univariate odds ratios (CT: 79.0 vs. chest radiographs: 20.3) and radiological pulmonary congestion was demonstrated more frequently on LDCT than on the chest radiographs (LDCT: 75% vs. chest radiographs: 67%, Table 2). Additionally, LDCT resulted in a higher true negative rate (LDCT: 96% vs. chest radiographs: 91%). Equivalent results were demonstrated for the secondary outcome echo-bnp AHF (Table 2).
| Parameters | Adjudicated AHF | Echo-bnp AHF | ||
|---|---|---|---|---|
| LDCT | Chest radiographs | LDCT | Chest radiographs | |
| Sensitivity (%) | 75 | 67 | 63 | 57 |
| Lower limit 95% CI | 63 | 54 | 52 | 45 |
| Upper limit 95% CI | 85 | 78 | 74 | 68 |
| Specificity (%) | 96 | 91 | 97 | 91 |
| Lower limit 95% CI | 92 | 85 | 93 | 86 |
| Upper limit 95% CI | 99 | 95 | 99 | 95 |
| PPV (%) | 89 | 74 | 92 | 78 |
| Lower limit 95% CI | 77 | 61 | 82 | 65 |
| Upper limit 95% CI | 96 | 85 | 98 | 87 |
| NPV (%) | 90 | 88 | 83 | 80 |
| Lower limit 95% CI | 85 | 82 | 76 | 73 |
| Upper limit 95% CI | 94 | 92 | 88 | 86 |
| PLR | 20.07 | 7.35 | 23.10 | 6.53 |
| Lower limit 95% CI | 9.03 | 4.40 | 8.65 | 3.75 |
| Upper limit 95% CI | 44.63 | 12.26 | 61.68 | 11.36 |
| NLR | 0.28 | 0.36 | 0.39 | 0.47 |
| Lower limit 95% CI | 0.18 | 0.25 | 0.29 | 0.36 |
| Upper limit 95% CI | 0.41 | 0.51 | 0.52 | 0.61 |
| Univariate odds ratio | 79.0 | 20.3 | 65.5 | 13.8 |
| Lower limit 95% CI | 31.3 | 9.9 | 23.3 | 6.9 |
| Upper limit 95% CI | 233.5 | 44.1 | 219.0 | 244.5 |
- AHF, acute heart failure; CI, confidence interval; LDCT, low-dose chest computed tomography; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
The clinical significance of using LDCT instead of chest radiographs was calculated as the numbers needed to examine where one more patient with adjudicated AHF is identified for every 12.5 patient scanned with LDCT. Additionally, if LDCT is used instead of chest radiographs, one false positive congestion diagnosis is prevented in every 20 patients scanned. For the alternative diagnose, echo-bnp AHF, the numbers needed to examine were 16 to identify one additional echo-bnp AHF, and in 17 patients, one false positive congestion is prevented.
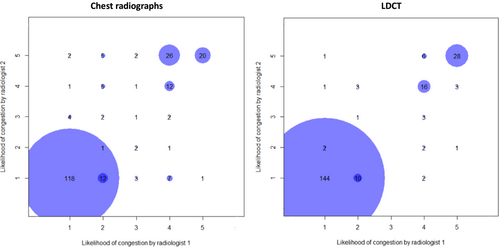
Interrater agreement of the computed tomography
Interrater reliability of the radiologists was evaluated separately on chest radiographs and LDCT (Figure 3). The radiologists were in more agreement to diagnose pulmonary congestion on LDCT [kappa: 0.88 (95% CI: 0.81, 0.95) compared with chest radiographs (kappa: 0.73 (95% CI: 0.63, 0.82)]. Some radiologic features, particularly vascular redistribution, peribronchial cuffing, and any pleural effusion had higher interrater agreement on LDCT (Table S3).
Timing of investigations and interventions
In our study, chest radiographs and LDCT were performed in continuation from each other, without lag in time, with a median time from admission of 2.5 h [IQR: 1.4–5.9]. The echocardiogram was performed with a median time of 4.0 h [IQR: 2.7–7.7] from admission.
Intravenous loop diuretics had been given to seven patients (11%) with adjudicated AHF before any of the radiologic examinations, and three patients (5%) had received intravenous diuretic between the radiologic examination and echocardiogram. For the secondary outcome, echo-bnp AHF, seven patients (9%) received intravenous diuretics before the radiologic examinations and four patients (5%) between the radiologic examination and echocardiogram.
Discussion
This is the first study that directly compares LDCT and chest radiographs for the association with pulmonary congestion due to AHF in adult patients seen in the emergence department with acute dyspnoea. We find that there is a higher probability of diagnosing pulmonary congestion as seen in AHF on LDCT than on chest radiographs in these dyspnoeic patients. In addition, in patients with AHF the number of patients with well-defined imaging signs of congestion is greater on LDCT (three-fourths of patients) than on chest radiographs (two-thirds of patients) and LDCT has better interrater reproducibility.
Chest radiographs versus low-dose chest computed tomography to detect pulmonary congestion
Our findings corroborate presumptions from a previous retrospective study by Carey et al.22 where chest radiographs were compared with a novel projection technique for ultra-low dose CT (thoracic tomograms) in 22 consecutive patients.
Although the study did not directly examine AHF, Carey et al. found that the area under the curve (AUC) was larger for thoracic tomograms than chest radiographs for non-focal lung disease (pulmonary oedema and interstitial lung disease) and effusions (pulmonary and pericardial), although the difference was not significant.
Furthermore, in comparison to our study, Barile et al.23 used electronic medical records to search for patients with a billing diagnosis of heart failure who, for various reasons, had both a non-contrast CT and chest radiographs within 3 h. They reported that radiologists often underestimate qualitative (visual) assessments of lung CT findings for pulmonary oedema and that quantitative CT Hounsfield unit measurements is a more accurate method for identifying this condition. However, the study by Barile et al only included patients with known heart failure and not consecutive dyspnoeic patients including both heart and pulmonary disease, and therefore, the different populations should be taken into consideration when comparing the two studies. Moreover, Barile et al.23 reported that a qualitative (visual) CT assessment was less sensitive than portable supine chest radiographs of the chest (sensitivity 100% and specificity 95%) in identifying pulmonary oedema. These results are inconsistent with ours, possibly because LDCT does not provide added benefit in acute cases when portable chest radiographs already indicate pulmonary congestion. The discrepancy may arise from the limited benefit of LDCT in critically ill patients with severe symptoms and signs, where pulmonary congestion is already evident on portable chest radiographs.
Our aim was to fill the knowledge gap if LDCT have a higher probability of confirming pulmonary congestion compared with chest radiographs. We chose to perform a head-to-head comparison on radiology only because a main pitfall of lung ultrasound is the clinical interpretation of B-lines in the absence of an established heart failure diagnosis,24 and there is a significant variance in diagnostic accuracy when it comes to lung ultrasound across various studies, with sensitivity ranging from 0.58 to 0.97 and specificity ranging from 0.69 to 0.94.25 As a result, chest radiographs are frequently ordered anyway during the acute phase in co-morbid elderly patients. We have demonstrated, in a previous study of 117 patients from the same population, that B-lines have a high specificity but low sensitivity, resulting in many unresolved dyspnoeic patients.26
Reproducibility
We observed that when radiologists identify pulmonary congestion, AHF is generally always diagnosed clinically by cardiologists. However, pulmonary congestion is often underestimated on radiology imaging of the chest. Furthermore, we found that the intermediate area is reduced with LDCT compared with chest radiographs (Figure 3). In our study, the reduced intermediate area on LDCT could be explained by the fact that more subtle signs of pulmonary congestion are more easily identified on chest LDCT, especially because the radiologists were blinded to all clinical data (e.g. symptoms and signs) normally available when performing the LDCT evaluation. Furthermore, the diagnosis of pulmonary congestion in our study required high level of interobserver agreement.
As a supportive finding, in addition to overall pulmonary congestion, we showed that the conditional odds ratios favoured LDCT as compared with chest radiographs, on several radiologic features of congestion, particularly enlarged heart, vascular redistribution, and any pleural effusion. The same signs had better interrater agreement, suggesting that some radiologic signs are more easily identified than other, and that radiologic signs of pulmonary congestion in general are better identified using a LDCT.
The reference standard for acute heart failure
We chose adjudicated AHF as the primary outcome as it aligns with the 2017 cardiovascular and stroke endpoint definitions for clinical trials consensus report (Appendix S1).18 It is a weakness that the adjudicated AHF diagnosis may have been indirectly affected by treatments initiated by the on-call clinician who had access to the performed and prior radiological images. However, neither the radiology images nor reports were available by the adjudicating cardiologists. Still, because information bias cannot be completely ruled out, and to mitigate the indirect effect of radiology, we verified our results by also analysing a secondary outcome, an observer-independent AHF diagnosis made independent of any radiologic examination or medical record review, thereby avoiding circular reasoning and consequently an overestimation of the results.
The diagnostic criteria of AHF constitute a challenge in all AHF studies due to the lack of a non-invasive gold standard, and because AHF comes with different forms of pulmonary congestion, intravascular and/or tissue congestion, which are often combined in clinical trials.5 Although the majority of patients with AHF have a combination of both intravascular and tissue congestion, radiology may oversee cases with right-sided heart failure without pulmonary congestion, very mild cases of predominant intravascular congestion, and chronic heart failure as these patients rarely have radiographic pulmonary oedema.5, 27 We demonstrated that approximately 30% of AHF patients do not have radiologic pulmonary congestion, and a higher number for the echo-bnp based AHF diagnosis (Figure 2).
To ensure that the study population represented consecutive patients, we did not exclude AHF patients without radiologic pulmonary congestion. However, these patients are best identified with elevated right atrial pressure, pulmonary capillary wedge pressure, jugular venous pressure, NT-proBNP, and elevated cardiac filling pressures5.28
Study strengths and limitations
This study had some limitations. First, due to logistic reasons, we only screened and included patients from night-time (2 am) until afternoons (3 pm), but not during evenings (3 pm until 2 am). Nonetheless, this enabled a 100% screening rate in the predetermined periods. We find it unlikely that the patients admitted during the evening would differ significantly from patients admitted during the day- or night-time. The eligibility rate is a common problem in acute studies. The main reason is the time limit for patients to understand and provide informed consent (Table S1). Another reason for the relatively low inclusion was patients with acute coronary syndrome29, 30 as these patients required uninterrupted telemetry and could not be transported to the additional LDCT. We did however include all patients with atrial fibrillation. Thus, the current results relate to acute patients with intermediate illness who often pose a considerable diagnostic challenge. Second, as with all cohort studies, definite conclusions of the clinical implication of LDCT in comparison to chest radiographs ideally needs to be verified in randomized-controlled trials. Third, pre-hospital treatment information was not reported or collected, and this could have had a minor impact on imaging finding if loop diuretics was administrated before hospital admission. Fourth, the modest sensitivity is most likely caused by patients with a final diagnosis of AHF but without any radiologic signs of pulmonary congestion, that is, patients with vascular congestion only or patients with right-sided heart failure but without pulmonary congestion. In these patients the chest radiographs and LDCT are most likely only useful for pulmonary differential diagnosis.
The current study had several strengths. First, this is the first study to compare imaging findings of pulmonary congestion of the lungs on chest radiographs and LDCT and their association with AHF in consecutive dyspnoeic patients seen in the emergency department. The acute clinical research setting represents real-world patients with a multitude of diagnoses and included a broad spectrum of consecutive patients with dyspnoea. Second, we only included patients above 50 years of age, as they represent the most relevant co-morbid population, because patients younger seldom develop AHF,10 and we wanted to avoid an unnecessary increased risk of radiation in younger individuals with a low probability of AHF. It is a limitation concerning universal applicability and generalizability in patients younger than 50 years of age, but we sought to avoid introducing bias by including younger, healthier individuals, a factor that could potentially affect the generalizability of our findings to an elderly, co-morbid population. Third, the radiological examinations and echocardiography were systematically performed in continuation of each other, within a few hours, securing a similar cardiovascular state. Fourth, the radiological results were a consensus between expert thoracic radiologists and the clinical diagnosis was confirmed using two different AHF definitions to minimize bias to the outcome. Fifth, conditional odds ratio was used providing less biased results when comparing two methods in the same patients.
Conclusions
In our study, LDCT was better than chest radiographs to identify pulmonary congestion due to AHF in consecutive dyspnoeic patients admitted to the emergency department, and interobserver variations were significantly lower for detecting imaging signs of pulmonary congestion using LDCT. These results indicate a modest clinical benefit of using LDCT instead of chest radiographs, but the cost–benefit advantage is still uncertain.
Aims
We aim to determine if there is a higher probability of detecting pulmonary congestion with LDCT compared with chest radiographs in consecutive adult patients with dyspnoea in the emergency department.
Acknowledgements
We would like to thank all our colleagues at Copenhagen University Hospital, Bispebjerg and Frederiksberg Hospital, for their work and collaboration.
Conflict of interest
Sponsor did not play a role in the design or conduct of the study, neither to collection, management, or analysis of data. Sponsor did not play a role in interpretation, preparation of the data, review or approval of the manuscript, or decision to submit the manuscript for publication. Christian Torp-Pedersen has received grants for studies from Bayer and Novo Nordisk unrelated to the current study and Olav Wendelboe Nielsen is, after the study was conducted, employed at Novo Nordisk. None declared for all remaining authors.
Funding
This work was supported by a research grant from the Danish Cardiovascular Academy, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406, and The Danish Heart Foundation.




