Sodium-glucose cotransporter 2 inhibitors are associated with reduced risk of mortality and readmissions in heart failure
Abstract
Aims
Compelling evidence from randomized trials has shown that sodium-glucose cotransporter 2 inhibitors (SGLT2is) are effective in heart failure (HF) across the spectrum of left ventricular ejection fractions. However, there are very few studies with real-world data.
Methods and results
A retrospective cohort analysis was performed based on patient-level data from the Swedish Heart Failure Registry (SwedeHF) linked with three other national registers. Patients included had an index registration between 3 September 2013 and 31 December 2020 in SwedeHF and were on treatment with guideline-recommended therapy without or with SGLT2i 3 months before or 6 months after their index registration. Endpoints were mortality or readmissions. Association between the use of SGLT2i and endpoints was studied using adjusted Cox models. In the overall cohort, 796/22 405 patients were included with/without SGLT2i. In patients with SGLT2i, 93.5% had diabetes mellitus. In the overall cohort, SGLT2i was statistically significantly associated with all-cause mortality {hazard ratio [HR]: 0.61 [95% confidence interval (CI) 0.48–0.79], P < 0.0001}, cardiovascular mortality [HR: 0.29 (95% CI 0.17–0.50), P < 0.0001], cardiovascular mortality or HF readmission [HR: 0.89 (95% CI 0.80–1.00), P = 0.046], and all-cause readmissions [HR: 0.90 (95% CI 0.81–0.99), P = 0.038]. Similar results were obtained for the diabetes cohort. However, no association with cause-specific readmissions was observed.
Conclusions
This nationwide real-world study indicates that patients with HF, in which majority coexisted with diabetes mellitus, who received SGLT2i were statistically significantly associated with lower risk for all-cause mortality, cardiovascular mortality, cardiovascular mortality or HF readmissions, and all-cause readmissions, in line with the randomized trials assessing SGLT2i.
Introduction
A vast body of research has shown the beneficial effects of sodium-glucose cotransporter 2 inhibitors (SGLT2is) in heart failure (HF) patients across the spectrum of left ventricular ejection fractions (LVEFs), with or without diabetes mellitus, and therefore supports a foundational role of SGLT2i in HF.1-5 To date, dapagliflozin and empagliflozin have shown significantly reduced cardiovascular (CV) death or HF events in HF regardless of LVEF.3-6 A comprehensive meta-analysis of five randomized controlled trials (RCTs) demonstrated that SGLT2i reduced the risk of composite CV death or hospitalization for HF, CV death, and all-cause mortality in a broad spectrum of patients with HF.6 In Sweden, SGLT2i was introduced in 2012, mainly in patients with type 2 diabetes mellitus (T2DM). However, because the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus (EMPA-REG OUTCOME) study in 2015 showed that empagliflozin reduced the risk of major adverse CV events, CV and all-cause death, and hospitalization for HF compared with placebo,7 the use of SGLT2i increased in HF patients.
Data on the real-world effectiveness of SGLT2i in HF are largely lacking except for a small study from 2016 to 2018.8 Despite RCTs remaining the gold standard for assessing efficacy, real-world analyses can provide evidence supplementing clinical trial data. Sweden has several comprehensive nationwide data sets that facilitate the identification of clinical outcomes in patients with HF. Given the granularity and coverage of these databases, registry-based findings in Sweden provide one of the world's most reliable real-world observations. This study used patient-level data from the Swedish Heart Failure Registry (SwedeHF) linked to clinical outcomes derived from three other National Administrative Health Registries (the National Patient Register, the Cause of Death Register, and the Swedish Prescribed Drug Register) to assess the real-world effectiveness of SGLT2i in patients with HF across the spectrum of ejection fraction (EF) on top of guideline-directed medical treatment.
Methods
Study design and data source
A retrospective cohort analysis was performed in patients with HF in Sweden based on patient-level data from the SwedeHF, including patients since 11 May 2000. SwedeHF has been described previously.9 The inclusion criterion was clinician-judged HF up to 2017 and since 2017 by the following International Classification of Diseases, 10th revision (ICD-10) codes: I50.0, I50.1, I50.9, I42.0, I42.6, I42.7, I25.5, I11.0, I13.0, and I13.2. Approximately 80 variables are registered at discharge from the hospital or after an outpatient clinic visit and are entered into SwedeHF.
The linking of SwedeHF and the registries above was approved by the Swedish Ethical Review Authority.
The study complied with the Declaration of Helsinki. For the SwedeHF, individual patient consent is not required, but patients are informed of entry into the SwedeHF and allowed to opt out of being included in the database. Eligible study patients were those recorded with an index registration (i.e. the date of registration in the SwedeHF between 3 September 2013 and 31 December 2020) and were treated with triple medications: angiotensin-converting enzyme inhibitor (ACE-I)/angiotensin receptor blocker (ARB)/angiotensin receptor neprilysin inhibitor (ARNI), beta-blocker (BB), and mineralocorticoid receptor antagonist (MRA) (overall cohort). This cohort was studied in the following subgroups: overall cohort with or without SGLT2i and diabetes mellitus cohort with or without SGLT2i. Use of SGLT2i was identified either <3 months before or 6 months after the index registration in the SwedeHF. Medications were retrieved from the SwedeHF and the Swedish Prescribed Drug Register based on dispensation date in relation to the index registration and defined according to the ICD-10 codes. Anatomic Therapeutic Chemical (ATC) codes and ICD-10 codes are listed in Supporting Information, Table S1.
Information about patients' comorbidities was obtained from the SwedeHF or the National Patient Register. Mortality data for Swedish residents, irrespective of citizenship or country of death, were obtained from the Cause of Death Register and used to determine the end of follow-up in patients who died during the study period.
Study outcomes
Outcomes included all-cause mortality, CV mortality, composite of CV mortality or HF readmissions, and all-cause and cause-specific (CV and HF) hospital readmissions. Incidence of outcomes and time to events were studied and followed up for 3 years from the index registration to censoring or respective event date to be comparable with clinical trials performed on SGLT2i.
Statistical methods
Descriptively, continuous data were presented as mean and standard deviation or median and range, as applicable; categorical data were summarized as number (n) and percentage (%). Baseline data between patients with and without SGLT2i were tested using the Mann–Whitney U-test for continuous variables, the Mantel–Haenszel χ2 trend test for ordered categorical variables, and Fisher's exact test for dichotomous variables.
Event rates were computed as the number of events divided by the number of follow-up years and expressed per 100 person-years. Exact Poisson limits were used to compute 95% confidence intervals (CIs) for event rates. Time-to-event data were studied using Cox regression, adjusting for age, sex, body mass index (BMI) (<18.5, 18.5–24.9, 25–29.5, 30–35, >35 kg/m2, or unknown), LVEF (<30%, 30–39%, 40–49%, ≥50%, or unknown), N-terminal pro-B-type natriuretic peptide (NT-proBNP) (≤900, 900–2500, 2501–5000, >5000 ng/L, or unknown), estimated glomerular filtration rate (eGFR) by using Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation that has been described previously10 (<60, ≥60 mL/min/1.73 m2, or unknown), hypertension, atrial fibrillation (AF), diabetes, implantable cardioverter-defibrillator (ICD), and cardiac resynchronization therapy (CRT). Proportional hazards were investigated and found satisfactory by a visual review of the log(−log(survival)) vs. log(follow-up time) for SGLT2i.
For the entire cohort in which all have HF (overall cohort), two sub-cohorts were created: one sub-cohort of HF patients with coexisting diabetes mellitus (diabetes mellitus cohort) and another sub-cohort of HF patients with diabetes mellitus and LVEF < 40%.
Pre-specified interaction analyses between baseline variables and the use of SGLT2i on all-cause mortality and the composite of CV mortality or HF readmissions were performed. The baseline variables included in the analyses were sex, age, BMI, LVEF, eGFR, NT-proBNP, AF, diabetes mellitus, ICD, CRT, and diuretics.
All statistical analyses were two-sided, and P-values of <0.05 were considered significant. All analyses were performed using SAS software Version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient population
In total, 50 663 patients were identified. Figure 1 depicts the study's inclusion and exclusion criteria. Finally, 22 405 (EF < 40%, 65.0%; EF 40–49%, 18.9%; EF ≥ 50%, 16.1%) in the overall group and 5948 (EF < 40%, 60.6%; EF 40–49%, 19.4%; EF ≥ 50%, 20.1%) in the diabetes mellitus group without SGLT2i were included. For those treated with SGLT2i, there are 796 patients (EF < 40%, 74.6%; EF 40–49%, 14.9%; EF ≥ 50%, 10.4%) in the overall group and 744 patients (EF < 40%, 74.0%; EF 40–49%, 15.4%; EF ≥ 50%, 10.5%) in the diabetes mellitus group.
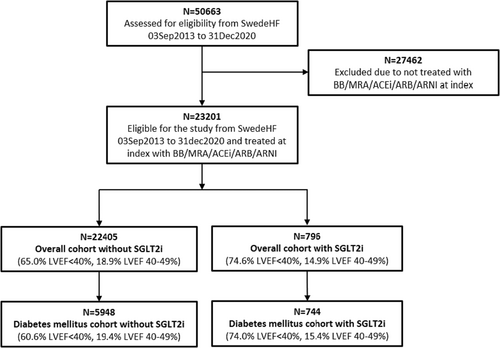
Baseline characteristics
Prescription of SGLT2i was 3.4% in the overall cohort and 11.1% in the diabetes mellitus cohort. In the overall cohort treated with SGLT2i, 93.5% had diabetes mellitus. Their baseline characteristics and medications are presented in Table 1. Baseline data for the diabetes mellitus subgroup with LVEF < 40% are presented in Supporting Information, Table S2. Those treated with SGLT2i have similar clinical characteristics and medications as in the diabetes mellitus sub-cohort with SGLT2i, except that they were more frequently treated with ARNI (45%).
| Variable | Overall cohort | Diabetes mellitus cohort | ||||
|---|---|---|---|---|---|---|
| Without SGLT2i | With SGLT2i | P-value | Without SGLT2i | With SGLT2i | P-value | |
| N = 22 405 | N = 796 | N = 5948 | N = 744 | |||
| Patient demographics | ||||||
| Male | 14 783 (66.0%) | 640 (80.4%) | <0.0001 | 4017 (67.5%) | 605 (81.3%) | <0.0001 |
| Age at SwedeHF index visit | 72.3 ± 11.7 | 67.3 ± 10.1 | <0.0001 | 73.6 ± 9.8 | 67.6 ± 9.9 | <0.0001 |
| Age ≥ 70 years | 14 289 (63.8%) | 350 (44.0%) | <0.0001 | 4099 (68.9%) | 335 (45.0%) | <0.0001 |
| Clinical data at the index visit | ||||||
| HF duration ≥ 6 months | 9842 (45.8%) | 351 (45.6%) | 0.97 | 3074 (54.3%) | 338 (47.1%) | 0.0003 |
| Weight (kg) | 83.4 ± 19.6 | 93.5 ± 21.2 | <0.0001 | 88.3 ± 19.6 | 93.7 ± 20.9 | <0.0001 |
| Body mass index (kg/m2) | 28.1 ± 9.2 | 30.4 ± 6.1 | <0.0001 | 30.0 ± 9.5 | 30.4 ± 6.0 | 0.0098 |
| Body mass index > 30 (kg/m2) | 5359 (30.4%) | 313 (47.7%) | <0.0001 | 2031 (42.8%) | 294 (48.1%) | 0.013 |
| Systolic blood pressure (mmHg) | 126.3 ± 20.7 | 122.8 ± 18.8 | 0.0001 | 127.4 ± 20.2 | 123.4 ± 18.7 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.4 ± 12.2 | 73.2 ± 10.5 | 0.10 | 72.9 ± 11.7 | 73.2 ± 10.5 | 0.23 |
| Heart rate (b.p.m.) | 72.7 ± 15.2 | 74.9 ± 14.5 | <0.0001 | 73.5 ± 14.2 | 75.2 ± 14.6 | 0.0080 |
| NYHA functional class | 0.21 | 0.0048 | ||||
| I | 1493 (9.2%) | 43 (6.8%) | 250 (6.2%) | 40 (6.8%) | ||
| II | 8027 (49.5%) | 319 (50.5%) | 1791 (44.4%) | 293 (50.0%) | ||
| III | 6353 (39.2%) | 259 (41.0%) | 1873 (46.4%) | 243 (41.5%) | ||
| IV | 349 (2.2%) | 11 (1.7%) | 121 (3.0%) | 10 (1.7%) | ||
| LVEF (%) | <0.0001 | <0.0001 | ||||
| ≥50% | 3411 (16.1%) | 81 (10.4%) | 1124 (20.1%) | 76 (10.5%) | ||
| 40 to <50% | 3997 (18.9%) | 116 (14.9%) | 1087 (19.4%) | 112 (15.4%) | ||
| 30 to <40% | 6722 (31.8%) | 283 (36.5%) | 1748 (31.2%) | 270 (37.2%) | ||
| <30% | 7025 (33.2%) | 296 (38.1%) | 1645 (29.4%) | 267 (36.8%) | ||
| Potassium (mmol/L) | 4.29 ± 0.43 | 4.31 ± 0.44 | 0.22 | 4.32 ± 0.45 | 4.32 ± 0.44 | 0.99 |
| NT-proBNP (ng/L) | 2335 (8–150 368) | 1517 (50–35 000) | <0.0001 | 2326 (8–150 368) | 1479 (50–35 000) | <0.0001 |
| NT-proBNP cat. (ng/L) | <0.0001 | <0.0001 | ||||
| ≤900 | 3812 (21.6%) | 207 (32.6%) | 914 (19.8%) | 197 (33.2%) | ||
| >900–2500 | 5434 (30.8%) | 211 (33.2%) | 1512 (32.7%) | 196 (33.1%) | ||
| >2500–5000 | 4056 (23.0%) | 115 (18.1%) | 1063 (23.0%) | 104 (17.5%) | ||
| >5000 | 4329 (24.6%) | 102 (16.1%) | 1132 (24.5%) | 96 (16.2%) | ||
| eGFR (CKD-EPI) | 67.1 ± 20.5 | 73.5 ± 20.3 | <0.0001 | 64.2 ± 21.1 | 73.2 ± 20.4 | <0.0001 |
| eGFR (CKD-EPI) ≥ 60 | 9513 (62.2%) | 445 (72.8%) | <0.0001 | 2089 (55.4%) | 411 (72.1%) | <0.0001 |
| Ischaemic aetiology | 6755 (39.0%) | 355 (56.2%) | <0.0001 | 2343 (49.4%) | 337 (56.9%) | 0.0006 |
| Medical history at the index visit | ||||||
| Hypertension | 16 484 (73.6%) | 674 (84.7%) | <0.0001 | 5316 (89.4%) | 646 (86.8%) | 0.039 |
| Atrial fibrillation | 12 894 (57.5%) | 374 (47.0%) | <0.0001 | 3552 (59.7%) | 355 (47.7%) | <0.0001 |
| Chronic obstructive pulmonary disease | 4120 (18.4%) | 130 (16.3%) | 0.15 | 1293 (21.7%) | 121 (16.3%) | 0.0005 |
| Diabetes mellitus | 5948 (26.5%) | 744 (93.5%) | <0.0001 | 5948 (100.0%) | 744 (100.0%) | |
| Stroke/TIA | 3110 (13.9%) | 121 (15.2%) | 0.30 | 1019 (17.1%) | 116 (15.6%) | 0.30 |
| Psychiatric diagnoses in the past 3 years before admission | 3102 (13.8%) | 104 (13.1%) | 0.57 | 818 (13.8%) | 96 (12.9%) | 0.57 |
| Musculoskeletal diseases in the past 3 years before admission | 4025 (18.0%) | 108 (13.6%) | 0.0011 | 1165 (19.6%) | 100 (13.4%) | <0.0001 |
| Malignant cancer in the past 3 years before admission | 2232 (10.0%) | 69 (8.7%) | 0.25 | 626 (10.5%) | 66 (8.9%) | 0.18 |
| ICD | 1792 (8.1%) | 103 (13.0%) | <0.0001 | 497 (8.5%) | 98 (13.3%) | <0.0001 |
| CRT | 1185 (5.4%) | 50 (6.3%) | 0.23 | 323 (5.5%) | 47 (6.4%) | 0.35 |
| Medications | ||||||
| ARNI dispensed 3 months before to 6 months after the index date | 2678 (12.0%) | 294 (36.9%) | <0.0001 | 658 (11.1%) | 264 (35.5%) | <0.0001 |
| ACE-I/ARB dispensed 3 months before to 6 months after the index date | 21 627 (96.5%) | 689 (86.6%) | <0.0001 | 5748 (96.6%) | 651 (87.5%) | <0.0001 |
| Diuretics | 13 423 (77.0%) | 572 (74.7%) | 0.15 | 3933 (85.6%) | 536 (75.1%) | <0.0001 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta-blocker; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SGLT2i, sodium-glucose cotransporter 2 inhibitor; SwedeHF, Swedish Heart Failure Registry; TIA, transient ischaemic attack.
- Data are presented as mean ± standard deviation or median (range) or number (percentage). For tests between two groups with respect for dichotomous variables, Fisher's exact test was used; for ordered categorical variables, the Mantel–Haenszel χ2 trend test was performed; and for continuous variables, the Mann–Whitney U-test was performed.
All-cause mortality with vs. without sodium-glucose cotransporter 2 inhibitors: overall cohort and diabetes mellitus cohort
In the overall HF cohort, all-cause mortality occurred in 4625/22 405 (20.6%) patients without SGLT2i and in 64/796 (8.0%) with SGLT2i within 3 years of index registration. SGLT2i was associated with a significant 39% reduction in all-cause mortality compared with those treated without SGLT2i [adjusted hazard ratio (aHR): 0.61 (95% CI 0.48–0.79), P = 0.0001]. This result was accompanied by a decrease in the crude event rate (95% CI) per 100 person-years from 9.9 (9.6–10.2) without SGLT2i to 5.2 (4.0–6.6) with SGLT2i (Table 2).
| Endpoint (followed up 3 years from the index date) | Without SGLT2i | With SGLT2i | With vs. without SGLT2i | |||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) events | Follow-up | Event rate (95% CI) per 100 person-years | n/N (%) events | Follow-up | Event rate (95% CI) per 100 person-years | Hazard ratio (95% CI)a | P-value | |
| Median (IQR) | Median (IQR) | |||||||
| Overall cohort | ||||||||
| All-cause mortality | 4625/22 405 (20.6%) | 2.38 (1.23–3.00) | 9.9 (9.6–10.2) | 64/796 (8.0%) | 1.37 (0.82–2.36) | 5.2 (4.0–6.6) | 0.61 (0.48–0.79) | 0.0001 |
| CV mortality | 2332/22 405 (10.4%) | 2.38 (1.23–3.00) | 5.0 (4.8–5.2) | 14/796 (1.8%) | 1.37 (0.82–2.36) | 1.1 (0.6–1.9) | 0.29 (0.17–0.50) | <0.0001 |
| CV mortality or HF readmission | 12 581/22 405 (56.2%) | 0.97 (0.26–2.31) | 43.7 (43.0–44.5) | 356/796 (44.7%) | 0.75 (0.25–1.48) | 45.1 (40.6–50.1) | 0.89 (0.80–1.00) | 0.046 |
| HF readmission | 12 114/22 405 (54.1%) | 0.97 (0.26–2.31) | 42.1 (41.4–42.9) | 355/796 (44.6%) | 0.75 (0.25–1.48) | 45.0 (40.4–49.9) | 0.92 (0.82–1.03) | 0.13 |
| CV readmission | 13 827/22 405 (61.7%) | 0.79 (0.20–1.95) | 54.0 (53.1–54.9) | 416/796 (52.3%) | 0.60 (0.20–1.31) | 58.6 (53.1–64.5) | 0.92 (0.84–1.02) | 0.13 |
| Any readmission | 14 285/22 405 (63.8%) | 0.75 (0.19–1.84) | 57.7 (56.8–58.7) | 422/796 (53.0%) | 0.60 (0.20–1.28) | 60.4 (54.8–66.5) | 0.90 (0.81–0.99) | 0.038 |
| Diabetes mellitus cohort | ||||||||
| All-cause mortality | 1633/5948 (27.5%) | 2.34 (1.20–3.00) | 13.3 (12.7–13.9) | 61/744 (8.2%) | 1.44 (0.85–2.46) | 5.1 (3.9–6.6) | 0.57 (0.44–0.74) | <0.0001 |
| CV mortality | 797/5948 (13.4%) | 2.34 (1.20–3.00) | 6.5 (6.0–7.0) | 14/744 (1.9%) | 1.44 (0.85–2.46) | 1.2 (0.6–2.0) | 0.27 (0.16–0.46) | <0.0001 |
| CV mortality or HF readmission | 3899/5948 (65.6%) | 0.77 (0.19–1.96) | 57.8 (56.0–59.7) | 340/744 (45.7%) | 0.78 (0.25–1.56) | 45.0 (40.4–50.1) | 0.88 (0.78–0.98) | 0.023 |
| HF readmission | 3747/5948 (63.0%) | 0.77 (0.19–1.96) | 55.6 (53.8–57.4) | 339/744 (45.6%) | 0.78 (0.25–1.56) | 44.9 (40.2–49.9) | 0.90 (0.81–1.01) | 0.08 |
| CV readmission | 4209/5948 (70.8%) | 0.59 (0.15–1.60) | 72.0 (69.8–74.2) | 397/744 (53.4%) | 0.64 (0.20–1.33) | 58.6 (53.0–64.7) | 0.91 (0.81–1.01) | 0.06 |
| Any readmission | 4313/5948 (72.5%) | 0.55 (0.15–1.52) | 76.4 (74.1–78.7) | 403/744 (54.2%) | 0.62 (0.20–1.32) | 60.5 (54.8–66.7) | 0.88 (0.79–0.98) | 0.017 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta-blocker; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium-glucose cotransporter 2 inhibitor; SwedeHF, Swedish Heart Failure Registry.
- All endpoints are followed up to 3 years from the index visit. CIs for unadjusted event rates per 100 person-years are obtained from exact Poisson confidence limits. Cox regression was used for time-to-event analysis and was presented by HR (95% CI).
- a Adjusted for age, sex, body mass index categorized (cat.), left ventricular ejection fraction, N-terminal pro-B-type natriuretic peptide cat., estimated glomerular filtration rate cat., hypertension, atrial fibrillation, diabetes, implantable cardioverter-defibrillator, and cardiac resynchronization therapy.
In the HF cohort with diabetes mellitus, all-cause mortality was seen in 1633/5948 (27.5%) patients without SGLT2i and in 61/744 (8.2%) with SGLT2i within 3 years of index registration. Patients treated with SGLT2i resulted in a significant 43% reduction in all-cause mortality compared with those without SGLT2i [aHR: 0.57 (95% CI 0.44–0.74), P < 0.0001] with a decrease in crude event rate (95% CI) per 100 person-years from 13.3 (12.7–13.9) without SGLT2i to 5.1 (3.9–6.6) with SGLT2i (Table 2).
In the HF and diabetes mellitus cohort and EF < 40%, patients treated with SGLT2i showed a significant (38%) reduction in all-cause mortality compared with those without SGLT2i [HR: 0.62 (95% CI 0.46–0.85), P = 0.0023]. We observed a decrease in the crude event rate (95% CI) per 100 person-years from 11.8 (11.0–12.6) without SGLT2i to 5.4 (4.0–7.3) with SGLT2i. The crude percentage of events decreased from 24.8% without SGLT2i to 8.4% with SGLT2i (Supporting Information, Table S3).
Cardiovascular death with vs. without sodium-glucose cotransporter 2 inhibitors: overall cohort and diabetes cohort
In the overall HF cohort, CV mortality occurred in 2332/22 405 (10.4%) patients treated without SGLT2i and in 14/796 (1.8%) with SGLT2i within 3 years of index registration. SGLT2i was associated with a significant (71%) reduction in CV mortality compared with those treated without SGLT2i [aHR: 0.29 (95% CI 0.17–0.50), P < 0.0001]. The crude event rate (95% CI) decreased from 5.0 (4.8–5.2) without SGLT2i to 1.1 (0.6–1.9) with SGLT2i per 100 person-years (Table 2).
In the diabetes mellitus cohort, CV mortality occurred in 797/5948 (13.4%) patients treated without SGLT2i and in 14/744 (1.9%) with SGLT2i within 3 years of index registration. SGLT2i was associated with a significant reduction of 73% in CV mortality compared with treatment without SGLT2i [aHR: 0.27 (95% CI 0.16–0.46), P < 0.0001]. A decrease in the crude event rate (95% CI) per 100 person-years from 6.5 (6.0–7.0) without SGLT2i to 1.2 (0.6–2.0) with SGLT2i was observed (Table 2).
In the HF and diabetes mellitus cohort and EF < 40%, SGLT2i was associated with a significant reduction (66%) in CV mortality compared with those treated without SGLT2i [HR: 0.34 (95% CI 0.19–0.60), P = 0.0002]. We found a decrease in the crude event rate (95% CI) per 100 person-years from 6.1 (5.5–6.7) without SGLT2i to 1.4 (0.7–2.5) with SGLT2i and a decrease in the crude percentage of events from 12.8% without SGLT2i to 2.2% with SGLT2i (Supporting Information, Table S3).
Cardiovascular mortality or heart failure hospitalization with vs. without sodium-glucose cotransporter 2 inhibitors: overall cohort and diabetes cohort
In the overall HF cohort, a composite endpoint of CV death or HF readmission occurred in 12 581/22 405 [56.2%, crude event rate 43.7 (95% CI 43.0–44.7)] patients without SGLT2i and in 356/796 [44.7%, crude event rate 45.1 (95% CI 40.6–50.1)] with SGLT2i. Patients treated with SGLT2i showed a significant reduction of 11% in this composite endpoint compared with those without SGLT2i [aHR: 0.89 (95% CI 0.80–1.00), P = 0.046] (Table 2).
In the diabetes mellitus cohort, a composite endpoint of CV death or HF hospitalization occurred in 3899/5948 [65.6%, crude event rate 57.8% (95% CI 56.0–59.7)] patients treated without SGLT2i and in 340/744 [45.7%, crude event rate 45.0 (95% CI 40.4–50.1)] with SGLT2i. SGLT2i was associated with a significant (12%) reduction in this endpoint compared with those treated without SGLT2i [aHR: 0.88 (95% CI 0.78–0.98), P = 0.023] (Table 2).
All-cause and cause-specific hospitalizations with vs. without sodium-glucose cotransporter 2 inhibitors: overall cohort and diabetes cohort
In the overall HF cohort, all-cause readmissions within 3 years from index registration occurred in 53.0% of patients treated with SGLT2i and 63.8% of patients without SGLT2i [aHR: 0.90 (95% CI 0.81–0.99), P = 0.038]. For treatment without SGLT2i, CV readmissions occurred in 61.7% of the patients and HF readmissions in 54.1%. For patients treated with SGLT2i, CV and HF readmissions occurred in 52.3% and 44.6% of patients, respectively. No statistically significant differences were detected.
Similarly, in the diabetes mellitus cohort, all-cause readmissions were significantly less frequent in patients treated with SGLT2i (54.2%) than in those without SGLT2i (72.5%) [aHR: 0.88 (95% CI 0.79–0.98), P = 0.017]. The number of CV and HF readmissions was numerically but not significantly lower in patients treated with SGLT2i than in those without SGLT2i (Table 2).
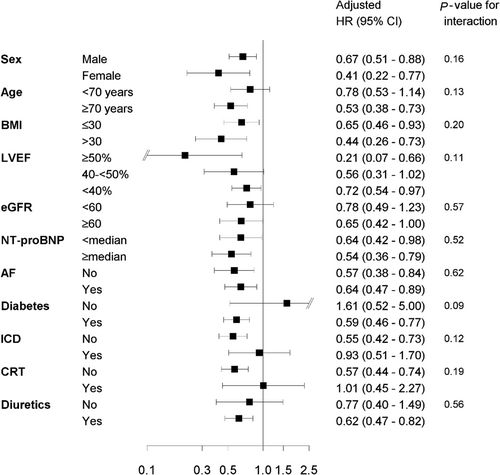
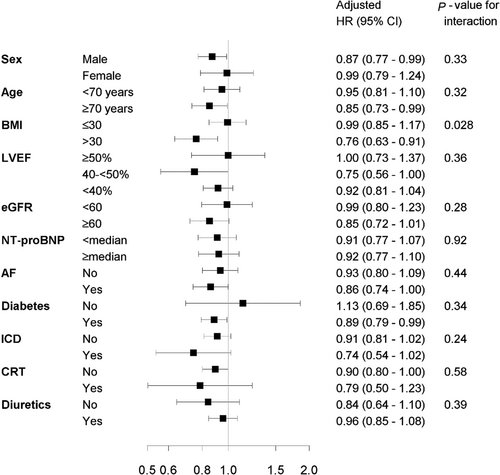
Interactions between sodium-glucose cotransporter 2 inhibitors and selected baseline characteristics on all-cause mortality, and cardiovascular mortality or heart failure readmissions
Associations related to SGLT2i and its interactions on all-cause mortality (Figure 2) and the composite endpoint of CV mortality or HF readmissions (Figure 3) were investigated for sex, age, BMI, LVEF, eGFR, NT-proBNP, AF, diabetes, ICD, CRT, and diuretics. No significant interactions were observed, except for that between SGLT2i and BMI (≤30 vs. >30 kg/m2) on the composite endpoint. For BMI ≤ 30 kg/m2, the aHR for SGLT2i vs. no SGLT2i was 0.99 (95% CI 0.85–1.17); for BMI > 30 kg/m2, the aHR for SGLT2i vs. no SGLT2i was 0.76 (95% CI 0.63–0.91), P for interaction = 0.028.
Discussion
Our study shows that SGLT2i in a real-world nationwide Swedish HF population was associated with a significant risk reduction in all-cause mortality, CV mortality, composite endpoint CV mortality or HF readmissions, and all-cause readmissions. We believe this is one of the few studies reporting the real-world effectiveness of SGLT2i in HF. These findings are in line with randomized trials with SGLT2i in HF, regardless of EF.
Our results are consistent in the overall and diabetes mellitus cohorts. Most patients treated with SGLT2i have diabetes mellitus because SGLT2i was indicated for the prevention of HF earlier in T2DM patients, as early as when the EMPA-REG OUTCOME study had shown significantly lower risks of death from any cause and for hospitalization for HF than those in a placebo group.7 However, the first outcome trial (DAPA-HF) in HF patients was published in 2019.3 Since 2016, SGLT2i treatment has been used in patients with both HF and T2DM in Sweden, and data for the current analysis were retrieved from the Swedish Prescribed Drug Register linked to the SwedeHF.
Our study differs from previous randomized trials in several aspects. First, we assess the association of effect with SGLT2i in all HF patients regardless of EF. Second, our patients are from a real-world setting without exclusions, implying a significantly higher event rate. Third, all our patients were on top of treatment with BB, MRA, and at least one of the ACE-I/ARB/ARNI medications to show the additional effect of SGLT2i. Fourth, 93.5% of our HF patients treated with SGLT2i have coexisting diabetes mellitus. Fifth, in our cohort, 54% had an HF duration < 6 months. Finally, our patients are sicker with higher NT-proBNP levels and 41% with New York Heart Association class III.
Our results show a greater reduction in all-cause and CV mortality than in randomized trials. We are aware that our study design cannot determine efficacy rather than association. However, the magnitude of the effectiveness of SGLT2i in the real world appears larger. There are undoubtedly many explanations for this event. One possible reason is that all our patients from the real world have much higher event rates compared with those reported in randomized trials. For instance, the incidence for the composite endpoint of CV mortality or HF readmission was 21.2% in the DAPA-HF, 24.7% in the EMPEROR-Reduced, 17.1% in the EMPEROR-Preserved, and 19.5% in the DELIVER. In contrast, in our study, which contains all HF regardless of the HF event, the incidence over a 3 year period is almost three times as high (i.e. 60.0%).3-5, 11
Similarly, for all-cause mortality, the incidence was 13.9% in the DAPA-HF, 14.2% in the EMPEROR-Reduced, 14.3% in the EMPEROR-Preserved, and 16.8% in the DELIVER, whereas in our study, it was 20.6%. This difference in incidence between our study and that in the randomized trials becomes more pronounced in our diabetes cohort. In our diabetes cohort, CV mortality was 13.4%, all-cause mortality 27.5%, and HF readmission 63.0%. Another explanation is that randomized trials with SGLT2i in HF included more stable HF patients with fewer or less severe comorbidities. This argument is supported by the higher proportion of CV mortality among all-cause mortality: 87% in the DAPA-HF, 76% in the EMPEROR-Reduced, 57% in the EMPEROR-Preserved, and 49% in the DELIVER. HF readmission, however, is only a small portion of all-cause readmissions. For instance, in the EMPEROR-Preserved, HF readmissions represented <20% of total readmissions, which is not the case in our study. In the overall cohort, CV mortality constitutes 46% of all-cause mortality, whereas HF readmissions represent more than two-thirds of all-cause readmissions. This observation is in accordance with our previous study showing that in a real-world cohort of HF patients, frequent hospital readmissions occurred in the early post-discharge period, mainly driven by worsening HF that constituted 63% of all-cause readmissions.12 Despite the possibility that miscoding of HF readmissions may occur in a registry database, it is still prominent in all-cause readmissions.
The event rates in our study are comparable with our recent study of the real-world effectiveness of ARNI in which the event rate of all-cause death was 9.3 (8.1–10.7), all-cause readmission 46.5 (43.0–50.2), and CV readmission 43.8 (40.4–47.3) per 100 person-years with ACE-I/ARB in a propensity score 1:1 ratio matched cohort with ARNI.13
Our results contrast with a previous publication from the SwedeHF database showing that SGLT2i was associated with lower mortality and morbidity.8 Still, our study extends this previous study by including more HF patients treated with SGLT2i, a longer study period, and the inclusion of three cohorts (overall cohort, diabetes mellitus cohort, and cohort with EF < 40%). Moreover, our study is strikingly different from a previous study in which patients treated with SGLT2i were eligible if a dispensation was recorded in the Dispensed Drug Registry in the 5 months before or 14 days after the date of registration in the SwedeHF.8 Our study included patients only if they were fully treated with ACE-I/ARB/ARNI, BB, and MRA and also with SGLT2i <3 months before or 6 months after their index registration in the SwedeHF. Six months after index registration was necessary because triple treatment is required to assess additional effects of SGLT2i. This is because it takes time to up-titrate triple treatments in elderly patients and real-world settings. In the former case, HF readmission is less prominent in all-cause readmissions (e.g. <20% of total hospitalizations in the EMPEROR-Preserved). However, in our cohort, HF hospitalization represents 87% of all-cause readmissions. Additionally, the difference in all-cause readmissions in those treated with vs. those treated without SGLT2i in both the overall cohort and the diabetes cohort indicates that SGLT2i was associated with reduced hospital readmissions without the influence of miscoding.
In our study, the majority of the study population is HF with reduced EF and diabetes mellitus, because of earlier indication of SGLT2i only in diabetes mellitus, in contrast with later HF trials that included only around 45% diabetes. However, the aim of this study is to provide information about the real-world effectiveness of SGLT2i in HF as they are largely lacking at the present moment. Therefore, we have neither intention nor possibility to study real-world effectiveness in an HF population comparable with SGLT2i HF trials because such indication is brand new and it takes some time to have data collected. Indeed, we showed that SGLT2i use was associated with significant relative risk reduction in CV mortality. However, no association with reduced HF hospitalization was observed. This is a common challenge with registry study as it does not permit validation at the patient level, being different from RCTs in which there is the Adjudication Committee to verify every endpoint event. Therefore, mortality is the most reliable outcome data for registry studies.
Strengths and limitations
The strengths of this study are the access to several large, high-quality national registries linked to each other and the identification of clinical data, data on drug dispensation, hospital readmissions, and mortality.
One major limitation is the small sample size of those treated with SGLT2i, which does not permit subgroup analysis for EF 41–49% and EF > 50%. This is partly because we enrolled only patients with EF > 50% who were on ACE-I/ARB/ARNI, BB, and MRA. If patients with EF > 50% who did not take ACE-I/ARB/ARNI, BB, or MRA had not been excluded, the number of HF with EF > 50% would have been much higher. Moreover, the overall patient group of chronic HF with SGLT2i consists mainly of the subgroup of patients with diabetes, which makes this study less representative to those without diabetes.
Another limitation is that we could not verify the diagnosis individually. For instance, there was undoubtedly wrong coding between type 1 diabetes mellitus and T2DM. However, because SGLT2i was initially approved to treat T2DM in 2012, T2DM was likely the most common in this patient cohort. This possibility should not affect our results, though. On the contrary, it can strengthen our finding that SGLT2i is effective in patients with HF, regardless of EF and type of diabetes mellitus. Because of the restriction of coverage of the SwedeHF, not all patients with HF-initiated SGLT2i were reported to the SwedeHF. To address this issue, we limited patients to those who received SGLT2i <3 months before and 6 months after the index date. In addition, we cannot be sure that patients continuously took medications throughout the study. Like many observational studies, socio-economic data were not included. Thus, there was no adjustment for these potentially confounding factors. Although the SwedeHF collects data from various variables, allowing us to perform extensive adjustments, we cannot rule out the presence of residual and unmeasured confounding. As mentioned, observational studies can only assess associations, not causal relationships. Previous studies have also shown that less sick patients, more males, and younger and better treated patients are enrolled in the SwedeHF,14 limiting the generalizability of our results.
Conclusions
In conclusion, this nationwide real-world study demonstrates that a population of patients with HF, in which majority coexisted with diabetes mellitus, who received SGLT2i as part of guideline-led Swedish clinical practice was associated with a statistically significant relative risk reduction in all-cause mortality, CV mortality, composite of CV mortality or HF hospitalization, and all-cause readmissions, in conformity with the outcomes of randomized trials assessing SGLT2i.
Conflict of interest
P.K. reports a fee for a lecture from Vifor and AstraZeneca. A.P. has nothing to disclose. U.D. reports research grants from AstraZeneca, Pfizer, Vifor, Boehringer Ingelheim, Boston Scientific, and Roche Diagnostics and consultancies/honoraria from Amgen, Pfizer, and AstraZeneca, all outside the submitted work. M.F. has received a research grant from AstraZeneca and a fee for lecture and as a member of the advisory board from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk, as well as the Swedish National Coordinator for study EMPULSE (Boehringer Ingelheim) and study STEP-HFpEF DM (Novo Nordisk).
Funding
None.




