Interferon-regulatory factor-1 boosts bevacizumab cardiotoxicity by the vascular endothelial growth factor A/14-3-3γ axis
Xuan-Ying Chen and Meng-Qi Xie are-co-first authors.
Abstract
Aim
Myocardial injury is a significant cause of death. This study investigated the role and underlying mechanism of interferon-regulatory factor-1 (IRF1) in bevacizumab (BVZ)-induced cardiomyocyte injury.
Methods and results
HL-1 cells and C57BL/6 mice receiving BVZ treatment were used to establish in vitro and in vivo models of myocardial injury. The relationship between VEGFA and 14-3-3γ was verified through co-immunoprecipitation and Glutathione S Transferase (GST) pull-down assay. Cell viability and apoptosis were analysed by MTT, propidium iodide (PI) staining and flow cytometry. The release of lactate dehydrogenase (LDH), cardiac troponins T (cTnT), and creatine kinase MB (CK-MB) was measured using the enzyme linked immunosorbent assay. The effects of knocking down IRF1 on BVZ-induced mice were analysed in vivo. IRF1 levels were increased in BVZ-treated HL-1 cells. BVZ treatment induced apoptosis, inhibited cell viability, and promoted the release of LDH, cTnT, and CK-MB. IRF1 silencing suppressed BVZ-induced myocardial injury, whereas IRF1 overexpression had the opposite effect. IRF1 regulated VEGFA expression by binding to its promoter, with the depletion of VEGFA or 14-3-3γ reversing the effects of IRF1 knockdown on the cell viability and apoptosis of BVZ-treated HL-1 cells. 14-3-3γ overexpression promoted cell proliferation, inhibited apoptosis, and reduced the release of LDH, cTnT, and CK-MB, thereby alleviating BVZ-induced HL-1 cell damage. In vivo, IRF1 silencing alleviated BVZ-induced cardiomyocyte injury by regulating the VEGFA/14-3-3γ axis.
Conclusion
The IRF1-mediated VEGFA/14-3-3γ signalling pathway promotes BVZ-induced myocardial injury. Our study provides evidence for potentially new target genes for the treatment of myocardial injury.
Introduction
Over the recent decades, several angiogenesis inhibitors have been applied in cancer therapy. Bevacizumab (BVZ) is a humanized monoclonal antibody to vascular endothelial growth factor A (VEGFA).1 BVZ has been widely used in many malignancies, such as breast and colorectal cancers.2, 3 However, studies have increasingly highlighted its clinical side effects, which mainly manifest as cardiovascular toxicity that consequently induces several adverse effects, such as hypertension, reduced left ventricular ejection fraction, and heart failure.4 BVZ has been found to significantly reduce cardiomyocyte viability and increase cell apoptosis in a dose-dependent manner, as well as promote mitochondrial dysfunction in cardiomyocytes.5 In addition, high serum troponin I (TP I) and creatine kinase MB (CK-MB) levels were found in BVZ-treated rats.6 Moreover, studies have shown that human cardiomyocytes treated with BVZ exhibited increased cytosolic calcium, apoptosis, and reactive oxygen species (ROS) production.7 However, the intracellular molecular mechanisms underlying BVZ-associated cardiotoxicity have yet to be fully understood. A previous study reported that VEGFA was involved in BVZ-induced cardiomyocyte toxicity.8 As a vascular bioregulatory substance, VEGFA has been reported to promote angiogenesis, increase blood flow, and play an important role in protecting cardiac function.9 Nevertheless, the specific regulatory mechanism by which VEGFA regulates BVZ-induced cardiomyocyte injury needs further exploration.
Interferon-regulatory factor-1 (IRF1), which belongs to the interferon-regulatory factor family, participates in a wide range of physiological and pathological processes.10 Recent studies have identified IRF1 as a transcription factor regulating the occurrence and development of cardiovascular diseases.11 In addition, IRF1 is an important endogenous mediator of ischaemic cardiomyopathy, with evidence suggesting that IRF1 overexpression could promote myocardial ischaemia–reperfusion injury.12, 13 Furthermore, IRF1 has been found to inhibit proliferation and exert a pro-apoptotic effect in hepatocellular carcinoma cells.14 A previous study found that IRF1 overexpression decreased VEGFA protein levels and suppressed osteosarcoma progression.15 However, further investigations are needed to confirm whether IRF1 aggravates BVZ-induced myocardial injury by regulating VEGFA expression.
14-3-3 proteins are a family of highly conserved proteins16 whose members have been reported to be involved in various biological processes, such as cell proliferation, differentiation, and apoptosis.17 14-3-3γ, a member of the 14-3-3 family, is involved in cardioprotection. For instance, one study showed that 14-3-3γ up-regulation was associated with the cardioprotective effects of curcumin against doxorubicin-induced myocardial injury.18 However, the role of 14-3-3γ in BVZ-induced cardiomyocyte injury remains unclear.
The present study elucidated the role of IRF1 in BVZ-induced myocardial injury. We demonstrated that IRF1 promoted BVZ-induced myocardial injury by mediating the VEGFA/14-3-3γ axis, which may help uncover novel targets for the future treatment of BVZ-induced myocardial injury.
Methods
Cell culture and treatment
HL-1 cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured at 37°C and 5% CO2 in DMEM/F12 (Sigma, St. Louis, MO, USA) supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA). Cells were incubated with different concentrations of BVZ (14.9, 149, and 745 μg/mL) for 24 h.5 BVZ (Avastin) was purchased from Selleck Chemicals, and the BVZ stock solution (25 mg/mL) was made using dimethyl sulfoxide (DMSO). DMSO (0.01%) was used for the control group. After treatment, cells were collected for subsequent analysis.
Cell transfection
Lentivirus plasmids including IRF1 overexpression plasmid (oe-IRF1), short hairpin RNA against IRF1 (sh-IRF1), and their corresponding negative controls were obtained from GenePharma (Shanghai, China). The lentiviral vector pLL-3.7 was co-transfected with packaging plasmids to produce lentivirus particles, which were filtered through 0.45-μm filters and immediately used for infection. HL-1 cells were infected with lentivirus at a multiplicity of infection of 50 for 24 h. Cells were selected with a medium containing 2 μg/mL of puromycin (Merck Millipore, Billerica, MA, USA) for 2 weeks to obtain stable expression. Full-length 14-3-3γ sequences were amplified through polymerase chain reaction and then inserted into the pcDNA3.1 vector (Life Technologies, USA) to generate the 14-3-3γ overexpression vector. 14-3-3γ short hairpin RNA (shRNA) (sh-14-3-3γ), VEGFA shRNA (sh-VEGFA), and negative control (sh-NC) plasmids were obtained from GenePharma. According to the manufacturer's instruction, 1 μg of plasmid and 2 μL of Lipofectamine 2000 (Invitrogen) were added to HL-1 cells at 70% confluence and incubated for 48 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HL-1 cells were seeded into 96-well plates at a density of 3 × 103 cells per well and cultured at 37°C in 5% CO2 for 12 h. At different time points, 5 mg/mL of MTT in phosphate-buffered serum (PBS) (Sigma) was added to the wells. The cells were then incubated at 37°C for 4 h. Finally, absorbance was determined at 570 nm (Molecular Devices, Sunnyvale, CA, USA).
Detection of lactate dehydrogenase release
Lactate dehydrogenase (LDH) activity in serum or cell supernatant was determined according to the instruction of the commercial assay kit (Jiancheng Bioengineering, Nanjing, China). Briefly, HL-1 cells (1 × 105 per well) were seeded into 24-well plates and cultured overnight. After treatment, 100 μL of culture supernatant was used for LDH activity measurement. Mouse blood samples were centrifuged at 3500 r/min for 5 min at 4°C to separate the serum. The supernatant was then collected for enzymatic reaction with substrates in 96-well plates. Finally, LDH activity was detected using a microplate reader (Bio-Rad Laboratories Inc, Hercules, CA, USA) at 450 nm.
Propidium iodide staining
Cells were cultured in 6-well plates (1 × 106 cells per well) for 24 h and fixed with formaldehyde. Thereafter, 10 μL of propidium iodide (PI; Sigma) was added into each well and incubated for 5 min. DAPI was used to dye nuclei. Images were acquired using a fluorescence microscope (Leica, Wetzlar, Germany) and analysed using ImageJ (NIH, USA).
Flow cytometry
Apoptosis was assessed using an Annexin V-FITC/PI Apoptosis Detection Kit (FACSCalibur, Becton Dickinson). Cells were harvested and fixed with pre-cold 70% ethanol. Cells were resuspended in binding buffer at 1 × 106 cells/300 μL, after which 10 μL of Annexin V-FITC and 5 μL of PI were added for 30 min in the dark. Apoptotic cells were detected via flow cytometry (Becton Dickinson, Franklin Lakes, NJ) and analysed using FlowJo software (version 7.6.5).
Western blotting
The total protein of cells and tissues was extracted using RIPA buffer. Protein concentrations were measured using the BCA assay (Beyotime). Equal amounts of protein were separated using 10% SDS-PAGE gel and transferred onto PVDF membranes (Millipore, Boston, MA, USA). The membranes were blocked using 5% skim milk for 1 h, followed by incubation with anti-IRF1 (ab230652, 1:1000, Abcam, Cambridge, UK), anti-VEGFA (ab46154, 1:1000, Abcam), and anti-14-3-3γ (#5522, 1:1000, Cell Signalling Technology, Danvers, USA) at 4°C overnight. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (ab205718, 1:10 000, Abcam) for 1 h at room temperature and developed using chemiluminescent HRP substrate (Abcam). Bands were analysed using ImageJ software (NIH).
Enzyme linked immunosorbent assay
cTnT and CK-MB levels were determined using the enzyme linked immunosorbent assay (ELISA) assay. Briefly, HL-1 cells or serum samples from C57BL/6 mice were centrifuged at 1300× g for 20 min at 4°C. Supernatants were detected using an ELISA kit (Invitrogen) following the manufacturer's instructions. The concentrations of cTnT and CK-MB were determined using a spectrofluorometer at 450 nm.
Dual luciferase reporter assay
The VEGFA promoter region sequences or mutant sequences were cloned into the pGL3-basic vector (Promega, WI, USA). Cells were seeded into 24-well plates, after which the reporter plasmids were co-transfected with sh-IRF1 or sh-NC using Lipofectamine 3000 reagent (Invitrogen) following the manufacturer's protocol. Luciferase activity was measured 48 h after transfection using the Dual luciferase reporter gene system (Promega).
Co-immunoprecipitation
Cells were lysed in RIPA buffer and centrifuged to remove cell debris. Supernatants were incubated with anti-VEGFA (#LS-C746723, 1:100, LSBio, Seattle, WA, USA), anti-14-3-3γ (#5522, 1:100, Cell Signalling Technology), or IgG (#2729, 1:100, Cell Signalling Technology) antibodies overnight at 4°C. Samples were then precipitated with Protein A/G agarose immunoprecipitation reagent (Santa Cruz) for 3 h. Equal amounts of protein were subjected to SDS-PAGE and analysed using western blotting with the primary antibody of VEGFA or 14-3-3γ.
Glutathione S Transferase pull-down assay
GST–VEGFA fusion proteins were produced in BL21 Escherichia coli and purified using glutathione agarose beads (Sigma). Full-length 14-3-3γ cDNA was inserted into pcDNA™6/myc-His vector to overexpress 14-3-3γ (His-14-3-3γ) (GenePharma). Cells expressing his-14-3-3γ were lysed with NP-40 buffer. The his-14-3-3γ protein was then purified using Ni-NTA agarose beads (Qiagen, Dusseldorf, Germany) and incubated with GST-VEGFA in pull-down buffer for 1.5 h at 4°C on a rotating wheel. After washing with pull-down buffer, relevant proteins were separated through SDS-PAGE and immunoblotted with anti-His tag antibody (#SAB4301134, 1:1000, Sigma).
Animal preparation
C57BL/6 mice (6 weeks old) were provided by ALF Biotechnology (Suzhou, China) and housed in a specific pathogen-free lab at a temperature of 22°C–23°C, humidity of 55%–60%, and a 12-h light/dark cycle with free access to food and water. The mice were randomly divided into four groups (n = 8/group): Control group, BVZ group, BVZ + sh-NC group, and BVZ + sh-IRF1 group. Thereafter, 100 μL of lentivirus carrying sh-NC or sh-IRF1 was injected into the left ventricular wall of mice. After 4 weeks, mice were treated with 10 mg/kg of BVZ via intraperitoneal injection twice a week for 2 weeks.19 Mice in the control group were injected with normal saline. Next, mice were anaesthetised with alfaxalone (50 mg/kg, intraperitoneally) and xylazine (5 mg/kg, intraperitoneally), placed in the supine position, monitored through echocardiography, and maintained at 37°C. Each group was evaluated using echocardiographic measurements (ATL-HDI5000, 15 MHz linear, and 12-MHz sectorial scanheads). The left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD) and heart rate were recorded. The index of left ventricular ejection fractions (LVEF), and left ventricular fraction shortening (LVFS) were calculated. Finally, mice were sacrificed by anaesthesia. Myocardial tissues were then obtained from the tip of the heart and cut into small pieces. Serum samples were also collected. Myocardial tissue samples were fixed in 4% paraformaldehyde and used for subsequent haematoxylin and eosin (H&E) staining. All experimental protocols were approved by Animal Ethics Committee of the First Affiliated Hospital, Nanchang University.
Hematoxylin and Eosin staining
Myocardial tissues from mice were fixed in 4% paraformaldehyde. Thereafter, tissues were dehydrated through gradient alcohols and embedded in paraffin. Consecutive sections (5 μm) were stained with haematoxylin for 5 min and eosin solution for 3 min. The mounted slides were observed under an optical microscope (Leica, Wetzlar, Germany).
TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining
Myocardial tissue sections (5 μm) were subjected to TUNEL staining using the TUNEL detection kit (Roche, Basel, Switzerland) according to the manufacturer's instruction. The nuclei were stained with DAPI. Subsequently, sections were examined under an olympus inverted microscope.
Immunohistochemistry
Paraformaldehyde-fixed mouse myocardial tissues were embedded in paraffin and cut into 5-μm-thick sections. Tissue slides were deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed in EDTA by microwave heating. After blocking with 1.5% normal goat serum, samples were incubated with IRF1 antibody (Cat. SR44-08, 1:200, Invitrogen) overnight at 4°C. After washing with PBS, sections were incubated with appropriate secondary antibody coupled with horseradish peroxidase for 1 h. Standard diaminobenzidine (DAB) staining (R&D Systems, Minneapolis, MN, USA) was conducted for chromogenic detection. The sections were observed under a microscope (Leica), and the average density of the images was analysed using ImageJ (NIH).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (Graph Pad, San Diego, USA). All data were expressed as mean ± standard deviation (SD). Each experiment was conducted in triplicate independent biological replicates. Student's t-test was used for comparison between two groups, whereas one-way ANOVA was used for comparisons among multiple groups. A P value < 0.05 indicated statistical significance.
Results
Bevacizumab promoted cardiomyocyte injury in HL-1 cells
We first established a cardiomyocyte injury model. HL-1 cells were cultured for 24 h and treated with BVZ. First, we determined the IC50 using a concentration range of BVZ equal ratio or greater, and the results showed that the IC50 treated by BVZ was 721.4 or 770.7 μg/mL (Figure 1). Subsequently, the concentration of BVZ was reduced around the IC50 range, and analysing the effects of different BVZ concentrations on HL-1 viability (14.9, 149 and 745 μg/mL). Our results showed that BVZ at 745 μg/mL inhibited cell viability by approximately 50%, as shown in Figure 1. Therefore, this BVZ concentration was used for subsequent cell treatment. Compared with the control group, HL-1 cells treated with BVZ showed stimulated LDH release (Figure 1). Next, PI staining showed that BVZ-treated HL-1 cells had greater red fluorescence than did the control cells (Figure 1). Moreover, flow cytometry analysis revealed that BVZ treatment promoted an obvious increase in apoptotic cells compared with the control condition (Figure 1). Moreover, compared with the control group, the BVZ group showed up-regulated levels of cleaved-casp3 and Bax but reduced Bcl-2 expression (Figure 1). Additionally, BVZ treatment increased the secretion of cTnT and CK-MB compared with control (Figure 1). Collectively, these results suggested that BVZ-induced cardiomyocyte injury.
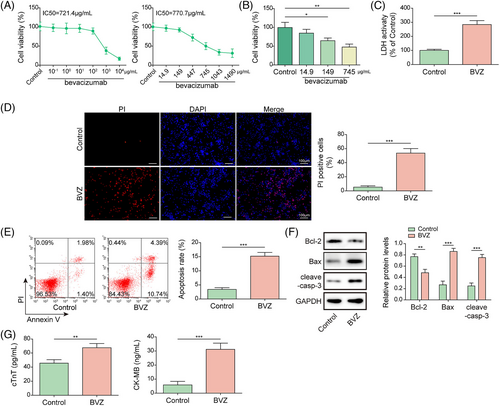
Interferon-regulatory factor-1 knockdown alleviated Bevacizumab-induced cardiomyocyte injury in HL-1 cells
To further analyse the role of IRF1 in BVZ-treated HL-1 cells, qPCR was performed. Our results showed that IRF1 expression was up-regulated in BVZ-induced HL-1 cells (Figure 2). Next, HL-1 cells were transfected with sh-IRF1 or oe-IRF1. Overexpression of IRF1 promoted IRF1 expression, whereas transfection of sh-IRF1 decreased IRF1 expression (Figure 2). Furthermore, IRF1 knockdown reversed the promotive effect of BVZ on IRF1 expression in HL-1 cells, whereas IRF1 overexpression enhanced the effects of BVZ on IRF1 expression (Figure 2). In BVZ-treated cells, IRF1 silencing increased cell viability and reduced LDH release, whereas IRF1 overexpression had the opposite effects (Figure 2). Additionally, in BVZ-treated HL-1 cells, IRF1 down-regulation suppressed apoptosis, decreased the expression of pro-apoptotic proteins (Bax and cleaved-casp3), and increased the expression of anti-apoptotic protein Bcl-2, whereas IRF1 overexpression enhanced BVZ-induced cell injury (Figure 2). Moreover, IRF1 knockdown inhibited the secretion of cTnT and CK-MB from BVZ-treated HL-1 cells, but IRF1 overexpression accelerated their secretion (Figure 2). Therefore, IRF1 promoted BVZ-induced HL-1 cell injury.
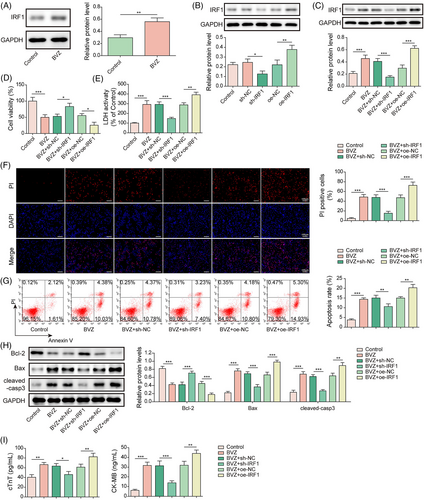
Interferon-regulatory factor-1 reduced vascular endothelial growth factor A expression and aggravated Bevacizumab-induced cardiomyocyte injury
To better understand the correlation between IRF1 and VEGFA in BVZ-induced cardiomyocyte injury, sh-IRF1 or sh-VEGFA was transfected into BVZ-treated HL-1 cells. Compared with the control group, VEGFA expression was down-regulated in BVZ-treated HL-1 cells (Figure 3). Next, data from JASPAR revealed that IRF1 bound to the VEGFA promoter (Figure 3). Dual luciferase reporter assay further confirmed that IRF1 bound to the VEGFA promoter region (Figure 3). Furthermore, we found that IRF1 deficiency induced VEGFA expression, whereas VEGFA knockdown reduced VEGFA expression (Figure 3). Moreover, sh-IRF1 induced VEGFA expression in BVZ-treated cells, whereas co-transfection with sh-VEGFA and sh-IRF1 reversed this effect (Figure 3). Additionally, IRF1 depletion improved cell viability and suppressed LDH release in BVZ-treated cells. However, the aforementioned effects were reversed after co-transfection with sh-VEGFA (Figure 3). Furthermore, co-transfection with sh-VEGFA reversed the repressive effects of sh-IRF1 on apoptosis in BVZ-treated HL-1 cells (Figure 3). In addition, IRF1 silencing decreased the cleaved-casp3 and Bax levels in BVZ-treated HL-1 cells and increased Bcl-2 expression. However, after co-transfection with sh-VEGFA, the opposite results were observed (Figure 3). Similarly, IRF1 knockdown suppressed the secretion of cTnT and CK-MB in BVZ-treated cells; however, co-transfection with sh-VEGFA reversed these inhibitory effects (Figure 3). Overall, IRF1 accelerated BVZ-induced cardiomyocyte injury by inhibiting VEGFA.
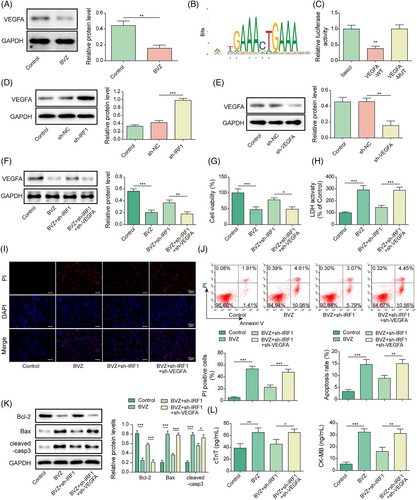
14-3-3γ participated in Bevacizumab-induced cardiomyocyte injury by interacting with vascular endothelial growth factor A
A decrease in 14-3-3γ expression was observed in BVZ-treated HL-1 cells (Figure 4). Meanwhile, Co-immunoprecipitation (Co-IP) and GST pull-down assays confirmed the binding relationship between VEGFA and 14-3-3γ (Figure 4). After transfecting oe-14-3-3γ and oe-NC into HL-1 cells, our results showed that 14-3-3γ overexpression promoted a remarkable increase in 14-3-3γ expression (Figure 4). In contrast, compared with control cells, BVZ-treated HL-1 cells showed a decrease in 14-3-3γ expression; however, this effect was effectively reversed by 14-3-3γ overexpression in BVZ-treated HL-1 cells (Figure 4). In addition, BVZ attenuated cell viability and increased LDH release, 14-3-3γ overexpression reversed these effects (Figure 4). Furthermore, BVZ treatment induced apoptosis, whereas transfection with oe-14-3-3γ overturned this effect in BVZ-treated HL-1 cells (Figure 4). BVZ also promoted the expression of cleaved-casp3 and Bax and decreased Bcl-2 levels, but these effects were eliminated by 14-3-3γ overexpression (Figure 4). Moreover, BVZ-treated HL-1 cells showed an increase in the secretion of cTnT and CK-MB, but 14-3-3γ overexpression reduced their levels (Figure 4). Overall, VEGFA interacted with 14-3-3γ and was involved in BVZ-induced myocardial injury.
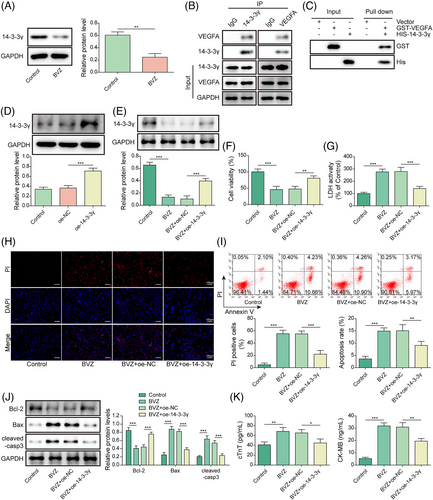
Interferon-regulatory factor-1 aggravated Bevacizumab-induced HL-1 cells injury by regulating the VEGFA/14-3-3γ axis
We further investigated whether IRF1 could regulate 14-3-3γ in BVZ-induced myocardial injury. Notably, qPCR indicated that 14-3-3γ was up-regulated after sh-IRF1 transfection but was down-regulated in the sh-14-3-3γ group (Figure 5). Moreover, we found that BVZ decreased 14-3-3γ expression in HL-1 cells, whereas sh-IRF1 increased 14-3-3γ levels; however co-transfection with sh-14-3-3γ and sh-IRF1 decreased 14-3-3γ expression (Figure 5). In addition, in BVZ-treated HL-1 cells, IRF1 silencing promoted cell viability and inhibited LDH release. However, co-transfection with sh-14-3-3γ and sh-IRF1 reversed these effects (Figure 5). We further found that down-regulation of IRF1 reduced BVZ-induced apoptosis, whereas co-transfection with sh-14-3-3γ and sh-IRF1 promoted apoptosis (Figure 5). Moreover, IRF1 knockdown suppressed the expression of cleaved-casp3 and Bax in BVZ-induced HL-1 cells but promoted the expression of Bcl-2. However, co-transfection with sh-14-3-3γ and sh-IRF1 reversed these effects in BVZ-treated HL-1 cells (Figure 5). Furthermore, IRF1 knockdown attenuated BVZ-induced cTnT and CK-MB secretion, whereas sh-14-3-3γ up-regulated their expression (Figure 5). Taken together, 14-3-3γ down-regulation reversed the effects of IRF1 silencing on BVZ-induced cardiomyocyte injury.
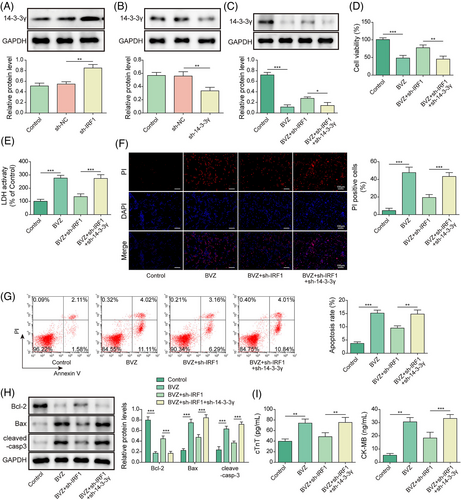
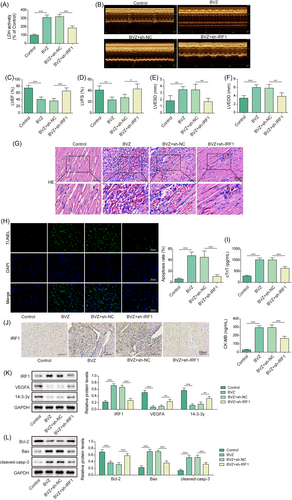
Knockdown of interferon-regulatory factor-1 alleviated Bevacizumab-induced cardiomyocyte injury in vivo
To explore the biological function of IRF1 in BVZ-mediated myocardial injury in vivo, C57BL/6 mice were injected with sh-IRF1 and treated with BVZ. We found that BVZ enhanced LDH release in the serum of mice, whereas IRF1 silencing suppressed LDH release in BVZ-treated mice (Figure 6). Meanwhile, BVZ demonstrated toxic effects on the myocardium, with changes in myocardial tissue morphology also having been observed. Additionally, echocardiography showed that compared with the control group, the BVZ group had decreased LVEF and LVFS but increased LVESD and LVEDD. However, transfection with sh-IRF1 alleviated the effects of BVZ treatment (Figure 6). As shown in Figure 6G, the myocardial structure and morphology of the control group were normal, and the myocardial fibres showed clear horizontal stripes, whereas the BVZ group exhibited clusters of infiltrating inflammatory cells in their myocardia, and disordered arrangement of cardiomyocytes. However, IRF1 knockdown significantly ameliorated the pathological changes mentioned. TUNEL staining further showed that compared with the control group, the BVZ group showed increased apoptosis, with IRF1 silencing ameliorating this effect (Figure 6). cTnT and CK-MB release was enhanced in the serum of BVZ-treated mice; however, IRF1 knockdown clearly repressed cTnT and CK-MB expression in BVZ-treated mice (Figure 6). Next, immunohistochemistry results demonstrated that compared with untreated mice, BVZ-treated mice showed IRF1 expression in their myocardial tissues, although this effect was abolished after IRF1 knockdown in mice (Figure 6). Additionally, compared with control mice, BVZ-treated mice showed increased IRF1 levels but decreased VEGFA and 14-3-3γ levels. However, IRF1 down-regulation reversed the effects of BVZ on the levels of these proteins (Figure 6). After BVZ treatment, cleaved-casp3 and Bax levels were significantly elevated, whereas Bcl-2 expression decreased. The opposite results were observed in BVZ-treated mice when IRF1 was knocked down (Figure 6). Overall, IRF1 expression was up-regulated in myocardial tissues of BVZ-treated mice, suggesting that it mediated BVZ-induced cardiomyocyte injury in vivo.
Discussion
While killing tumour cells, anti-tumour drugs also damage normal tissues and organs within the human body, with a particular focus having been placed on myocardial damage, which is often caused by cardiac complications rather than tumours.20, 21 BVZ, the most commonly used VEGFA inhibitor, is an effective anti-angiogenic agent that has been proven to delay the progression of tumours in patients.22, 23 However, BVZ treatment may lead to microvascular damage, resulting in cardiovascular disease, thromboembolic events, and gastrointestinal perforation,24-26 suggesting that BVZ induced cardiotoxicity may also be caused by endothelial cell damage. In addition, Previous studies reported that myocardial ischaemia is a potential life-threatening and not uncommon complication of 5-fluorouracil or capecitabine chemotherapy. And 5-fluorouracil can cause endothelial cell aging and dysfunction, which is one of the reasons for its cardiovascular side effects,27 indicating that endothelial cell damage also has a potential role in cardiotoxicity. After its initiation in clinical practice, several cardiovascular side effects involving left ventricular dysfunction and subsequent heart failure have been reported due to BVZ-induced cardiotoxicity.28-30 One case report involving a 69-year-old woman with cervical cancer presented with acute decompensated heart failure. Two years before the onset of heart failure, she was diagnosed with cervical cancer and received chemotherapy combined with bevacizumab (4.9 mg/kg/week) and paclitaxel (1.0 mg/kg/week) for 4 months.29 In addition, a study found that HLX04 (a analogue of bevacizumab) synergizes with XELOX or mFOLFOX6, it has a similar therapeutic effect in treating rectal cancer as bevacizumab. In addition, pharmacokinetics showed that no significant differences were observed between the HLX04 (about 75 μg/mL) and bevacizumab (about 85 μg/mL) groups in the samples collected before administration at weeks 18, 36, and 48, indicating that the serum concentrations of HLX04 and bevacizumab reached a stable state at week 18,31, indicating that when the concentration in the patient's serum is 85 μg/mL, it has a protective effect on tumour. In present study, we used BVZ to treat HL-1 cells and found that BVZ inhibited HL-1 cells viability in a concentration dependent manner (Figure 1). And when the concentration of BVZ treated HL-1 was <100 μg/mL, it had almost no effect on cell viability. However, when the BVZ concentration was about 745 μg/mL, the cell inhibition rate reached 49%. In another study, rats were injected with 10 mg/kg of BVZ intraperitoneally to induce cardiotoxicity in vivo.6 Moreover, published reports have revealed that 10 mg/kg of BVZ causes immature skeletal development in rats,32 and BVZ (7 mg/kg for 10 days) plays an anti-tumour role in the treatment of intramedullary spinal cord tumour,33 indicating that administering BVZ at a dose exceeding a certain threshold could trigger side effects on the patient's organs. BVZ could induce cardiotoxicity by promoting mitochondrial dysfunction, oxidative stress, endoplasmic reticulum stress, and ERK inactivation.5, 34 Interestingly, one research has shown that miR-140-5p could mediate BVZ-induced cytotoxicity to cardiomyocytes by targeting the VEGFA/14-3-3γ signalling pathway.8 In the present study, we revealed that IRF1 accelerated BVZ-induced cardiomyocyte injury by regulating the VEGFA/14-3-3γ axis.
IRF1 is involved in immune responses, tumour suppression, and apoptosis in multiple cell types.35-37 Recent studies have shown that IRF1 functions as an important factor in cardiac hypertrophy, fibrosis, and cardiac dysfunction.11 A recent study based on microarray data analysis suggested that IRF1 may be a candidate biomarker gene for ischaemic cardiomyopathy,38 while another study showed that IRF1 induced macrophage pyroptosis in patients with acute coronary syndrome.39 Notably, the literature reports that BVZ promoted IRF1 expression in glioma cells.19 In the present study, we found that IRF1 expression was significantly increased in HL-1 cells and mice treated with BVZ. Furthermore, the changes in mitochondrial activity or the promotion of apoptosis may reflect the toxicity of myocardial cells or the inhibition of cell proliferation rate. Another study showed that RRM2 expression was reduced in cardiac tissues and primary cardiomyocytes treated with doxorubicin and that RRM2 overexpression significantly inhibited excessive cell apoptosis and autophagy, thereby reducing doxorubicin-induced cardiotoxicity.40 Meanwhile, evidence has suggested glyphosate caused cardiotoxicity in zebrafish, which induced DNA and mitochondrial damage while reducing the proliferative ability of zebrafish cardiomyocytes, resulting in decreased cardiomyocytes.41 Herein, IRF1 down-regulation promoted cell proliferation and suppressed apoptosis, thereby inhibiting BVZ-induced cardiomyocyte injury.
Studies have suggested that IRF1 inhibited proliferation, invasion, and migration of osteosarcoma cells through VEGFA.15 Jiang et al. reported that IRF1 was required for cardiac remodelling in response to pressure overload by binding to the promoter region of the iNOS gene.42 Huang et al. demonstrated that IRF1 was responsible for the transcriptional inhibition of PGC1α by directly binding to the promoter region of PGC1α in cardiorenal syndrome type 4.43 We also verified that IRF1 bound to the VEGFA promoter region and inhibited VEGFA expression. A previous study reported that VEGFA induced proliferation of endothelial cells and contributed to tumour angiogenesis.44 Meanwhile, another study showed that VEGFA was decreased in BVZ-treated cardiomyocytes and that the inhibition of VEGFA enhanced BVZ-mediated cell injury and apoptosis.8 We further found that depletion of VEGFA reversed the effects of IRF1 knockdown on BVZ-induced cardiotoxicity, suggesting that IRF1 plays an important role in BVZ-induced cardiomyocyte injury by regulating VEGFA expression.
Studies have shown that the 14-3-3γ protein plays an essential role in signal transduction,45 with reports suggesting that 14-3-3γ is involved in cardiotoxicity by inhibiting oxidative stress and preventing mitochondrial dysfunction.18 In fact, one study showed that tetramethylpyrazine achieved its cardioprotective effects by increasing 14-3-3γ expression.46 Furthermore, quercetin had been found to alleviate doxorubicin-triggered cardiomyocyte toxicity by up-regulating 14-3-3γ expression.47 In the current study, we demonstrated that 14-3-3γ had a protein interaction with VEGFA. After BVZ treatment, we found that 14-3-3γ levels in HL-1 cells decreased and that 14-3-3γ overexpression repressed BVZ-induced cardiomyocyte injury. In addition, 14-3-3γ silencing reversed the effects of IRF1 knockdown on BVZ-induced myocardial injury.
In conclusion, our data showed that IRF1 could induce apoptosis, inhibit cell proliferation, and promote the release of LDH, cTnT, and CK-MB by mediating the VEGFA/14-3-3γ axis in BVZ-triggered myocardial injury. Our findings suggest that IRF1 may be a novel therapeutic target for preventing BVZ-induced myocardial injury. Nevertheless, some limitations of this study are worth noting. First, the lack of clinical data was the primary limitation of the current study. Moreover, the results of the MTT assay measured not only cell viability but also the overall mitochondrial metabolic activity of the cell. The reduced response of proliferating cells in the MTT assay reflects their direct cytotoxicity due to proliferation inhibition and cell death (the ratio of which will depend on the proliferation rate and direct cytotoxicity). In addition, future in vivo experiments should focus more on cardiomyocyte specific staining as a good method for evaluating BVZ-mediated cardiomyocyte injury. Additionally, in in vivo experiments, BVZ, as an IgG1 monoclonal antibody, may have different therapeutic effects in different immunized mice. Accordingly, we intend to conduct a follow-up study to determine whether the corresponding homologous controls should be designed when studying the in vivo effects of BVZ.
Funding
This work was supported by National Natural Science Foundation of China (No. 82060679) and Jiangxi Provincial Health Department Project (No. SKJP220212447).
Conflict of interest
The authors declare that they have no conflict of interest.




