Changes over time in patient-reported outcomes in patients with heart failure
Abstract
Aim
This paper describes the trajectory during 1 year of four patient-reported outcomes (PROs), namely, sleep, depressive symptoms, health-related quality of life (HrQoL), and well-being, in patients with heart failure (HF), their relationship and the patient characteristics associated with changes in these PROs.
Methods and results
Data analyses of PROs from 603 patients (mean age 67 years; 29% female, 60% NYHA II) enrolled in the HF-Wii study. On short term, between baseline and 3 months, 16% of the patients experienced continuing poor sleep, 11% had sustained depressive symptoms, 13% had consistent poor HrQoL, and 13% consistent poor well-being. Across the entire 1-year period only 21% of the patients had good PRO scores at all timepoints (baseline, 3, 6, and 12 months). All others had at least one low score in any of the PROs at some timepoint during the study. Over the 12 months, 17% had consistently poor sleep, 17% had sustained symptoms of depression, 15% consistently rated a poor HrQoL, and 13% poor well-being. Different patient characteristics per PRO were associated with a poor outcomes across the 12 months. Age, education, New York Heart Association, and length of disease were related to two PRO domains and submaximal exercise capacity (6 min test), co-morbidity, and poor physical activity to one.
Conclusion
In total, 79% of the patients with HF encountered problems related to sleep, depressive symptoms, HrQoL, and well-being at least once during a 1-year period. This underscores the need for continuous monitoring and follow-up of patients with HF and the need for dynamic adjustments in treatment and care regularly throughout the HF trajectory.
Introduction
Heart failure (HF) is a chronic condition with stable phases and episodes of acute deterioration. HF can significantly influence the patient's emotional, physical, spiritual, and social well-being, with fluctuations in symptoms and health-related quality of life (HrQoL) over time.1-6 Changes in symptoms and HrQoL are described to be predictive of clinical outcomes, and the persistence of symptoms or deterioration is a predictor of poor prognosis or rehospitalization.1-3 Changes in symptoms or deterioration of HrQoL can be expected in patients with chronic illness because the disease often slowly progresses. Changes in symptoms may also reflect a more acute deterioration of HF or the occurrence of co-morbidities. Therefore, it is critical to examine changes in the patient's health over time.
Patients' perspectives on their health state or behaviour are provided via patient-reported outcomes (PROs), defined as ‘any report of the state of a patient's health condition that is triggered by the patient, without any evaluation of the patient's response by a clinician or healthcare provider’.7 Recently, several associations of the European Society of Cardiology made a statement placing patient-reported outcomes at the centre of cardiovascular clinical practice.8 They recommended to extend the definition of a PRO with aspects of health behaviour and health care and redefined to ‘any report of the status of a patient's health condition, health behaviour, or experience with healthcare that comes directly from the patient, without interpretation of the patient's response by a clinician or anyone else’. To measure PROs, it is recommended by the International Consortium for Health Outcomes Measurement (ICHOM) to use a broad area of PROs, using a measurement set that includes multiple variables, such as sleep, depressive symptoms, HrQoL, and well-being.9
Separate PROs can be associated and changes in one outcome might have consequences for others over time. For example, a deterioration of depressive symptoms might have consequences for other symptoms, such as sleep problems or might cause a deterioration of well-being and HrQoL. Furthermore, interventions can have consequences for one outcome (e.g. change in depressive symptoms) but not for other outcomes (e.g. HrQoL and sleep). Most studies report cross-sectionally on patient-reported outcomes. We had data available of a stable group of patients with HF at 4 different timepoints on four PROs that were collected with validated questionnaires.
The objectives of this study were to describe the trajectory of the PROs of sleep, depression, HrQoL, well-being in patients with HF, their relationship and to describe patient characteristics associated with change over time of these PROs.
Methods
This study used PRO data from the HF-Wii study. The design and outcomes of the HF-Wii study have been published,10, 11 however the outcomes of the PROs are not reported previously. No significant differences were found in PROs between the intervention and control groups, therefore in the current study we analysed the patients in the HF-Wii study as one group. The HF-Wii study was an international multicenter, randomized controlled trial designed to evaluate the effect of access to an exergame in patients with stable chronic HF. Data was collected in Sweden, Italy, Germany, the Netherlands, Israel and the USA. Eligible participants (>18 years, no upper age limit) had been diagnosed with HF [New York Heart Association (NYHA) class I–IV] independent of ejection fraction. In addition, they spoke the language of the country participating in the study. Patients were excluded if they could not use the exergame platform due to visual impairment, hearing impairment, severe cognitive impairment, motor impairment, were unable to complete questionnaires or had a life expectancy <6 months. The HF-Wii main study randomized patients to exergame (intervention) or physical activity advice with motivational support (control). All patients in the study received physical activity advice from a HF team member and telephone follow-up calls. Patients in the intervention group also had access to an exergame, received an exergame training session, installation at home and tailored exergame advice for 3 months.
The study was conducted according to the principles of the Declaration of Helsinki (2008) in accordance with the Medical Research Involving Human Subjects Act. In Sweden, ethical approval was obtained centrally (DNR 2012/247-31), and additional approval was obtained from the local medical ethical committees (the Netherlands NL48647.068.14/METC141085; Italy 0052838/272/U.V.F/1 (2014); Israel 0022-13-RMC; Germany S22(a)/2015; USA UCI IRB HS# 2016-2955). The trial is registered on ClinicalTrials.gov (NCT01785121).11
Measurement
PROs included as secondary endpoints in the HF-Wii study were sleep, symptoms of depression, HrQoL and well-being. Data on PROs was collected at baseline, 3, 6, and 12 months after inclusion.
Sleep problems were assessed with the minimal insomnia sleep scale (MISS), a brief three-item instrument focusing on difficulties initiating sleep, maintaining sleep and non-restorative sleep. The MISS score of ≥ 6, as published by the scale constructors, was used as a cut-off for sleep problems.12
Depressive symptoms were measured with the hospital anxiety and depression scale (HADs), consisting of 14 items (response scale 0–3), divided into two subscales of seven items, each measuring anxiety and depression. The presence of depression was defined as a subscale score of ≥7.13
HrQoL was examined by the Minnesota Living with Heart Failure Questionnaire (MLHFQ), with higher scores indicating worse HrQoL (21 items, score range 0–105).14 Poor HrQoL was defined as the MLHFQ score >45 with reference to a previous study.15
Well-being was assessed with Cantril's Ladder of Life,16 which measured life satisfaction by first asking the patient to imagine life in the best possible light with a picture of a ladder numbered from 0–10 and then asking the patient to score where they stand at present. Poor well-being was defined as a score of ≤ 5.
In addition, the following demographic and clinical variables for patients were collected from questionnaires and medical records to describe the sample: age, gender, marital status, education, NYHA classification, aetiology and duration of HF, LV function, submaximal exercise capacity assessed by a six-minute walk test, heart rate, blood pressure, serum haemoglobin, serum creatinine, body mass index (BMI), co-morbidities and medications. Cognitive impairment was assessed with the Montreal Cognitive Assessment (MoCA), a brief screening instrument to detect mild cognitive impairment. A score of 26–30 was considered normal; 18–25 was considered a mild cognitive impairment; 10–17 was a moderate cognitive impairment, and <10 was considered a severe cognitive impairment.17 Self-reported physical activity was measured by a single-item question: ‘Over the past week (even if it's not a typical week), how much time did you exercise or were you physically active (e.g. strength training, walking, swimming, gardening, or other types of training)?’ Answer possibilities were none, <30 min a week, 30–60 min a week, 1 to 3 h a week, or more than 3 h/week).18
Analysis
- To describe the trajectory of a PRO between baseline and 3 months, a threshold score of each PRO (described under ‘measurement’) was used to determine if patients had a good or poor score on the PRO. According to the threshold score of the PRO at baseline and after 3-month follow-up, patients were classified into four trajectory groups: Consistently good, deterioration, improvement, and consistently poor. For example, if the MISS score was <6 at baseline and at 3 months, the patient was classified as consistently good (Table 1).
- To describe the trajectory of the PROs at each of the four timepoints (baseline, 3, 6, and 12 months), one point was assigned to a good outcome per PRO (i.e. no depression, good QoL, good well-being, and no sleep problems) as described in step 1. For each PRO, the score was from 0 (Poor PRO at all four-timepoints) to 4 (good PRO at all four timepoints).
- Multivariate logistic regression analyses were performed to identify risk factors associated with a lower score on the PROs. First, we did univariate analysis adding variables that are theoretically related to the PRO. Consequently, entered were: age, gender, marital status, presence of grandchildren, education, NYHA class, HF aetiology, duration of HF, LVEF function, 6MWT, atrial fibrillation, serum haemoglobin, serum creatinine, body mass index, co-morbidity [stroke, diabetes, chronic obstructive pulmonary disease (COPD), and cancer], smoking, cognitive function, self-reported physical activity. Those variables that were related to the outcomes in univariate analysis (P < 0.15)19 were entered in the multivariate analysis.
| Trajectory | Patient-reported outcomes | |
|---|---|---|
| At baseline | At 3 months | |
| Consistently good | Good (1) | Good (1) |
| Deterioration | Good (1) | Poor (0) |
| Improvement | Poor (0) | Good (1) |
| Consistently poor | Poor (0) | Poor (0) |
- Patients were classified into four trajectory groups: consistently good, deterioration, improvement, and consistently poor.
- HrQoL, health-related quality of life.
All statistical tests were two-tailed, and statistical significance was defined as P < 0.05. All analyses were performed with SPSS version 25 and SAS version 9.4 for Windows (SAS Institute Inc., Cary, North Carolina, USA).
Results
There were 605 patients in the HF-Wii study. Two patients were excluded from the analysis because these patients did not answer any PROs at any timepoint. This analysis used data from the remaining 603 participants in the HF-Wii trial.
Characteristics of study participants
The mean age of the 603 participants was 67 years; 29% were female and mainly classified as NYHA II (60%). Most patients were married or living with a partner (71%). Co-morbidities such as diabetes (26%) and COPD (18%) were common, and 10% of the participants had a history of stroke (Table 2).
| Estimate (95% confidence limits) | |
|---|---|
| Age, years, mean | 67 (66.0–67.8) |
| Gender, female, n (%) | 175 (29%) |
| Married/living with a partner, n (%) | 428 (71%) |
| Education, n (%) | |
| Low (only primary school) | 151 (25%) |
| Medium (high school) | 271 (45%) |
| High (university/college) | 181 (30%) |
| NYHA functional classification | 2.2 (2.2–2.3) |
| I, n (%) | 60 (10%) |
| II, n (%) | 362 (60%) |
| III/IV, n (%) | 181 (30%) |
| Ischaemic aetiology of HF, n (%) | 253 (42%) |
| Duration of HF ≥ 2 years | 337 (56%) |
| LV function, n (%) | |
| Normal | 103 (17%) |
| Mild dysfunction | 187 (31%) |
| Moderate to severe dysfunction | 314 (52%) |
| Heart rate, b.p.m., mean | 71.0 (64.1–77.9) |
| Systolic BP, mmHg, mean | 123.2 (121.8–124.6) |
| Diastolic BP, mmHg, mean | 72.5 (71.5–73.4) |
| A 6-min walk test distance, mean | 403.0 (391.7–414.3) |
| Atrial fibrillation, n (%) | 133 (22%) |
| Serum haemoglobin, g/dL, mean ± SD | 13.4 (13.3–13.6) |
| Serum creatinine, μmol/L, mean ± SD | 104.0 (100.7–107.) |
| Body mass index, kg/m2, mean ± SD | 28.2 (27.8–28.6) |
| Co-morbidity, n (%) | |
| Stroke | 60 (10%) |
| Diabetes | 157 (26%) |
| COPD | 109 (18%) |
| Cancer | 72 (12%) |
| Medical therapy, n (%) | |
| ACEI/ARB | 506 (84%) |
| Beta-blocker | 522 (87%) |
| MRA | 290 (49%) |
| CRT | 68 (11%) |
| ICD | 141 (24%) |
| Current smoker, n (%) | 50 (8.3%) |
| Cognitive impairment | |
| MoCA score | 24.3 (23.8–24.8) |
| No cognitive problems 26–30 | 265 (44%) |
| Mild cognitive problems 18–25 | 308 (51%) |
| Moderate cognitive problems 10–17 | 28 (4.7%) |
| Severe cognitive problems <10 | 2 (0.3%) |
| Poor physical activity | 211 (35%) |
| HADS depression | 5.2 (4.9–5.5) |
| Sleep | 4.4 (4.1–4.6) |
| Total MLHFQ | 34.7 (33.0–36.5) |
| Well-being | 6.2 (6.1–6.4) |
- Poor physical activity is defined as physical activity of <60 min a week.
- ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HADS, Hospital Anxiety and Depression Scale; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, Left ventricular ejection fraction; MLHFQ, the Minnesota Living with Heart Failure Questionnaire; MoCA, Montreal Cognitive Assessment; MRA, mineralocorticoid receptor antagonists; NYHA functional classification, New York Heart Association functional classification.
Trajectories of sleep, depression, health-related quality of life, and well-being
Baseline to 3 months
When looking at the trajectories, 58% of the patients had consistently good sleep (at baseline and 3 months (Figure 1), 71% of the patients no depressive symptoms, 63% had consistently good HrQoL, and 53% reported consistently good well-being. Meanwhile, 16% had consistently poor sleep, 11% of patients had sustained symptoms of depression, 13% consistently rated a poor HrQoL, and poor well-being respectively. Between 5% (HrQoL) and 13% (well-being) of the patients deteriorated in outcome during the first 3 months. Meanwhile, between 10% (depression) and 20% (well-being) of the patients improved in outcome during the first 3 months.
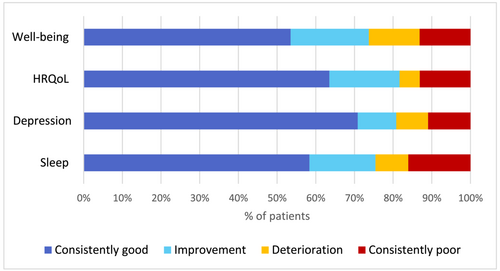
Trajectory of patient-reported outcomes over 12 months
As shown in Table 3, in all the measured PROs during the 12-month follow-up a considerable number of patients scored above the cut off defined as good. Good sleep was reported between 66% and 76% at the four timepoints, 74%–80% did not have symptoms of depression, 68%–82% had good HrQoL at all timepoints and 66–79% had good well-being during the 12-month follow-up.
| Baseline | 3 months | 6 months | 12 months | |
|---|---|---|---|---|
| Good sleep | 398 (66%) | 452 (75%) | 458 (76%) | 440 (73%) |
| No depression | 458 (76%) | 482 (80%) | 446 (74%) | 476 (79%) |
| Good HrQoL | 410 (68%) | 488 (81%) | 494 (82%) | 488 (81%) |
| Good well-being | 398 (66%) | 446 (74%) | 476 (79%) | 458 (76%) |
- Sleep problem is defined as a score of the minimal insomnia sleep scale (MISS) of ≥6. Depression is defined as a subscale score of the HADS scale of ≥7. Poor HrQoL is defined as the MLHFQ score >45. Poor well-being is defined as a score of the Cantrils ladder of life of ≤5.
To examine the results over time for all four timepoints, we allocated one point for each good score for each timepoint. Therefore, the theoretical range for the mean scores of each of the individual PROs was from 0 (representing poor at all four timepoints) to 4 (reflecting good at all four timepoints). As a result, we found a score of 3.1 for depression, 3.1 for HrQoL, 3.0 for well-being, and 2.9 for sleep. Over the 12 months, 17% had consistently poor sleep, 17% had sustained symptoms of depression, 15% consistently rated a poor HrQoL, and 13% poor well-being (a score of ≤1 (Table 4).
| Scores | Estimate | 95% CI | 0 | 0 < score ≤ 1 | 1 < score ≤ 2 | 2 < score ≤ 3 | 3 < score ≤ 4 |
|---|---|---|---|---|---|---|---|
| Sleep | 2.9 | 2.8–3.0 | 55 (9.1%) | 48 (8.0%) | 84 (14%) | 133 (22%) | 283 (47%) |
| Depressive symptoms | 3.1 | 2.9–3.2 | 78 (13%) | 24 (3.9%) | 54 (9.0%) | 72 (12%) | 380 (63%) |
| HrQoL | 3.1 | 3.0–3.2 | 49 (8.1%) | 43 (7.1%) | 36 (5.9%) | 127 (21%) | 350 (58%) |
| Well-being | 3.0 | 2.9–3.1 | 30 (4.9%) | 51 (8.5%) | 96 (16%) | 163 (27%) | 259 (43%) |
- CI, confidence interval; HrQoL, health-related quality of life.
Adding all four PROs at four different timepoints (theoretical range of 0–16) showed that 21% (N = 126) patients had good scores in all of the four PROs at all timepoints (score 16) (Figure 2).
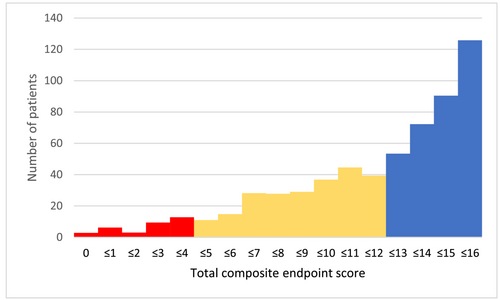
Relationship between the patient-reported outcomes
Table S1 shows a strong correlation between the HADS depression score and the HrQoL score measured by MLHFQ (r = 0.58). In addition, a medium correlation was observed between depression and well-being (Ladder of Life) (r = −0.43); depression and sleep (r = 0.44); HrQoL and sleep (r = 0.50); and well-being and sleep (r = −0.28).
Patient characteristics associated with poor patient-reported outcomes
Multivariate logistic regression analysis showed that depressive symptoms independently associated younger age, odds ratio [OR = 0.96, 95% confidence interval (CI) = 0.94–0.98], HF duration of 2 years or more (OR = 1.94, 95% CI = 1.21–3.13), and shorter distance in meters of the 6-min walk test (OR = 0.995, 95% CI = 0.994–0.997) (Table 5).
| Poor sleep | Depressive symptoms | Poor HrQoL | Poor well-being | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age | 0.96 | 0.94–0.98 | 0.96 | 0.94–0.98 | ||||
| Low education (high school or less) | 1.78 | 1.06–3.00 | 2.53 | 1.30–4.93 | ||||
| HF duration ≥2 years | 1.94 | 1.21–3.13 | 1.78 | 1.07–2.96 | ||||
| NYHA III/IV | 3.64 | 2.20–6.05 | 3.04 | 1.83–5.03 | ||||
| A history of stroke | 2.09 | 1.02–4.27 | ||||||
| A history of COPD | 1.87 | 1.05–3.36 | ||||||
| Poor physical activity | 1.72 | 1.04–2.87 | ||||||
| Six-minute walk test | 0.995 | 0.994–0.997 | ||||||
- Dependent variable for the logistic regression analysis was each PRO score of ≤1.
- CI, confidence interval; OR odds ratio.
Poor sleep over time was associated with low education (OR = 1.78, 95% CI = 1.06–3.00). Furthermore, poor HrQOL was related to younger age (OR = 0.96, 95% CI = 0.94–0.98), HF duration of 2 years or more (OR = 1.78, 95% CI = 1.07–2.96), NYHA III/IV (OR = 3.64, 95% CI = 2.20–6.05), a history of stroke (OR = 2.09, 95% CI = 1.02–4.27), and a history of COPD (OR = 1.87, 95% CI = 1.05–3.36). With regard to well-being, low education (OR = 2.53, 95% CI = 1.30–4.93), NYHA III/IV (OR = 3.04, 95% CI = 1.83–5.03), and lower levels of physical activity (OR = 1.72, 95% CI = 1.04–2.87) were related to poor well-being in the long-term.
Discussion
This paper describes the changes over time for four PROs recognized as important in HF care.8, 9 We found that patients who participated in the study had high PRO scores and four key observations were found.
First, we found that although most patients had a constantly good or improved score, some outcomes deteriorated during the first 3 months of follow-up or even remained consistently poor. Although at all timepoints good PRO scores were reported by most patients, looking closely at good scores per patient, only 21% of the patients had good outcomes at all four timepoints of the 1-year follow-up. 79% of patients had at least one ‘poor’ score for one of the PROs during the year of follow-up, indicating that they deteriorated or developed new symptoms. This is reflecting the instability of PROs per patient and indicated the constant need to be alert for deterioration. This supports the need for continuous and long-term follow-up of patients with HF and the need to adapt treatment and care regularly during the HF trajectory.20 The integration of using PROs into the routine of HF management could improve the monitoring of disease progression and HrQoL, not only during changes in treatment but also in the ‘at first sight’ stable phases of follow up.9, 21
Second, our results can help identify which patients need support to improve their outcomes. Persistent depressive symptoms or new onset of depressive symptoms or sleep problems are described to be predictive of rehospitalization5, 22 and have increased risk of cardiovascular death.22 In our study patients who were younger, those with longer HF duration and low submaximal exercise capacity had more often persistent depressive symptoms and these patients might need additional treatment or support. Our study also showed that different baseline characteristics correlated with a long-term poor outcome on the PROs during 12 months. We found that co-morbidity (stroke and COPD), NYHA, submaximal exercise capacity (6MWT), and duration of HF were related to different PROs. We did not find gender differences or differences related to ejection fractions as recently found by Seckin and colleagues.23
Thirdly, we showed that although there was a strong correlation between the PROs (the highest correlation between the HADS depression score and the HrQoL score measured by MLHFQ was 0.58), these did not overlap totally, confirming that these instruments have commonalities but are measuring different concepts and that a comprehensive approach to measuring the outcomes of patients with HF is needed. The international consortium for health outcomes measurement (ICHOM) advised to use a set of PROs such as the KCCQ-12, the Patient-Reported Outcomes Measurement Information System (PROMIS) Physical Function Short Form 4a and the Patient Health Questionnaire-2 (PHQ2).9 However, depending on the case-mix of a clinic, the goal of a study or the data already collected in a registry, researchers and clinicians do not always follow to these recommendations.
Finally, we found that there is still a long way to go to improve PROs interpreting scores and the relationship between the different PROs. Defining a PRO as ‘good’ is challenging, since there are now valid cut offs for every PRO. The minimal clinically meaningful difference is considered a clinically useful metric, but this is hardly ever established in most questionnaires. Additional studies are needed to establish the minimal clinically meaningful differences in different types of cardiac PROs and to improve the clinical interpretability and validity of these PRO measurements.23
Strengths and limitations
A strength of this study is that we could use data on several relevant PROs that had been collected in an international study at four different timepoints. However, since these data were used from a trial focussing on physical activity, not all relevant PROs might have been collected with instruments advised by the ICHOM.8 We also had data from a quite stable population with a considerable number of patients having no sleep problems, depressive symptoms, low HrQoL, and poor well-being. In total 64% of patients in this sample had mild to moderate cognitive impairment at baseline. As shown in other studies,24 this is reflective of a patient sample that is seen in daily practice in HF clinics.
Conclusion
In total, 79% of the patients with HF had problems related to sleep, depression, HrQoL and well-being during one timepoint during a year of follow up. This supports the need for continuous and long-term follow-up of patients with HF and the need to adapt treatment and care regularly during the HF trajectory.
Acknowledgements
Norrköping: L Nestor, C Norrman, M Viklander, A Waldemar, RM Petterson; M. Wärfman, Jönköping: E Lundberg, H Sköldbäck, M Sahlin. Linköping: A Gylling, M Huss, M Jonsson, P Wodlin, L Hjelmfors. Stockholm: U Lennmark. Nyköping: E Säfström. Italy: R Corsi, G Alberto Ortali. The Netherlands: HP Brunner-La Rocca, M Spanjers, A van de Voorde, G Cleuren. Israel: S Donanhirsh, Y Navon, V Yaari. Germany: A Walther. USA: J Ardo, J Nguyen, M Cacciata.
Conflict of interest
None declared.
Funding
This work is supported by the Swedish National Science Council (K2013-69X-22302-01-3 and 2016-01390); Swedish National Science Council/Swedish Research Council for Health, Working Life and Welfare, VR-FORTE (2014-4100); the Swedish Heart and Lung Association (E085/12); the Swedish Heart and Lung Foundation (20130340 and 20160439); the Vårdal Foundation (2014-0018); and the Medical Research Council of Southeast Sweden (FORSS 474681).




