Effects of septal myectomy on left atrial and left ventricular function in obstructive hypertrophic cardiomyopathy
Abstract
Aims
Mechanical function of the left atrium (LA) and the left ventricle (LV) has been demonstrated to be a prognostic factor in patients with hypertrophic cardiomyopathy (HCM). We explore whether myocardial mechanical function can be improved by septal reduction therapy in symptomatic obstructive HCM.
Methods and results
Among 65 patients who underwent septal myectomy for symptomatic obstructive HCM from 2006 to 2022, 44 were analysed after excluding those who underwent simultaneous valve repair or replacement or maze operation. LA and LV functional variables including LA strain and LV global longitudinal strain were evaluated by two-dimensional and speckle-tracking echocardiography and compared before and 1 year after surgery. After septal myectomy, LA volume index (58.1 ± 18.3 vs. 45.3 ± 14.6 mL/m2, P = 0.001) decreased significantly. As LV end-systolic dimension increased after surgery, the LV ejection fraction decreased (73.8 ± 6.7 vs. 62.9 ± 8.3%, P < 0.001). LA strain (24.4 ± 9.3 vs. 30.5 ± 13.6%, P = 0.004) improved after septal myectomy, but LV global longitudinal strain deteriorated (−12.6 ± 3.6 vs. −11.6 ± 4.3%, P = 0.033), mainly related to worsening non-septal longitudinal strain (−14.4 ± 4.3 vs. −10.9 ± 8.4%, P = 0.005).
Conclusions
As haemodynamic loads due to LV outflow tract obstruction was relieved through surgical septal reduction therapy in patients with symptomatic obstructive HCM, there was a significant reduction in LA volume and restoration of LA mechanical dysfunction. However, LV mechanical dysfunction deteriorated even after surgical septal reduction therapy.
Introduction
Septal reduction therapies including septal myectomy and septal ablation generally accepted to be the gold standard treatment for symptomatic patients with obstructive hypertrophic cardiomyopathy (HCM) who are refractory to medical treatment.1, 2 Exercise intolerance improves after septal myectomy by increasing forward stroke volume and reducing left ventricular (LV) afterload, relieving the pressure gradient of the LV outflow track.1, 2 Previous studies have demonstrated myocardial reverse remodelling and functional changes after septal myectomy.3, 4 However, in the era of novel medical treatment for obstructive HCM, it is necessary to clarify the benefits of septal myectomy on myocardial function.
Impairment of LV global longitudinal strain (GLS) in HCM is a significant prognostic factor of future clinical events such as sudden cardiac death, heart failure, and ventricular arrhythmia.5-8 Left atrium (LA) size and function affected by progression of LV diastolic dysfunction also are prognostic factors in patients with HCM.9-12 Therefore, studying LA and LV mechanical functional changes after septal myectomy would be clinically important to predict prognosis in patients with obstructive HCM.
After septal myectomy, various factors such as reduction in the LV outflow track (LVOT) pressure gradient, extension of the QRS duration, and change in septal wall thickness are likely to have a combined effect on changes in mechanical function of the LA and LV. Therefore, we explore whether LA and LV functional variables, including strain values, improve after septal myectomy in patients with symptomatic obstructive HCM.
Methods
Study population
A total of 65 patients who underwent surgical septal myectomy for symptomatic obstructive HCM at a single tertiary centre from 2006 to 2022 were identified retrospectively. Baseline clinical records and electrocardiograms and echocardiograms before and after surgery were obtained from electronic medical records. To be eligible for septal myectomy, patients with symptomatic obstructive HCM should have echocardiographic findings of a peak LVOT pressure gradient ≥30 mmHg at rest or ≥50 mmHg during Valsalva manoeuvres and refractory symptom despite maximally tolerated medical treatment. All patients underwent transaortic extended septal myectomy. Patients who underwent concomitant valve repair or replacement or who had received a maze procedure were excluded. The postoperative echocardiograms 1 year after surgery were selected for analysis. Patients were excluded if preoperative and postoperative echocardiograms were not available for review. The final analysis involved 44 patients. The study was approved by the Institutional Review Board of Severance Hospital (IRB no. 4-2022-1575) and carried out in accordance with the principles of the Declaration of Helsinki. As this was a retrospective study and the data were analysed anonymously, informed consent of the study subjects was not required.
Echocardiography
Standard two-dimensional and Doppler measurements were performed following American Society of Echocardiography guidelines.13 Echocardiography follow-up was performed regularly after septal myectomy according to routine institutional practices, even without worsening symptoms or special reasons. HCM was clinically diagnosed based on the presence of unexplained myocardial hypertrophy (wall thickness ≥15 mm) in the absence of local or systemic aetiologies capable of producing the extent of hypertrophy evident.1 LVOT peak velocity at rest and during Valsalva manoeuvres were measured by continuous-wave Doppler echocardiography and LVOT peak pressure gradient were estimated using a simplified Bernoulli method: peak pressure gradient = 4 V2, where V is the LVOT peak velocity.14 LA volume was derived from standard apical four- and two-chamber views using the biplane method for disks.13 An LA volume index was calculated by dividing LA volume by body surface area.13 In patients with atrial fibrillation, Doppler echocardiographic parameters were derived from the average of three cardiac cycles when the R-R interval was relatively regular.
Two-dimensional speckle-tracking echocardiography
Speckle-tracking echocardiography was used to evaluate LA and LV myocardial function. The analysis was performed offline using vendor-independent post processing software (TomTec; Image Arena 4.6, Munich, Germany) by an independent investigator blinded to the clinical information. LV endocardial and epicardial borders were traced and user-defined to include the optimal myocardial layer at end-diastole and end-systole phases according to practical guidelines.15, 16 LV GLS was calculated from the average of 16 segmental strain values at each frame, LV septal strain was acquired by averaging four segmental (basal anteroseptal, mid anteroseptal, basal inferoseptal, and mid inferoseptal) values, and LV non-septal strain was obtained by averaging the other 12 segmental values. We defined the absolute value of LV GLS, septal longitudinal strain (LS), and non-septal LS as |LV GLS|, |septal LS|, and |non-septal LS|, respectively, to avoid confusion during statistical analysis. Apical four-chamber non-foreshortened views were used to measure LA strain as recommended in the consensus report.17, 18 LA endocardial borders were traced manually on the end-systolic frame and tracked throughout the cardiac cycle, generating a composite LA longitudinal strain curve. LA reservoir strain was measured at end-systole, just before mitral valve opening.11, 18 A representative case of LA and LV strain measurements is demonstrated in Figure 1. For patients with sinus rhythm, strain measurements were averaged over two cardiac cycles. In patients with atrial fibrillation, the values obtained from three cardiac cycles were averaged.
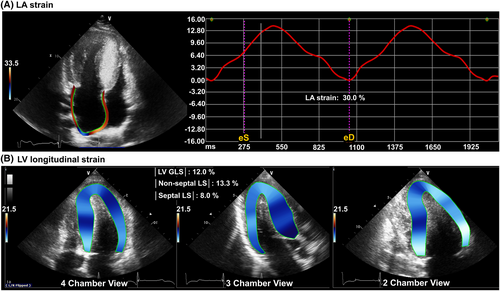
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation, and categorical data were presented as frequency and percentage. Echocardiographic and electrocardiographic parameters before and after septal myectomy were compared by paired Student's t-tests for continuous variables and χ2 tests for categorical variables. Pearson's correlation tests using correlation coefficient (r) were performed to evaluate the associations of preoperative or postoperative characteristics and myocardial functional variables. To determine the factors associated with reduction of LA volume index, univariate and multivariate regression analyses were performed. The variables selected for entry into multivariate analysis were those with a P-value <0.10 in univariate analysis and variables judged to have an effect on the dependent variable. P-values <0.05 were considered statistically significant. Data were analysed using SPSS (version 26.0, SPSS, Chicago, IL, USA).
Results
Baseline characteristics
Baseline characteristics of the study population are shown in Table 1. The mean age at the time of surgery was 52.8 ± 17.2 years, and females constituted half of the study group. Patients with hypertension accounted for 43.3% of the total, and nine patients (20.5%) showed atrial fibrillation. Most of the patients revealed New York Heart Association functional class II or III. The average LVOT peak pressure gradient before septal myectomy was 71 ± 37 mmHg at rest and 90 ± 34 mmHg at Valsalva provocation, while 79.5% patients were taking beta-blockers and 65.9% were taking calcium channel blockers.
| N = 44 | |
|---|---|
| Age at surgery, years | 52.8 ± 17.2 |
| Female, n (%) | 22 (50.0) |
| Body mass index, kg/m2 | 25.1 ± 4.4 |
| Hypertension, n (%) | 19 (43.2) |
| Diabetes mellitus, n (%) | 1 (2.3) |
| Dyslipidaemia, n (%) | 2 (4.5) |
| Atrial fibrillation, n (%) | 9 (20.5) |
| Systolic blood pressure, mmHg | 121.7 ± 14.2 |
| Diastolic blood pressure, mmHg | 71.4 ± 12.7 |
| Heart rate, b.p.m. | 63.3 ± 10.8 |
| NYHA functional class, n (%) | |
| Class I | 2 (4.5) |
| Class II | 18 (40.9) |
| Class III | 23 (52.3) |
| Class IV | 1 (2.3) |
| LVOT peak PG at rest, mmHg | 71 ± 37 |
| LVOT peak PG during Valsalva, mmHg | 90 ± 34 |
| Medications | |
| RAAS inhibitors, n (%) | 10 (22.7) |
| Loop diuretics, n (%) | 10 (22.7) |
| Beta-blockers, n (%) | 35 (79.5) |
| Calcium channel blockers, n (%) | 29 (65.9) |
| Anticoagulant, n (%) | 8 (18.2) |
| Antiplatelet, n (%) | 15 (34.1) |
- LVOT, left ventricular outflow track; NYHA, New York Heart Association; PG, pressure gradient; RAAS, renin-angiotensin-aldosterone system.
Change in left atrium structure and mechanical function
Preoperative and postoperative echocardiographic variables are compared in Table 2. Follow-up echocardiography tests were performed after a median of 12.0 months (interquartile range 9.0–15.0) after myectomy. The LA volume index decreased significantly after septal myectomy (61.5 ± 19.6 vs. 49.1 ± 17.8 mL/m2, P < 0.001). In terms of LA mechanical function, strain improved significantly after septal myectomy (24.4 ± 9.3 vs. 30.5 ± 13.6, P = 0.004). When subgroup analysis was conducted according to rhythm status, the decrease in LA volume index and the improvement of LA mechanical function after surgery were more evident in the sinus group (Figure 2 and 2).
| Preoperative | Postoperative | P-value | |
|---|---|---|---|
| Two-dimensional and Doppler echocardiography | |||
| LV EDD, mm | 44.4 ± 4.6 | 45.5 ± 5.2 | 0.131 |
| LV ESD, mm | 26.6 ± 3.9 | 30.6 ± 4.3 | <0.001 |
| LV septal thickness, mm | 23.8 ± 5.9 | 16.2 ± 4.5 | <0.001 |
| LV ejection fraction, % | 73.8 ± 6.7 | 62.9 ± 8.3 | <0.001 |
| LA volume index, mL/m2 | 61.5 ± 19.6 | 49.1 ± 17.8 | <0.001 |
| E, m/s | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.011 |
| A, m/s | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.062 |
| Deceleration time, ms | 214.7 ± 52.4 | 235.6 ± 69.0 | 0.072 |
| Septal e′, m/s | 3.5 ± 1.0 | 3.4 ± 0.9 | 0.392 |
| Septal E/e′ | 25.7 ± 10.5 | 23.0 ± 9.6 | 0.049 |
| TR max velocity, m/s | 2.6 ± 0.5 | 2.4 ± 0.5 | 0.065 |
| PASP, mmHg | 32.4 ± 9.5 | 30.5 ± 10.3 | 0.218 |
| Speckle tracking echocardiography | |||
| LV GLS, % | −12.6 ± 3.6 | −11.6 ± 4.3 | 0.033 |
| Septal LS, % | −7.1 ± 4.8 | −7.4 ± 3.8 | 0.631 |
| Non-septal LS, % | −14.4 ± 4.3 | −10.9 ± 8.4 | 0.005 |
| LA strain, % | 24.4 ± 9.3 | 30.5 ± 13.6 | 0.004 |
| Electrocardiography | |||
| Sinus rhythm, n (%) | 35 (79.5) | 36 (81.8) | 0.767 |
| Atrial fibrillation, n (%) | 9 (20.5) | 4 (9.1) | 0.096 |
| Permanent pacing, n (%) | 0 (0) | 4 (9.1) | <0.001 |
| QRS duration, ms | 107.9 ± 19.0 | 157.2 ± 21.8 | <0.001 |
- A, late diastolic mitral inflow velocity; E, early diastolic mitral inflow velocity; E/e, ratio of early diastolic mitral inflow velocity to early diastolic mitral annular tissue velocity; e′, early diastolic mitral annular tissue velocity; EDD, end-diastolic dimension; ESD, end-systolic dimension; GLS, global longitudinal strain; LA, left atrium; LS, longitudinal strain; LV, left ventricle; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation.

Change in left ventricular structure and mechanical function
All patients were confirmed cases of septal hypertrophy at baseline and showed significant decreases in septal thickness after septal myectomy (23.8 ± 5.9 mm at pre-operation vs. 16.2 ± 4.5 mm at post-operation, P < 0.001). While the LV end-diastolic thickness presented no significant interval change (44.4 ± 4.6 mm at pre-operation vs. 45.5 ± 5.2 mm at post-operation, P = 0.131), the LV end-systolic thickness was increased (26.6 ± 3.9 mm at pre-operation vs. 30.6 ± 4.3 mm at post-operation, P < 0.001) compared with thickness before surgery. LV ejection fraction was decreased after surgery (73.8 ± 6.7 vs. 62.9 ± 8.3%, P < 0.001) (Figure 2). For LV mechanical function, |LV GLS| was further aggravated after surgery (12.6 ± 3.6% at pre-operation vs. 11.6 ± 4.3% at post-operation, P = 0.033). When we analysed LV regional LS by septum and non-septal wall separately, |septal LS| demonstrated no alteration (7.1 ± 4.8% at pre-operation vs. 7.4 ± 3.8% at post-operation, P = 0.631), while |non-septal LS| showed a difference (14.4 ± 4.3% at pre-operation vs. 12.8 ± 4.9% at post-operation, P = 0.009) after septal myectomy (Figure 2).
Factors associated with cardiac reverse remodelling after septal myectomy
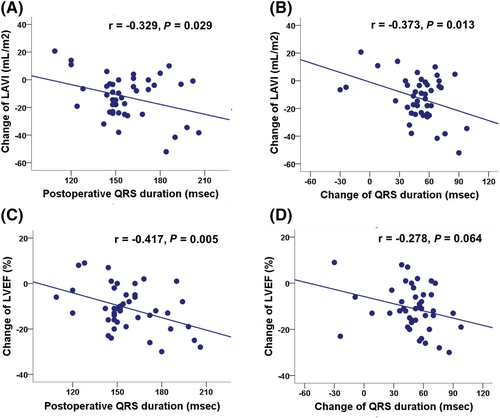
In simple correlation, the pre-operative LVOT peak pressure gradient reflecting preoperative haemodynamic loads was related to the change in LA volume index with marginal statistical significance (r = −0.290, P = 0.057). In addition, postoperative QRS duration and change of QRS duration reflecting effective septal reduction correlated closely with change in LA volume index (r = −0.329, P = 0.029; r = −0.373, P = 0.013, respectively) (Figure 3 and 3). In multivariate analysis, the amount of LA volume reduction was independently correlated with preoperative LVOT peak pressure gradient and change of QRS duration (Table 3). In terms of LA mechanical function, change of LA strain was not correlated with either preoperative LVOT peak pressure gradient (r = −0.050, P = 0.749) or change of QRS duration (r = −0.022, P = 0.888).
| Change of LAVI | Univariate | Multivariate | ||
|---|---|---|---|---|
| t | P-value | t | P-value | |
| Age at surgery | 0.094 | 0.925 | ||
| Female sex | −0.393 | 0.696 | ||
| Atrial fibrillation | 0.365 | 0.717 | ||
| Preoperative septal thickness | 0.555 | 0.582 | 0.335 | 0.739 |
| Preoperative LVOT PG | −1.961 | 0.057 | −2.035 | 0.049 |
| Preoperative LV GLS | −0.175 | 0.862 | −0.768 | 0.447 |
| Preoperative E/e′ | 0.311 | 0.757 | 1.435 | 0.159 |
| Postoperative QRS duration | −2.258 | 0.029 | ||
| Change in QRS duration | −2.609 | 0.013 | −2.188 | 0.035 |
- GLS, global longitudinal strain; LAVI, left atrial volume index; LV, left ventricular; LVOT, left ventricular outflow track; PG, pressure gradient.
Similarly, postoperative QRS duration correlated closely with change of LV ejection fraction (r = −0.417, P = 0.005) and change of QRS duration correlated with change of LV ejection fraction (LVEF) (r = −0.278, P = 0.064) with marginal significance (Figure 3 and 3). However, the decrease in |LV GLS| was not significantly associated with changes in QRS duration.
Discussion
The principal findings of the present study are as follows. First, LA enlargement and impaired LA mechanical function were reversibly restored 1 year after surgical septal reduction in patients with symptomatic obstructive HCM. Second, LV mechanical dysfunction in patients with symptomatic obstructive HCM did not improve and deteriorated 1 year after septal myectomy. Third, LA reverse remodelling tended to be achieved in patients with high preoperative haemodynamic afterloads and greater QRS widening after septal myectomy. Fourth, the postoperative QRS duration paradoxically affected LA reverse remodelling and LV systolic function. Indeed, that QRS widening, an indicator of effective septal myectomy, was significantly associated with LA volume reduction but may promote LV systolic dysfunction after septal myectomy. In summary, septal myectomy did not improve LV mechanical function by relieving the haemodynamic load of the LV but reduced LA volume and improved LA mechanical function.
Diastolic dysfunction is a hallmark of HCM, and the clinical importance of LA enlargement and mechanical function, which are indicators of chronic LV diastolic dysfunction and thromboembolic risks,19-22 is well known in patients with HCM.9-12, 23 While actively applying an implantable cardioverter defibrillator in patients at high risk of sudden cardiac death or performing septal myectomy in patients with symptomatic obstructive HCM, the residual risk in HCM patients is that of heart failure and stroke according to underlying LA and LV functions.1 In addition, because interest in medical septal reduction therapy using cardiac myosin inhibitors has recently increased,24 the effect of reducing LVOT obstruction on myocardial mechanics is expected to become an important issue.
Left atrial dysfunction in hypertrophic cardiomyopathy and effect of septal myectomy
It was demonstrated that patients with obstructive HCM have significantly greater impairment in LA function and larger LA volume, as assessed by cardiac magnetic resonance (CMR) and velocity vector imaging, compared with those with nonobstructive HCM.25 The CMR data also showed that LA volume decreased and reservoir and booster strain increased significantly 8.6 months after septal myectomy in patients with obstructive HCM.25 LA mechanical function in patients with obstructive HCM is influenced by increased LA afterload due to LVOT obstruction, atrial myopathy itself, and accompanying atrial fibrillation. Among the various factors affecting LA, haemodynamic loads caused by LVOT obstruction were resolved with septal myectomy, and LA volume reduction and LA mechanical functional improvement were evident and consistent across several studies.4, 26, 27 In the present study, LA volume reduction after septal myectomy and improvement of the LA strain evaluated by two-dimensional speckle-tracking were demonstrated. It is mechanistically acceptable that the degree of LA volume reduction increases as the preoperative LVOT pressure gradient and postoperative QRS duration increase. LA volume reduction and improvement of LA strain were obvious in patients with sinus rhythm. However, patients with underlying AF did not show significant LA reverse remodelling or improvement in LA mechanical function. It is possible that the number of AF patients in the present study was very small, limiting statistical significance, but it is an acceptable result that LA reverse remodelling would be difficult in patients with AF. We need to study the effect of AF on LA reverse remodelling after septal myectomy in a larger number of patients in the future.
Left ventricular dysfunction in hypertrophic cardiomyopathy and effect of septal myectomy
In the present study, LV GLS tended to worsen after septal myectomy. HCM is a primary genetic myocardial disease, and LV in patients with obstructive HCM is hypertrophied with advanced myocardial fibrosis and clinically significant LV longitudinal mechanical dysfunction.5, 28 It is uncertain whether there will be a benefit to the underlying diseased myocardium by relieving the haemodynamic load of LV by eliminating the pressure gradient of LVOT though septal myectomy and combining the adverse effects of the surgical procedure. QRS widening such as an acquired left bundle branch block through septal myectomy can have a chronic negative effect on LV mechanical function.29, 30 It is difficult to determine whether the reduction in LVEF is a result of a decrease in LV contraction itself or a natural consequence of septal myectomy due to an increase in LV end-systolic dimension, and the reduction remains within the normal range. LV GLS is particularly useful in that it well detects LV mechanical systolic dysfunction and has a prognostic implication for the state in which the LVEF is preserved.5-7 Although we expected that LV GLS would be unchanged or slightly better as the haemodynamic load of LV decreased, this was not the case, and deterioration of non-septal longitudinal strain suggests that septal myectomy may have a negative effect on diseased myocardium in HCM.
Our findings are partially consistent with results from previous studies.31 The systolic functional deterioration after septal myectomy in obstructive HCM may be due to inherent characteristics of myectomy and ongoing and irreversible disease.4,31 A recent CMR study demonstrated that late gadolinium enhancement in the LV increased despite septal myectomy in patients with obstructive HCM.4 The data indicated that septal myectomy can correct haemodynamic changes but cannot alter the genetic mutation of these patients and therefore cannot stop progressive myocardial fibrosis.4 Another study demonstrated worsening of septal systolic strain rates and no improvements in global and regional longitudinal strain after septal myectomy despite improvement in exercise capacity in 10 patients with obstructive HCM.32 In this study, the amount of late gadolinium enhancement showed no significant increase after septal myectomy.32 The reduction of the hypertrophied LV and wall thinning after septal myectomy may also have contributed to the impaired LV longitudinal systolic function. However, there was no difference in the septal wall strain in our study. Therefore, the functional change of the non-septal wall seems to be more important.
In addition to disease progression, we can additionally consider the possibility that QRS widening was an obstacle to the improvement of LV systolic function after relieving LVOT obstruction. A left bundle branch block is common after septal myectomy but did not influence postoperative mortality in a study of 2482 patients.30 Although a left bundle branch block indicates effective septal reduction in patients receiving septal myectomy and is a common finding, this may be a consequence of a decrease in LVEF and LV GLS after septal myectomy, particularly in the non-septal segment. Current guidelines recommend evaluating the effects of therapy after septal reduction therapy, not only the decrease in LVOT gradient, but also systolic function of the LV.1 Our results suggest that LA functional assessment can be used as an indicator of functional improvement after septal myectomy because LV systolic function continues to deteriorate after septal myectomy.
Future perspectives
Recently, a novel pharmacological strategy in patients with symptomatic obstructive HCM who are eligible for invasive septal reduction therapy has emerged. Mavacamten, a cardiac-specific allosteric myosin inhibitor that hampers actin-myosin cross-bridges, has been shown to alleviate symptoms and reduce LVOT gradients in patients with symptomatic obstructive HCM.24 In addition, it is evident that myosin inhibition improves LV diastolic function in patients with symptomatic obstructive HCM.33, 34 Future studies are warranted on how reducing the LVOT pressure gradient without inducing significant QRS widening by administration of mavacamten affects LV mechanical function. Specifically, the focus of future studies would be on comparing the differences in LV GLS, regional LS, and ejection fraction after invasive septal reduction therapy versus mavacamten administration, and whether mavacamten can improve LV systolic function compared with invasive strategy.
Study limitations
First, this was a retrospective, observational study from a single tertiary centre, which could have potential selection bias. Second, the number of patients included in the final analysis was small. This is because patients who underwent valve surgery or maze procedure were excluded to confirm the effect of septal myectomy on the LA and LV. Third, CMR data were not included in the analysis. However, evaluation of LA strain and LV GLS is widely used in HCM, and its role as a prognostic factor is well known. Moreover, we believe that the main results of the study will not change even if the CMR data are included.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number HI22C0154).
Conflict of interest
None declared.




