Safety of sodium-glucose cotransporter 2 inhibitors drugs among heart failure patients: a systematic review and meta-analysis
Hamidreza Soleimani and Behrad Saeedian contributed equally as co-first authors.
Abstract
Sodium–glucose cotransporter-2 inhibitors (SGLT2is) reduce morbidity and mortality for heart failure (HF) patients and are recommended as cornerstones for their medical therapy. Utilization in clinical practice remains low for multiple reasons, one of which may be adverse events. We investigated the incidence of these events to see if they are associated with SGLT2i use. A systematic search was performed in databases, including PubMed, Embase, Cochrane Library, Clinicaltrials.gov, and WHO's International Clinical Trials Registry Platform. Relevant randomized controlled trial studies assessing the safety outcomes of SGLT2i in HF patients were included in this study. We conducted the common-effect meta-analysis to estimate the relative risk (RR) and 95% confidence interval (CI) of safety outcomes in SGLT2i compared with placebo. Eighteen studies were included in the meta-analysis composed of 12 925 HF patients taking an SGLT2i and 12 747 taking a placebo. The meta-analysis indicated that the all-cause mortality and serious adverse events (SAEs) were lower in the SGLT2i group (RR, 0.91; 95% CI, 0.85–0.97; P = 0.005, I2 = 0%; and RR, 0.92; 95% CI, 0.90–0.95; P < 0.001, I2 = 43%, respectively). Volume depletion and genitourinary infections were more prevalent in the SGLT2i group (RR, 1.17; 95% CI, 1.06–1.28; P = 0.001, I2 = 0%; and RR, 1.27; 95% CI, 1.13–1.43; P < 0.001, I2 = 17%, respectively). Our meta-analysis demonstrated that using SGLT2is in HF patients was correlated with reduced mortality and SAEs, with a more prominent effect in HF with reduced ejection fraction patients and those taking dapagliflozin.
Introduction
Heart failure (HF) is a global pandemic, with increasing hospitalization, morbidity, and mortality rates worldwide.1 The four pillars of goal-directed medical therapy for HF include beta-blockers, renin–angiotensin–aldosterone system inhibitors, aldosterone antagonists, and, most recently, sodium–glucose cotransporter-2 inhibitors (SGLT2is).2 Despite these clinically effective agents' availability, outcomes for HF patients remain unacceptable. These poor outcomes are partly due to poor utilization of proven therapies, with early uptake of SGLT2i being particularly sluggish.3 This has devastating implications for patients, with major clinical and cost burdens.4, 5
The SGLT2i drug class was originally developed as an antidiabetic agent and functions by inhibiting glucose reabsorption in the proximal renal tubule, resulting in glucosuria and decreased plasma glucose concentrations. The underlying mechanisms responsible for favourable cardiovascular outcomes are not yet fully understood but may be due to glucose-independent mechanisms that regulate volume status and sodium balancing.6-9
Cost and clinical inertia are frequently cited as key drivers of SGLT2i underutilization.3, 10 Another potential contributor to restricted use that has been inadequately investigated is the side effects of SGLT2i. Some studies have documented clinical cases wherein SGLT2i medications were discontinued early due to various complications such as bacterial infections, hypotension, acute kidney injury, limb ischaemia, and others, leading to unfavourable clinical outcomes.11 A comprehensive appraisal of the currently available evidence on adverse effects on SGLT2i is needed to inform efforts to minimize the associated side effects, reduce discontinuation, and improve uptake. Thus, the current analysis aims to systematically review and perform a pooled analysis of the major safety outcomes of four drugs in the SGLT2i class of medications, including dapagliflozin, empagliflozin, canagliflozin, and sotagliflozin in comparison with placebo, in patients across the whole spectrum of HF with or without diabetes mellitus.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.12 Study protocol details were registered in the International Prospective Register of Systematic Reviews (PROSPERO)13 (PROSPERO ID: CRD42023424911).
Criteria for inclusion/exclusion of studies in the review
Type of studies
Randomized controlled trials (RCTs) reporting the use of a drug from the SGLT2i class in patients with HF (reduced and preserved) were eligible for inclusion. Abstracts, case reports, review articles, trial design protocols, non-comparative studies, and conference abstracts were dismissed.
Participants and interventions
All men and women aged 18 years and above with the New York Heart Association functional classes II–III and chronic HF, either with reduced ejection fraction [defined as left ventricular ejection fraction (LVEF) < 40%] or mildly reduced or preserved ejection fraction (defined as LVEF > 40%), were included in this study. Patient exclusion criteria differed in each study protocol. Still, they mostly consisted of patients with disorders that could change their clinical course independent of HF or with conditions that could jeopardize their safety or limit their participation in the respective trial.
Outcomes
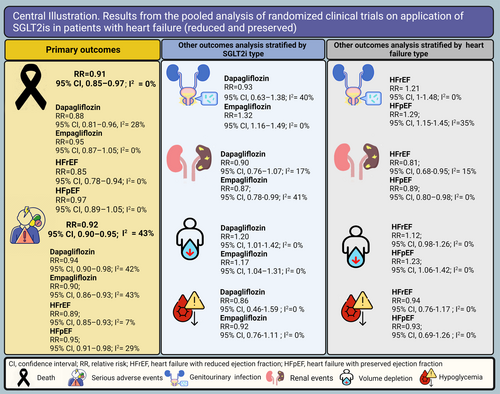
In this systematic review, only outcomes classified as adverse events by the respective trial were reported. Outcomes related to the efficacy of the drug were excluded from the analysis. Prespecified adverse events were different across the studies. The primary outcomes of interest in this study were death and incidence of serious adverse events (SAEs). Other adverse events studied specifically in this study were adverse events serious enough to lead to discontinuation of the drug, serious renal adverse events, volume depletion, urinary tract infections, major hypoglycaemia, ketoacidosis in diabetic patients, and adverse events related to the lower limb including fractures, amputations, and Fournier's gangrene. The definition of all the mentioned events is based on the standard coding in the latest version of the Medical Dictionary for Regulatory Activities (MedDRA) at the time of each trial's enrolment period (Central Illustration 1).
Search strategy
After identifying relevant keywords and search terms (Supporting Information, Table S1), we performed a systematic search in the following electronic databases: PubMed, Embase, Cochrane Library, Clinicaltrials.gov, and WHO's International Clinical Trials Registry Platform, from 1 January 2014 until 1 March 2023. No language restriction was applied. A reference list of eligible studies and relevant reviews on the topic were also screened.
Data extraction and management
The results of a systematic search were imported into EndNote software Version 20.0 (Clarivate PLC, London, UK) and screened by two independent authors (A. P. and B. S.), who reviewed the title and abstract of the studies. Any conflicts were resolved by a third reviewer (H. R. S.). Full texts of selected studies were retrieved, and data were extracted using a predesigned form. The data extraction sheet included information about the first author's name, study site, study type, sample size of each comparison group, baseline characteristics, incidence, and type of adverse events.
Risk of bias assessment
The quality of the RCTs included in the study was assessed using Version 2.0 of the Cochrane Risk of Bias Assessment Tool for Randomized Trials (RoB2).14-16 Two authors (N. B. and B. S.) assigned stars in each category, and conflicts were resolved through consensus.
Data synthesis
The included studies for the meta-analysis were pooled into two sections. The first section evaluated studies considering the prespecified primary outcomes of this meta-analysis (i.e. death and SAEs). The death and SAEs occurrence were compared between SGLT2i and placebo recipients in the subgroups of SGLT2i types and HF types [i.e. HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF)]. In the second section, other prespecified outcomes of this meta-analysis were compared between SGLT2i and placebo recipients in the subgroups of different SGLT2i types and HF types.
Statistical analysis
All statistical analyses were conducted using R programming language (R for Windows, Version 4.1.3, Vienna, Austria) and RStudio Version 1.1.463 (Posit PBC, Boston, MA, USA) utilizing the ‘tidyverse’ and ‘meta’ statistical packages. Relative risks (RRs) with 95% confidence intervals (CIs) were calculated for binary variables, while mean and standard deviation (SD) were calculated for continuous variables. In studies that reported median and interquartile range, we used the method developed by Luo et al.17 and Wan et al.18 to calculate the mean and SD. Heterogeneity was assessed using the I2 statistic, with significant heterogeneity defined as I2 > 50%. If the heterogeneity was significant, we used a random-effect model to estimate the effect size of the pooled data; otherwise, a common-effect model was used. Sensitivity and subgroup analyses were conducted to identify sources of heterogeneity when possible. Funnel plots were produced for variables reported in more than 10 studies.
Certainty of evidence assessment
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was utilized to assess the overall quality of the evidence regarding outcomes of interest.19-21 Based on GRADE, the evidence is rated as high, moderate, low, and very low. The evidence is evaluated on different domains, including risk of bias, inconsistency, indirectness, imprecision, and publication bias. For RCTs, domains may be downgraded for one or two levels due to either ‘serious’ or ‘very serious’ risks. Two reviewers (B. S. and Y. P.) assessed each domain for each outcome, and any discrepancy was solved by consensus. All decisions were documented regarding upgrading or downgrading the certainty of evidence to ensure transparency (Supporting Information, Table S2).
Results
Study selection
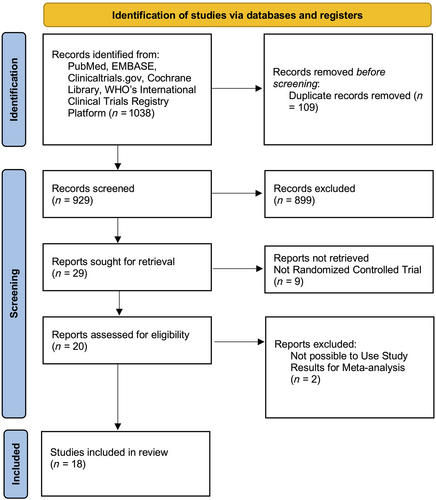
All five databases searched yielded 1038 records. Following both manual and automated deduplication procedures, the number of remaining records available for screening was 929. The PRISMA flow diagram elucidates the precise methodology employed for the selection process (Figure 1).
Study characteristics
Eighteen trials with 42 318 participants (21 338 received an SGLT2i and 20 980 received a placebo) met our inclusion criteria and were included. Out of the total 21 338 individuals who received an SGLT2i, dapagliflozin was administered to 14 398 patients (67.5%), empagliflozin to 6110 patients (28.6%), sotagliflozin to 608 patients (2.8%), and canagliflozin to 222 patients (1.0%). A summary of studies included in the meta-analysis is described in Table 1. Among these 18 trials, three recruited patients with HFpEF, six targeted HFrEF, and nine recruited both. Most studies included in this systematic review have been conducted in North America and Europe.
| First author and year | Study region | Study type | Number of patients | Age (years) (mean ± SD) | Male sex (%) | Drug type | EF type | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGLT2i | Placebo | SGLT2i | Placebo | ||||||||||
| D. Fitchett 2016 | Canada–Germany–USA–Norway | RCT-randomized | 462 | 244 | 64.5 ± 8.8 | 64.5 ± 8.9 | 70 | Empagliflozin | Reduced and preserved | ||||
| E. T. Kato 2019 | Japan–USA–Israel–Canada–UK–Sweden | RCT-randomized, double-blind | HFrEF | Non-HFrEF | HFrEF | Non-HFrEF | 64.46 ± 6.46 | 64.46 ± 6.46 | 66.4 | Dapagliflozin | Reduced and preserved | ||
| 317 | 8256 | 353 | 8216 | ||||||||||
| J. J. V. McMurray 2019 | UK–USA–Denmark–Argentina–Poland–Czech Republic | RCT-randomized | 2373 | 2371 | 66.2 ± 11.0 | 66.5 ± 10.8 | 76.7 | Dapagliflozin | Reduced | ||||
| M. E. Nassif 2019 | USA–Canada | RCT-randomized, double-blind | 131 | 132 | 62.2 ± 11.0 | 60.4 ± 12.0 | 73.4 | Dapagliflozin | Reduced | ||||
| M. Packer 2020 | UK–Germany–USA–Greece–Canada–Japan | RCT-randomized, double-blind | 1863 | 1863 | 67.2 ± 10.8 | 66.5 ± 11.2 | 77 | Empagliflozin | Reduced | ||||
| D. L. Bhatt 2020 | USA–Canada–Belgium–Netherlands–UK–Denmark–Finland–Sweden | RCT-randomized, double-blind | 608 | 614 | 69 ± 6 | 70 ± 6 | 66.29 | Sotagliflozin | Reduced and preserved | ||||
| K. Damman 2020 | Netherlands–Australia | RCT-randomized, double-blind | 40 | 39 | 79 ± 6 | 73 ± 12 | 67 | Empagliflozin | Mean left ventricular ejection fraction of 36% | ||||
| J. S. S. Singh 2020 | UK | RCT-randomized | 28 | 28 | 66.9 ± 7.0 | 67.4 ± 6.8 | 76.1 | Dapagliflozin | Reduced and preserved | ||||
| T. Abraham 2021 | USA–Poland–Italy–Greece–Cyprus | RCT-randomized, double-blind | HFrEF | Non-HFrEF | HFrEF | Non-HFrEF | HFrEF | Non-HFrEF | HFrEF | Non-HFrEF | 65.6 | Empagliflozin | Reduced and preserved |
| 156 | 157 | 156 | 158 | 69 ± 8 | 74 ± 5 | 70 ± 7 | 75 ± 4 | ||||||
| S. D. Anker 2021 | Germany–USA–France–Greece–Brazil–Netherlands–Korea–India–Mexico | RCT-randomized, double-blind | 2997 | 2991 | 71.8 ± 9.3 | 71.9 ± 9.6 | 55.4 | Empagliflozin | Preserved | ||||
| J. Jensen 2021 | Denmark | RCT-randomized, double-blind | 60 | 60 | 68 ± 10 | 67 ± 10 | 83 | Empagliflozin | Reduced | ||||
| M. E. Nassif 2021 | USA | RCT-randomized, double-blind | 162 | 162 | 69 ± 8 | 71 ± 7 | 43 | Dapagliflozin | Preserved | ||||
| M. E. Nassif 2021 | USA | RCT-randomized, double-blind | 33 | 32 | 69.5 ± 12.0 | 62.9 ± 13.3 | 63.1 | Empagliflozin | Reduced and preserved | ||||
| S. G. Santos-Gallego 2021 | USA–Spain | RCT-randomized, double-blind | 42 | 42 | 64.2 ± 10.9 | 59.9 ± 13.1 | 64 | Empagliflozin | Reduced | ||||
| S. D. Solomon 2022 | USA–UK–Netherlands–Singapore–Spain | RCT-randomized, double-blind | 3131 | 3132 | 71.8 ± 9.6 | 71.5 ± 9.5 | 56.14 | Dapagliflozin | Left ventricular ejection fraction of more than 40% | ||||
| A. A. Voors 2022 | Netherlands–Germany–USA–Poland–France–Singapore | RCT-randomized, double-blind | 265 | 265 | 62 ± 7 | 70 ± 8 | 66.23 | Empagliflozin | Reduced and preserved | ||||
| J. A. Spertus 2022 | USA | RCT-randomized, double-blind | 222 | 226 | 62.9 ± 13.19 | 64.0 ± 13.45 | 55.1 | Canagliflozin | Reduced and preserved | ||||
| M. J. Hundertmark 2023 | UK–Germany | RCT-randomized, double-blind | 35 | 37 | 68.3 ± 11.9 | 68.4 ± 10.96 | 58.64 | Empagliflozin | Reduced and preserved | ||||
- EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; RCT, randomized controlled trial; SD, standard deviation; SGLT2i, sodium–glucose cotransporter-2 inhibitor.
Quality appraisal
Twelve of the 18 studies had a low risk of bias, and 8 were unclear in criterion D5: bias in the selection of the reported results according to the RoB2 but were low risk in the rest of the criteria (Supporting Information, Figure S1).
Results of the primary outcome analysis
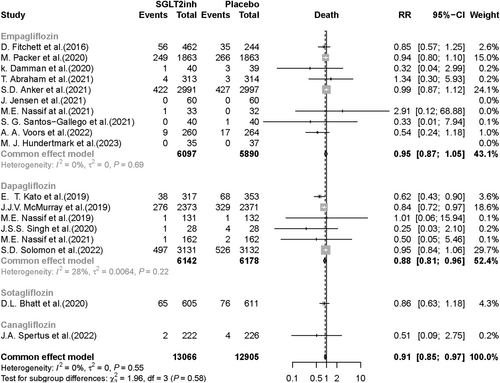
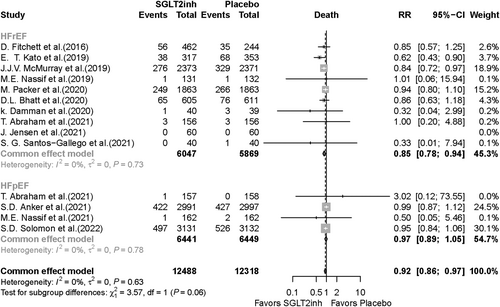
Eighteen RCTs reporting death as an outcome were included in the meta-analysis. Death was significantly lower in the SGLT2i group compared with the placebo group (RR, 0.91; 95% CI, 0.85–0.97; P value = 0.005). In the subgroup analysis based on SGLT2i type, death was significantly lower in the dapagliflozin group compared with the placebo group (RR, 0.88; 95% CI, 0.81–0.96; P value = 0.003), but there was no significant difference between patients taking empagliflozin compared with those who received placebo (RR, 0.95; 95% CI, 0.87–1.05; P value = 0.288) (Figure 2). Fourteen RCTs were included in the death meta-analysis based on HF type. Of note, in HFrEF patients, death was 15% lower in SGLT2i recipients compared with placebo recipients (RR, 0.85; 95% CI, 0.78–0.94; P value < 0.001). In HFpEF patients, there was no significant difference in death occurrence (RR, 0.97; 95% CI, 0.89–1.05; P value = 0.479) between the SGLT2i vs. placebo groups (Figure 3).
The meta-analysis included 16 RCTs reporting SAEs as an outcome in patients receiving SGLT2i or placebo. SAEs were significantly lower in the SGLT2i group compared with the placebo group (RR, 0.92; 95% CI, 0.90–0.95; P value < 0.001) (Figure 4). In the subgroup analysis based on SGLT2i type, SAEs were 6% lower in the dapagliflozin group vs. the placebo group (RR, 0.94; 95% CI, 0.90–0.98; P value = 0.004) and 10% lower in the empagliflozin group vs. the placebo group (RR, 0.90; 95% CI, 0.86–0.93; P value < 0.001) (Figure 4). Twelve studies were included in the meta-analysis based on HF type; in HFrEF patients, SAEs occurrence was significantly lower in the SGLT2i group compared with the placebo group (RR, 0.89; 95% CI, 0.85–0.93; P value < 0.001). In the HFpEF group, SAEs were also significantly lower in the SGLT2i recipients compared with the placebo recipients (RR, 0.95; 95% CI, 0.91–0.98; P value = 0.006) (Figure 5). Publication bias for death and SAEs is shown visually via funnel plots in Supporting Information, Figures S2 and S3.
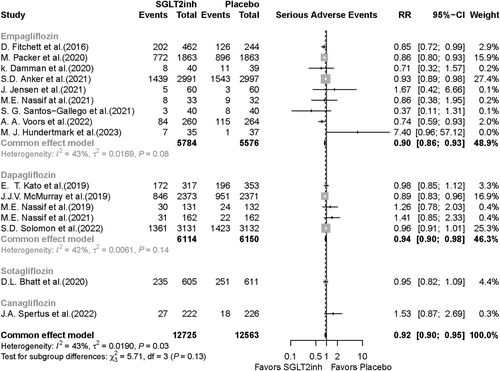
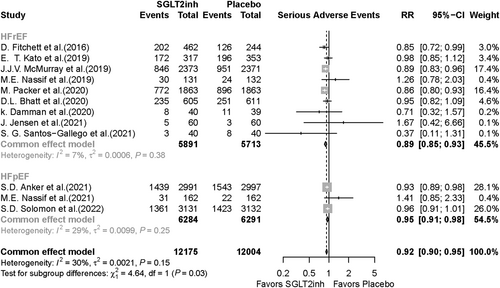
Results of the other outcomes analysis
Results of the other outcomes analysis based on the type of the sodium–glucose cotransporter-2 inhibitor
Due to the small number of studies regarding sotagliflozin and canagliflozin, only studies considering dapagliflozin and empagliflozin are brought into this context.
The incidence of renal events was 13% lower in the empagliflozin group compared with the placebo group (RR, 0.87; 95% CI, 0.78–0.99; P value = 0.02). In the dapagliflozin group, the incidence of renal events showed no significant difference compared with the placebo group (RR, 0.90; 95% CI, 0.76–1.07; P value = 0.229) (Supporting Information, Figure S4). The overall incidence of renal events was 12% lower in the SGLT2i group compared with the placebo group (RR, 0.88; 95% CI, 0.80–0.97; P value = 0.009) (Supporting Information, Figure S4). Genitourinary infections were significantly higher in the empagliflozin group in comparison with the placebo group (RR, 1.32; 95% CI, 1.16–1.49; P value < 0.001), but there was no significant difference in the incidence of genitourinary infections in dapagliflozin recipients compared with placebo recipients (RR, 0.93; 95% CI, 0.63–1.38; P value = 0.729) (Supporting Information, Figure S5). Pooled genitourinary infection incidence was significantly higher in SGLT2i recipients vs. placebo recipients (RR, 1.27; 95% CI, 1.13–1.43) (Supporting Information, Figure S5). Volume depletion occurrence was significantly higher in the dapagliflozin group compared with the placebo group (RR, 1.20; 95% CI, 1.01–1.42) and 17% higher in the empagliflozin group compared with the placebo group (RR, 1.17; 95% CI, 1.04–1.31; P value < 0.001) (Supporting Information, Figure S6). Overall, volume depletion occurrence was 17% higher in the SGLT2i group vs. the placebo group (RR, 1.17; 95% CI, 1.06–1.28; P value = 0.001) (Supporting Information, Figure S6).
Drug discontinuation was not significantly different in either empagliflozin compared with the placebo subgroup or dapagliflozin compared with the placebo subgroup (RR, 0.97; 95% CI, 0.89–1.04; P value = 0.451; and RR, 0.99; 95% CI, 0.92–1.06; P value = 0.793, respectively) (Supporting Information, Figure S7). Major hypoglycaemia was not significantly different in either of the empagliflozin compared with placebo nor dapagliflozin compared with placebo subgroups (RR, 0.92; 95% CI, 0.76–1.1; P value = 0.383; and RR, 0.86; 95% CI, 0.46–1.59, respectively) (Supporting Information, Figure S8).
Outcomes related to ketoacidosis, fracture, amputation, and Fournier's gangrene were compared between the SGLT2i and placebo arms, and no significant difference was found between dapagliflozin and empagliflozin in these events. The outcomes are described in detail in Supporting Information, Figures S9–S12. Publication bias for all the aforementioned outcomes is shown visually in Supporting Information, Figures S13–S20.
Results of the other outcomes analysis based on the heart failure type
In the HFrEF group, the incidence of renal events was 19% lower in the SGLT2i group compared with the placebo group (RR, 0.81; 95% CI, 0.68–0.95; P value = 0.01), and in the HFpEF group, renal events incidence was 11% lower in the SGLT2i group compared with the placebo group (RR, 0.89; 95% CI, 0.80–0.98; P value = 0.02) (Supporting Information, Figure S21). Genitourinary infections in the HFrEF subgroup were not significantly different between the SGLT2i and placebo arms (RR, 1.21; 95% CI, 1–1.48; P value = 0.056). In the HFpEF subgroup, genitourinary infections were significantly higher in SGLT2i recipients vs. placebo recipients (RR, 1.34; 95% CI, 1.16–1.54; P value < 0.001) (Supporting Information, Figure S22). In HFrEF patients, no significant difference was detected in volume depletion between the SGLT2i and placebo groups (RR, 1.12; 95% CI, 0.98–1.26; P value = 0.07). Of note, in the HFpEF subgroup, the occurrence of volume depletion in SGLT2i recipients was significantly higher compared with placebo recipients (RR, 1.23; 95% CI, 1.06–1.42; P value = 0.005) (Supporting Information, Figure S23). The incidence of drug discontinuation was not significantly different in either HFrEF or HFpEF subgroups between the arms of comparison (RR, 0.93; 95% CI, 0.83–1.03; P value = 0.183; and RR, 1.03; 95% CI, 0.94–1.13; P value = 0.540, respectively) (Supporting Information, Figure S24). Major hypoglycaemia was not significantly different across the comparison arms in none of the HFrEF (RR, 0.92; 95% CI, 0.76–1.1; P value = 0.383) and HFpEF (RR, 0.86; 95% CI, 0.46–1.59; P value = 0.646) subgroups (Supporting Information, Figure S25).
Regarding ketoacidosis and lower limb outcomes (i.e. fracture and amputation), comparisons between the SGLT2i and placebo arms showed no significant difference regarding HF subtypes and are described in detail in Supporting Information, Figures S26–S28.
Certainty of evidence
Among eight outcomes considered for the certainty of evidence with the GRADE tool, seven had high quality, and ketoacidosis showed moderate quality (Supporting Information, Table S2). The inconsistency of SAEs was rated serious because of a 43% heterogeneity, and genitourinary infections and volume depletion had serious publication bias based on their respective funnel plots (Supporting Information, Figures S14 and S15). However, none of these were significant enough to downgrade the overall quality. Ketoacidosis had a moderate quality and serious imprecision risk due to the broad 95% CI and the inability to estimate the absolute effect accurately.
Discussion
We conducted a meta-analysis to evaluate the safety of SGLT2i drugs in HF treatment. Treatment with SGLT2i reduced the risk of death, SAEs, and renal events without being associated with discontinuation due to adverse effects, major hypoglycaemia, ketoacidosis, or adverse limb events. However, the risk of genitourinary infections and volume depletion was higher compared with placebo. Additionally, patients with HFpEF had a higher incidence of adverse events than patients with HFrEF, and dapagliflozin appeared to have the best adverse effect profile compared with other SGLT2i. These findings provide valuable insights into the safety profile of SGLT2i and considerations around their potential use in patients with HF.
The use of SGLT2i has been increasing in recent years due to their promising effects on cardiovascular and renal outcomes. However, there are concerns about potential adverse events in patients with HF. In this context, our study aimed to evaluate the association between SGLT2i use and mortality, SAEs, and other key safety outcomes.
We found that the SGLT2i group had significantly lower rates of all-cause mortality and SAEs than the placebo group, consistent with previous meta-analyses.22-29 Most all-cause deaths were attributed to cardiovascular events, suggesting that a reduction in cardiovascular deaths drove the reduction in all-cause mortality. Among the other safety outcomes, only renal events, genitourinary infections, and volume depletion significantly correlated with SGLT2i treatment. The risk of renal events was lower among patients receiving SGLT2i, which is consistent with prior studies and a key consideration in HF patients who frequently have comorbid renal insufficiency.22, 24, 26, 28, 30, 31 Genitourinary infections were found to be more frequent in patients using SGLT2i, possibly due to the higher urinary excretion of glucose.7, 8 However, the two major trials for dapagliflozin, DAPA-HF and DELIVER, did not show significant differences in genitourinary infections compared with placebo, while the EMPEROR-Reduced and EMPEROR-Preserved trials for empagliflozin favoured placebo. The overall analysis leaned towards favouring the placebo due to the weight of the two latter trials. This contradicts some previous studies in diabetic patients that found dapagliflozin to have a higher risk of genitourinary infections.32, 33 Both dapagliflozin and empagliflozin were found to increase the risk of volume depletion to the same extent, particularly in HFpEF patients. There was no increased risk of other key potential side effects of SGLT2i treatment, including hypoglycaemia, ketoacidosis, Fournier's gangrene, and limb-related events such as amputation or fracture. Perhaps most importantly, there was no difference in drug discontinuation rates between those treated with SGLT2i and placebo, reassuring clinicians that these medications are safe and well tolerated by most patients.
Our subgroup analysis revealed that dapagliflozin exhibited a stronger protective effect for mortality compared with other SGLT2i, particularly empagliflozin. This may be due to the lower selectivity ratio for the SGLT2:SGLT1 receptor of dapagliflozin, which suggests that it may have a greater potential to prevent HF, as SGLT1 up-regulation in the myocardium is seen in myocardial ischaemia and hypertrophy.34-36 The neutral effect of dapagliflozin on plasma aldosterone and norepinephrine levels may also have potential benefits in preventing HF.37 Pharmacokinetic differences may also explain the observed variation in results between dapagliflozin and empagliflozin as dapagliflozin reaches peak concentration faster and has a longer half-life compared with the same dose of empagliflozin.38-44 Other adverse events showed no significant difference in risk between patients taking dapagliflozin and empagliflozin.
While overall mortality was reduced in patients taking SGLT2i, a more significant effect was observed in those with HFrEF compared with those with HFpEF. This finding is consistent with a previous meta-analysis26 and supports that SGLT2i offers greater benefits for patients with HFrEF. The reduction in SAE was also more pronounced in the HFrEF group than in the HFpEF group. Other safety outcomes had small or no significant difference in risk between HFrEF and HFpEF patients.
Strength and limitations
Our systematic review and meta-analysis is the most comprehensive one to date, including the largest number of randomized clinical trials with over 20 000 participants. The precise and comprehensive search of major databases and independent screening by two reviewers ensured reliability. Our subgroup analysis for SGLT2i drug type and HF status gives clinicians more information about adverse events, allowing for personalized therapy. However, several limitations should be considered. There is moderate heterogeneity between studies, as studies varied in size and were conducted in different regions with varying standards, potentially introducing bias. Not all studies reported all the analysed adverse events and lacked important safety outcomes. Also, multiple adverse events, such as SAEs, renal events, volume depletion, and major hypoglycaemia, varied in definition between the studies. Lastly, most studies did not adhere to new guidelines classifying patients with HF into three categories (HFrEF, HF with mildly reduced ejection fraction, and HFpEF), which, due to their possible different pathophysiologies, may require specific analysis for safety outcomes.
Conclusions
This large contemporary meta-analysis of randomized trials investigating the treatment of HF patients with SGLT2i trials demonstrated a significantly reduced risk of death and SAEs with SGLT2i treatment, with a more prominent effect observed in patients with HFrEF. Compared with other SGLT2i, dapagliflozin exhibited a stronger protective effect in preventing mortality without a significant difference in SAEs. SGLT2i treatment was also associated with a lower risk of renal events, balanced against a higher risk of genitourinary infections and volume depletion. Crucially, there was no difference in the rate of drug discontinuation between the SGLT2i treatment and placebo groups. These results provide valuable insights into the potential benefits and risks of SGLT2i drugs in patients with HF.
Conflict of interest
Dr M.G.N. reports funding from the American College of Cardiology (George F and Ann Harris Bellows Foundation), the Patient-Centered Outcomes Research Institute, and the National Institute on Aging/National Institutes of Health (GEMSSTAR award: R03AG074067); and consulting from HeartFlow, Inc. Other authors had nothing to disclose.
Funding
None.




