Transcatheter edge-to-edge repair in papillary muscle injury complicating acute myocardial infarction
Abstract
Aims
Acute mitral regurgitation (MR) in the setting of myocardial infarction (MI) may be the result of papillary muscle rupture (PMR). This condition is associated with high morbidity and mortality. We aim to evaluate the feasibility of transcatheter edge-to-edge mitral valve repair (TEER) in this acute setting.
Methods and results
We analysed data from the International Registry of MitraClip in Acute Mitral Regurgitation following acute Myocardial Infarction (IREMMI) of 30 centres in Europe, North America, and the middle east. We included patients with post-MI PMR treated with TEER as a salvage procedure, and we evaluated immediate and 30-day outcomes. Twenty-three patients were included in this analysis (9 patients suffered complete papillary muscle rupture, 9 partial and 5 chordal rupture). The patients' mean age was 68 ± 14 years. Patients were at high surgical risk with median EuroSCORE II 27% (IQR 16, 28) and 20 out of 23 (87% were in cardiogenic shock). All patients were treated with vasopressors, and 17 out of 23 patients required mechanical support. TEER procedure was performed on the median 6 days after the index MI date IQR (3, 11). Procedural success was achieved in 87% of patients. The grade of MR was significantly decreased after the procedure. MR reduction to 0 or 1 + was achieved in 13 patients (57%), to 2 + in 7 patients (30%), P < 0.01. V-Wave was reduced from 49 ± 8 mmHg to 26 ± 10 mmHg post-procedure, P < 0.01. Sixteen out of 23 patients (70%) were discharged from hospital and 5 of them required reintervention with surgical mitral valve replacement. No additional death at 1 year was documented.
Conclusions
TEER is a feasible therapy in critically ill patients with PMR due to a recent MI. TEER may have a role as salvage treatment or bridge to surgery in this population.
Introduction
Acute mitral regurgitation (MR) the setting of myocardial infarction (MI) is often accompanied by haemodynamic instability and has been linked to poor prognosis.1, 2
It can be caused by primary MR due to papillary muscle rupture (PMR) or due to the rapid remodelling of the infarcted left ventricle (LV) causing apical and inferior displacement of the papillary muscles (secondary MR).3, 4 The rapid onset of regurgitant volume to a non-compliant left atrium (LA) results in a rapid rise in LA pressure, increased pulmonary pressures, pulmonary congestion, and forward left ventricular (LV) failure. When acute MR is caused by papillary muscle injury the outcomes are often fatal due to rapid deterioration.5
The current management of this condition includes haemodynamic stabilization, medical therapy, and urgent surgical mitral valve repair or replacement. Urgent mitral valve intervention is essential, particularly in patients with primary acute MR.6 However, Data from the SHOCK Trial Registry suggest that most patients were treated conservatively due to a prohibitive surgical risk and that mortality rates among them reaches 80%. In fact, even in patients who were treated surgically the mortality extents to 50%.7
Mitral transcatheter edge-to-edge repair (TEER) is an important tool in treating high-risk patients with severe MR. Emerging data stresses its role in acutely decompensated patients including those in cardiogenic shock.8 TEER was also shown to be beneficial in acute MI patients who develop severe MR.9-11
Our group previously demonstrated that TEER was successful in decreasing post-MI secondary MR, improving haemodynamic parameters with lower complication rates when compared with surgical intervention.12 The clinical improvement achieved as a result of successful TEER was a bridge to recovery and avoidance of deterioration in selected decompensated patients.12 However, the available data concerning the role of TEER in post-MI primary MR caused by PMR is based on a limited number of case reports. In this study of an international multicenter registry, we evaluated the role of TEER in post-MI acute primary MR patients. Our goal was to assess the feasibility of TEER in this population.
Methods
Study population and registry
The International Registry of MitraClip in Acute Mitral Regurgitation following Acute Myocardial Infarction (IREMMI) evaluates the outcomes and safety of patients who underwent TEER in an acute setting after MI. We reached out to all the participating centres to review the information on patients who experienced post-MI symptomatic MR. Our current study cohort included patients who had at least symptomatic grade 3 MR within 90 days after acute MI (both ST-elevation and non-ST-elevation) between December 2009 and September 2022, in 30 centres in North America, Europe, and the Middle East. In this analysis we included patients that were treated with TEER in the acute setting due to primary aetiology including complete papillary muscle rupture, partial muscle rupture and chordal (head) rupture.
Patients were at severe condition despite receiving medical treatment and support. They were evaluated by a multidisciplinary team that included a cardiothoracic surgeon, an interventional cardiologist, and a non-interventional cardiologist. All patients were treated in accordance with accepted clinical guidelines. Since all patients were deemed to be at a high surgical risk, salvage TEER procedures were carried out to enable stabilization and recovery. We evaluated immediate and 30-day outcomes in those patients.
The study was conducted in accordance with the Declaration of Helsinki Ethical Principles and was approved by individual local ethics committees.
Echocardiographic evaluation
The severity of MR, left ventricle ejection fraction (LVEF), systolic pulmonary artery pressure (sPAP), and mitral valve (MV) gradient were measured and graded according to the American Society of Echocardiography guidelines.13, 14 The severity of MR was assessed by an integrated multiparametric visual evaluation tool in accordance with standard clinical practice (incorporating 2D, spectral and colour Doppler images), using an ordinal scale (grading 0 - no MR, 1 + mild MR, 2 + moderate MR, 3 + moderate to severe MR, 4 + severe MR). A trans-oesophageal echo (TEE) was completed in all patients prior to the procedure. MR grade, mitral valve area (MVA) and MV gradient, calcium at grasping area and coaptation features were assessed in order to evaluate TEER feasibility.
Transcatheter edge-to-edge mitral valve repair
TEER procedure was performed under general anaesthesia, fluoroscopy, and TEE were routinely used for guidance. After trans-septal puncture, the delivery system was advanced to the left atrium (LA), LV and retracted back to grasp the anterior and posterior leaflets. Procedural success was defined as successful grasping of the anterior and posterior leaflets together with reduction of at least 2 MR grades. V-wave and left atrial pressures (LAP) were measured and recorded immediately before and after the TEER implantation. Procedures were completed using MitraClip device (Abbott Vascular; Menlo Park, CA, USA).
Outcomes
Outcomes of interest were procedural success, safety, 30 days mortality, clinical outcomes, and haemodynamics. Acute procedural success was defined as successful implantation of one or more clips with a reduction of the MR to <2+.
Safety outcomes included procedural and periprocedural complications: clip detachment, cerebrovascular event, MI, bleeding requiring transfusion, cardiac tamponade, and urgent cardiovascular surgery.
Clinical outcomes included in-hospital and 30-days mortality, intensive care unit (ICU), length of stay, and NYHA class at follow-up.
Haemodynamic parameters LAP and V-wave were measured during the procedure. In cases where right heart catheterization was used, pulmonary artery wedge pressure (PCWP) was measured simultaneously with the procedure. After discharge, patients were followed up by individual centres in outpatient clinic visits in which clinical evaluation and echocardiography were performed.
Statistical analysis
Results are reported according to variable properties. Continuous variables (LVEF, PCWP, V-wave, and PAP) are reported according to distribution: mean ± standard deviation for normal distribution (differences between the groups were tested using Student's t-test) and median (interquartile range [IQR]) for non-normal distribution (differences between the groups were tested Mann–Whitney U test. Categorical variables (MR grade) are reported as number (%), and the differences between the groups were tested with chi-square test. Differences were considered statistically significant at P values <0.05. IBM Statistical Package for the Social Sciences (SPSS) Statistics 25.0 (IBM Corp., Armonk, NY) was utilized to perform the analyses.
Results
Patient characteristics
A total of 655 patients were included in the IREMMI. Of those 187 (29%) were treated percutaneously with TEER. Twenty-three out of 187 (12%) cases of post-MI severe MR were due to primary aetiology (Figure 1). The final analysis included these 23 patients. The baseline patient characteristics are summarized in Table 1. Patients' mean age was 68 ± 14 years, and there were 10 women and 13 men. Thirteen out of 23 patients (57%) presented with STEMI, and among these patients, 7 out of 23 (30%) had an anterior wall MI. For patients with inferior wall MI (70%), the infarct-related artery was the right coronary artery (RCA) in nine patients and the left coronary artery (LCX) in seven patients. Seventy percent of the patients had multi-vessel coronary artery disease (CAD), and none of the patients had previously undergone coronary artery bypass graft (CABG).
| Patient no. | Age/sex | ACS | IRA | Time to PCI | Euroscore II (%) | PMR mechanism | KILLIP class | LVEF (%) | sPAP (mmHg) | MV | LV support |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 F | NSTEMI | RCA | 24-48 h | 27 | Complete | 4 | 25 | 50 | Yes | IABP |
| 2 | 42 M | STEMI | RCA | <24 h | 16 | Complete | 4 | 35 | 60 | Yes | IABP- > ECMO |
| 3 | 71 M | STEMI | LCX | <24 h | 28 | Complete | 4 | 72 | 59 | Yes | IMPELLA |
| 4 | 66 M | STEMI | RCA | <24 h | 78 | Complete | 4 | 55 | 38 | Yes | ECMO + IMP |
| 5 | 53 M | STEMI | LAD | <24 h | 47 | Complete | 4 | 55 | 47 | Yes | Pressors |
| 6 | 57 F | NSTEMI | RCA | <24 h | 33 | Complete | 4 | 61 | 48 | Yes | ECMO + IMP |
| 7 | 66 M | STEMI | LM | <24 h | 13 | Chordal | 3 | 25 | 40 | Yes | Pressors |
| 8 | 87 F | STEMI | LAD | <24 h | 72 | Chordal | 4 | 35 | 70 | Yes | IABP |
| 9 | 81 F | STEMI | RCA | <24 h | 16 | Partial | 4 | 65 | 42 | Yes | Pressors |
| 10 | 82 M | STEMI | LCX | <24 h | 28 | Partial | 4 | 45 | 30 | Yes | IABP |
| 11 | 50 M | NSTEMI + OHCA | LAD | <24 h | 22 | Chordal | 4 | 20 | 60 | Yes | ECMO + IMP |
| 12 | 63 M | STEMI | LAD | <24 h | 27 | Partial | 4 | 33 | 53 | Yes | IMPELLA |
| 13 | 78 F | NSTEMI | LAD | 24-48 h | 26 | Complete | 4 | 50 | 39 | No | Pressors |
| 14 | 86 F | NSTEMI | LAD | 24-48 h | 42 | Complete | 4 | 45 | 42 | Yes | IABP |
| 15 | 53 M | NSTEMI | LCX | 24-48 h | 11 | Partial | 4 | 62 | 61 | Yes | IABP |
| 16 | 68 M | STEMI | LCX | <24 h | 23 | Chordal | 4 | 30 | 60 | Yes | IABP |
| 17 | 55 F | NSTEMI | LCX | <24 h | 16 | Complete | 4 | 60 | 32 | Yes | IABP |
| 18 | 55 M | STEMI | RCA | <24 h | 13 | Partial | 4 | 55 | 77 | Yes | IABP |
| 19 | 57 M | NSTEMI | RCA | 24-48 h | 13 | Partial | 4 | 47 | 36 | Yes | IABP |
| 20 | 77 M | NSTEMI | RCA | 24-48 h | 15 | Partial | 3 | 35 | 46 | No | Pressors |
| 21 | 81 F | STEMI | LCX | > 48 h | 20 | Partial | 4 | 30 | 55 | No | IABP |
| 22 | 66 F | STEMI | RCA | <24 h | 23 | Partial | 3 | 55 | 60 | No | Pressors |
| 23 | 88 F | NSTEMI | LCX | > 48 h | 18 | Chordal | 4 | 20 | 50 | Yes | IABP |
| Mean/rate |
68 ± 14 10/23 (43%) F |
13/23 (57%) STEMI |
9 RCA 7 LCX 7 LAD/LM |
15/23 (65%) < 24 h |
27 IQR (16, 28) |
9 Complete 9 Partial 5 Chordal |
20/23 (87%) in CS |
45 IQR (32, 55) |
47 IQR (41, 60) |
19/23 (83%) Ventilated |
17/23 MCS (74%) 4/23 ECMO (17%) |
- Values are given as mean ± standard deviation, n (%), or median [interquartile range].
- ACS, acute coronary syndrome; IABP, intra-aortic balloon pump; IRA, infarct related artery; LAD, left anterior descending artery; LCX, left circumflex artery; LM, left main coronary artery; LV, left ventricle; LVEF, left ventricle ejection fraction; MR, mitral regurgitation; MV, mitral valve; NSTEMI, non-ST elevation myocardial infarction; RCA, right coronary artery; sPAP, systolic pulmonary artery pressure; STEMI, ST elevation myocardial infarction.
Patients were at high surgical risk with EuroSCORE II 27% IQR (16, 28) and 20 out of 23 (87%) were in cardiogenic shock (CS). All patients were treated with vasopressors, 83% were ventilated and 17 out of 23 patients were supported by mechanical support (11 IABP, 2 IMPELLA, and 4 VA-ECMO). Echocardiography findings confirmed that all patients had significant primary MR. The underlining primary MR mechanism was complete PMR in nine patients (39%), partial PMR in nine patients, and chordal rupture in five patients. The median LVEF of the cohort was 45% IQR (35, 56). During hospitalization, all patients were treated in intensive care units. PCI was performed in less than 24 h in 65% of cases.
Five patients received Mitraclips from the first generation (Generation 1 or NT), eight received third-generation clips, and ten received fourth generation Mitraclips.
Procedural and safety outcome
Procedural outcomes and follow-up data are summarized in Table 2. TEER procedure was performed on median 6 days after the index MI date IQR (3, 11). 65% of patients underwent TEER within a week of presentation. Total average procedure time was less than 2 hours, a median of 117 minutes IQR (65, 150).
| Patient no. | MI to TEER (days) | Procedure time (min.) | Implants no. | Acute procedural success | MR post-procedure | MV gradient (mmHg) | Total ICU stay (days) | In-hospital mortality | FU-30 days | MVR conversion |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 60 | 1 | Yes | 1 | 3 | N/A | Day 3, CV | — | No |
| 2 | 16 | 43 | 1 | Yes | 1 | 4 | N/A | No | Alive | No |
| 3 | 6 | 200 | 3 | Yes | 0 | 4 | N/A | No | Alive | No |
| 4 | 4 | 360 | 3 | Yes | 2 | 4 | N/A | No | Day 28 | No |
| 5 | 7 | 150 | 3 | Yes | 1 | 4 | N/A | No | Alive | No |
| 6 | 15 | 550 | 2 | No, LR | 3 | 6 | 16 | No | Alive | No |
| 7 | 22 | N/A | 1 | Yes | 1 | 3 | 37 | No | Alive | No |
| 8 | 2 | N/A | 1 | Yes | 1 | 5 | 10 | Day 7, sepsis | — | No |
| 9 | 6 | 60 | 2 | Yes | 1 | 6 | 18 | Day 18, N/A | — | No |
| 10 | 1 | 50 | 2 | Yes | 1 | 4 | 16 | No | Alive | No |
| 11 | 8 | 90 | 1 | Yes | 1 | 3 | 18 | Day 32, HF | — | No |
| 12 | 11 | 90 | 1 | Yes | 1 | 3 | 14 | No | Alive | No |
| 13 | 5 | 150 | 4 | Yes | 2 | 4 | 8 | Day 23, CV | — | No |
| 14 | 6 | 60 | 2 | Yes | 2 | 4 | 8 | Day 8, CV | — | No |
| 15 | 1 | 140 | 2 | Yes | 2 | 8 | 34 | No | Alive | Yes, 1 month |
| 16 | 2 | 180 | 2 | Yes | 2 | 7 | N/A | No | Alive | No |
| 17 | 1 | 117 | 1 | Yes | 2 | 2 | 6 | No | Alive | Yes, 9 months |
| 18 | 4 | 69 | 2 | Yes | 2 | 3 | 15 | No | Alive | Yes, 8 months |
| 19 | 11 | 73 | 2 | No, LR | 3 | 5 | 6 | No | Alive | Yes, 4 months |
| 20 | 35 | 150 | 2 | Yes | 1 | 6 | N/A | No | Alive | No |
| 21 | 15 | 133 | 2 | Yes | 1 | 5 | 7 | No | Alive | No |
| 22 | 8 | N/A | 1 | Failed | 4 | 2 | 5 | No | Alive | Yes, 2 months |
| 23 | 1 | N/A | N/A | Yes | 1 | 5 | 4 | Day 4, shock | — | No |
| Mean/rate | 6 IQR (3, 11) | 117 IQR (65, 150) | 14 out of 22 > 1 clip | 20/23 (87%) | See text | 5/23 (17%) > 5 mmHg | 10 IQR (7, 17) | 7/23 (30%) | 15/23 (65%) | 5/16 MVR (31%) |
- FU, follow-up; ICU, intensive care unit; LR, low reduction; MR, mitral regurgitation; MV, mitral valve; MVR, mitral valve replacement; N/A, not available.
- Values are given as mean ± standard deviation, n (%), or median [interquartile range].
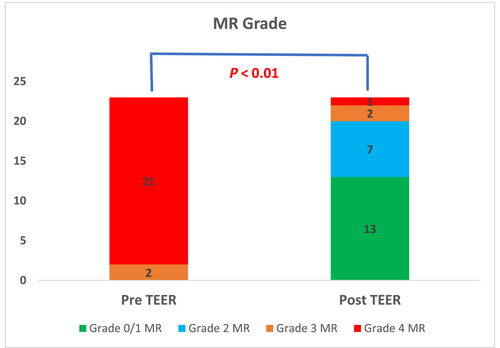
One to four clips were implanted in each case. Acute procedure success was achieved in 87% (20 of 23 patients). MR grade was significantly reduced after the procedure, P < 0.01. MR reduction to 0 or 1 + was achieved in 13 patients (57%), to 2 + in 7 patients (30%), and 3 or 4 in 3 patients (Figure 1). MR reduction was accompanied by improved haemodynamic features; the left atrial V-wave was reduced from 49 ± 8 mmHg to 26 ± 10 mmHg post-procedure, P < 0.01 (Figure 2). sPAP was reduced from 50 ± 12 mmHg to 40 ± 13 mmHg, P = 0.02, (Figure 3). Mitral valve stenosis, MV gradient > 5 mmHg, was observed in 5 cases (22%). No significant change in LVEF was observed.
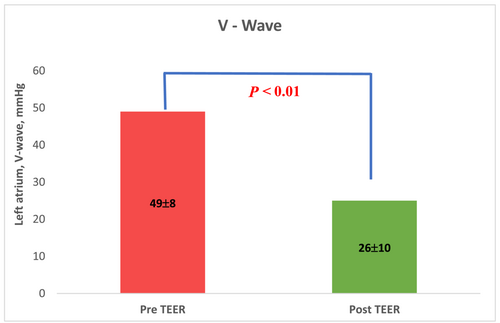
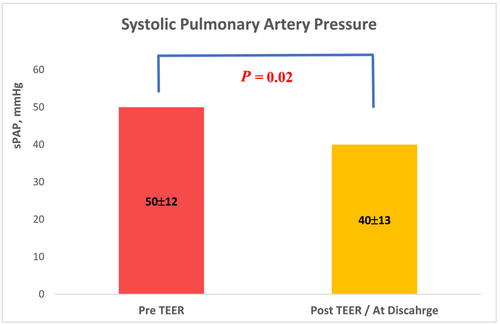
Severe adverse events were documented in two cases (9%). In one case, partial PMR escalated to a complete rupture during grasping attempt. Two clips were implanted, and MR grade was reduced to moderate to severe with some clinical improvement. The patient was discharged after 10 days and required MVR after 4 months. In another case, the patient suffered access site bleeding requiring blood products.
Sixteen out of 23 patients (70%) were discharged from hospital. The median ICU stay was 10 days IQR (7, 17). In-hospital deaths (7 of 23 patients) were majorly related to cardiovascular causes (heart failure and shock complications). Time from presentation to in-hospital mortality was a median of 8 days, IQR (6, 21). Clinical improvement was observed at discharge (Figure 4). Among survivors, 11 patients (69%) were in NYHA class 1 or 2, and 5 patients were in NYHA 3.
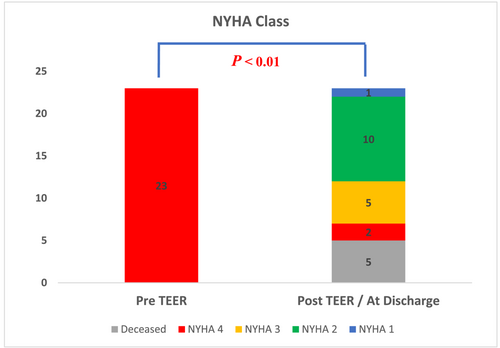
It is noteworthy that three patients who experienced unsuccessful TEER did not experience mortality or necessitate further intervention. Nevertheless, haemodynamic improvement during the procedure was observed.
Follow-up
Fifteen out of 23 patients (65%) were alive at 30 days post-procedure; 7 patients died in-hospital and one patient died out-of-hospital due to an unknown cause on day 28. Surgical mitral valve replacement (MVR) was required for 5 patients, and among these cases, 2 involved unsuccessful TEER procedures. Out of the three patients who underwent successful TEER and subsequently had surgery, one patient presented with moderate mitral stenosis, while the other two exhibited a worsening of mitral regurgitation.
The median time to MVR was 4 months IQR (2, 8). No additional death at 1 year was documented. Clinical and echocardiographic results at 30 days did not significantly change from immediate post-procedure or discharge. No single leaflet detachment was reported.
Discussion
In an international multicentre study, we collected the largest published series of 23 patients who underwent TEER procedure after acute MI complicated by primary MR caused by papillary muscle injury. The majority of the patients were men with multivessel coronary disease. Patients were more likely to have an inferior ST-elevation myocardial infarction with mildly reduced LV function. All patients developed acute heart failure; nearly 90% of them were in cardiogenic shock (CS) and required TEER for stabilization. The salvage TEER procedure significantly reduced the MR and left atrial V wave in most patients, allowing recovery. Still, in-hospital mortality was high with only two-thirds of the cohort who survived.
Although complete PMR is uncommon, occurring in 1–3% of MI patients, it is associated with severe clinical presentation, rapid deterioration, and poor prognosis. It usually occurs within 5 days of an MI and has a mortality rate of up to 80%, if not treated immediately. In order to stabilize patients, the medical management of acute MR includes intravenous diuretics, vasodilators, inotropic support, and potentially mechanical support.6
In decompensated patients with PMR, surgical mitral valve intervention is the preferred therapy, and it is associated with better outcomes when compared to medical therapy. In the APEX-AMI trial, 0.26% of the cohort (15 patients) had papillary muscle rupture, and those who underwent surgical repair outlived those who received conservative therapy (69% vs. 33%).15
However, in some patients, the surgical risk is extremely high. In fact, in the SHOCK Trial Registry (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock), only 38% of patients were offered mitral valve surgery. In a more recent analysis of AMI admissions from the National Inpatient Sample, 932 (0.029%) of the 3 244 799 admissions were complicated by PMR, and only 58% of them underwent mitral valve surgery. Patients who did not have surgery were older and had more comorbidities, and the overall mortality rate was 36%.16 There is a scarcity of data on surgery in acute MR caused by PMR. In a relatively large case series of 48 consecutive patients gathered over 22 years, 79% of patients underwent mitral valve replacement, with intraoperative mortality of 4.2% and in-hospital mortality of 25%. In-hospital mortality was predicted by EuroSCORE II, complete PMR and intraoperative IABP.17
Furthermore, the risk of surgical intervention in the acute setting is high, particularly among post-MI patients. Lorusso et al. evaluated the outcomes of 279 patients who underwent emergency surgery for acute MR due to AMI, degenerative mitral valve disease, or acute endocarditis and reported an overall 30-day mortality of 22.5%; ranging from 14.8% among patients with degenerative valve disease to 26.9% in post-AMI patients.18
The emerging role of TEER, particularly in high-risk patients early after MI requiring urgent intervention, has demonstrated feasibility and promising efficacy. TEER therapy is well tolerated and improves haemodynamic parameters. In a recent analysis, our group showed the In-hospital and 1-year mortality rates were significantly higher in the surgical mitral intervention compared with TEER (16% vs. 6%, P = 0.03, 31% vs. 17%, P = 0.04, respectively) among patients with post-MI functional MR and patients with PMR were excluded.
The role of TEER in primary post-MI MR is undetermined and mainly obtained from case series. Most case studies report patients in their 5th to 7th decade of life presenting with myocardial infarction and rapid deterioration with pulmonary oedema and cardiogenic shock within days of presentation.
Patients were treated successfully with 1 to 3 clips and good clinical recovery.19-28 Two case series were published, So et al. performed an institutional review and found 8 patients who underwent emergency TEER, 4 of which were related to post-MI acute MR. 75% had chordal rapture, 75% required MCS and the procedural was successful in 5 of 8 cases.27 Recent case series by Chang et al. included five patients with post-MI acute MR in CS, supported by MCS. Procedural success was achieved in all cases, and the procedure was performed within 3 days in all cases; however, only one patient survived to hospital discharge.28
Patients with primary MR are often present in extreme clinical and require urgent intervention. Our findings confirm that TEER is a safe and effective procedure in acute settings for a specific high-risk group and that the effectiveness of mitral intervention was maintained in most patients during long-term follow-up. Importantly, TEER does not rule out the possibility of later mitral valve surgery and thus could serve as a bridge for patient stabilization and definitive surgical replacement; indeed, seven patients from our group ultimately underwent surgical treatment. Our data suggest that most patients underwent the procedure within a week after presentation which is in line with surgical literature. This finding may be related to the availability of TEER as an urgent intervention, and it also might be due to a potential confounding factor implying of a potential positive selection in the patients treated with TEER.
The study's limitations include small sample size and the use of observational data, which could be influenced by unmeasured variables. In addition, the data derived from different centres participating in this multinational retrospective registry spread over a long period of time with different generation of TEER devices. The possibility exists that fourth-generation devices, which permit independent leaflet grasping, could potentially improve procedural success or quality. This aspect should be evaluated in further studies. It should be acknowledged that TEER in PMR is a challenging procedure and the technical feasibility of performing these cases requires rapid team organization, treating patients in extreme conditions, and overcoming anatomic challenges like small left atrium, and large flails.
In addition, left ventricular remodelling modifying the mitral valve geometry could lead to MR recurrence after TEER in the mid or long-term.
As a result, the study's findings should be interpreted with caution. The results of this population in this severe clinical setting should be further evaluated to confirm these findings.
Finally, this population is among the highest risk for surgical treatment. It is worth considering TEER in selected cases, as a bridge to elective or salvage mitral valve surgery if required.
Conclusions
In this multicentre registry, we have demonstrated that TEER is a feasible therapy in critically ill patients with acute severe primary mitral regurgitation due to a recent MI who are deemed inoperable. Most patients have shown haemodynamic and clinical improvement. TEER may have a role as salvage treatment or bridge to surgery in this population.
Acknowledgements
We would like to thank the contributors to the IREMMI: Ander Regueiro, Andrea Scotti, Andres Iniguez-Romo, Antonio Mangieri, Arthur Kerner, Azeem Latib, Carlos Vergara-Uzcategui, Claudia Scardino, Dabit Arzamendi, Estefanıa Fernández-Peregrina, Fabien Praz, Federico De Marco, Felipe Fernandez -Vazquez, Francesco Condello, Francesco Giannini, Gabriele Crimi, Jacob George, Julio Echarte-Morales, Haim Danenberg, Igal Moaraf, Isaac Pascual, Italo Porto, Konstantinos Spargias Lian Krivoshei, Marco Toselli, Marco Gennari, Marianna Adamo, Mattia Di Pasquale Neil Fam, Noe´ Corpataux, Tomas Benito-Gonzalez, Ronen Beeri, Vlasis Ninios Xavier Freixa.
Conflict of interest
None declared.
Disclosures
Dan Haberman is an Abbott CEC member. Rodrigo Estévez-Loureiro is consultant for Abbott. Andrew Czarnecki received speaker fee from Abbott and consultant for Caisson Interventional and Baylis Medical. Paolo Denti is a consultant for InnovHeart and receives speaker fee from Abbott and Edwards Lifescience Luis Nombela-Franco has served as a proctor of Abbott Vascular and received speakers' honoraria from Boston Scientific and Abbott Vascular. Giuseppe Tarantini consultant free from Abbot, Boston, Edwards, Medtronic and St. jude. Francesco Maisano has served as a consultant for Abbott Vascular, Edwards Lifesciences, Cardiovalve, Valtech, and Medtronic; and is cofounder of 4tech. Maurizio Taramasso is Consultant for Abbott, Boston Scientific, 4tech and receives personal fees from Edwards Lifescience, CoreMedic, Swissvortex and Mitraltech. Mony Shuvy is a proctor and consultant for Abbot and consultant for Edwards Lifesciences. All other authors have reported that they have no relationship relevant to the contents of this paper to disclose.




