Alcohol septal ablation in hypertrophic cardiomyopathy: For which patients?
Abstract
Percutaneous and surgical therapies for septal reduction for hypertrophic cardiomyopathy have been going head-to-head for the past 20 years with similar outcomes and mortality rates, although contemporary myectomy seems to materialize its superiority. However, on closer analysis, the external validity of studies advocating myectomy does not translate to all centres. The aim of this review was to examine the most recent data on septal reduction therapy and to attempt to phenotype the appropriate patient for each of the two treatments. The key to similar low mortality rates between ventricular septal myectomy and alcohol septal ablation appears to be proper patient selection performed in high volume clinical environments. Furthermore, we analyse the role of mavacampten (the recently approved cardiac myosin inhibitor) in replacing or complementing the two septal reduction therapies.
Introduction
Treatment of obstructive hypertrophic cardiomyopathy (HCM) remains challenging. Medical therapy, surgical therapy, and pacemaker therapy have been shown to be beneficial in some patients over the years. Several therapeutic targets are being pursued at the same time: medical therapy for heart failure, management of ventricular arrhythmias and sudden cardiac death risk and septal reduction therapy. In case of failure (advanced heart disease and symptoms that are refractory to all other interventions), a transplant referral is indicated. Patients with HCM have a dynamic disease that should be followed closely in specialized centres. Indeed, in recent years, this has been greatly encouraged by the formation of dedicated HCM outpatient clinics and centres of expertise with experience in the invasive treatment of HCM.
Symptoms such as heart failure and chest pain are caused by a combination of diastolic dysfunction, left ventricular outflow tract (LVOT) obstruction, mitral regurgitation, and microvascular dysfunction and myocardial ischaemia secondary to ‘small vessel’ disease.1 The most visible hallmark of HCM is LVOT obstruction, which is also the most important mechanism responsible for aggravating heart failure symptoms in this disorder. In the obstructive type of HCM, there is systolic anterior motion (SAM) of the mitral valve towards the interventricular septum. This produces LVOT obstruction by pulling the anterior leaflet of the mitral valve into the LVOT during systole by the Venturi phenomenon and a drag effect. The majority of HCM patients (70%) have a resting or exercise-induced LVOT gradient.1 At rest, LVOT obstruction is seen in approximately 25% of patients, and is an independent predictor of poor prognosis.2 Although negative inotropic drugs can efficiently alleviate symptoms in many cases, 5%–10% of patients with HCM remain refractory to pharmaceutical therapy.3 As a result, patients with LVOT gradient of ≥50 mmHg at rest or with provocation, and advanced and limiting heart failure symptoms that are refractory to drug schemes should undergo septal reduction therapy.4, 5 An invasive septal reduction therapy is required in approximately 5% of all patients with HCM and up to 30% in tertiary referral populations.3 Septal myectomy and alcohol septal ablation (ASA) are both performed in clinical practice in order to reduce the obstructive myocardial mass. Both have the same level of recommendation in the guidelines, although randomized trials comparing the two approaches have not been performed and are unlikely to ever occur (due to logistical impracticability and ethical issues). Attempts were made, but no trial was completed due to selection bias and some patients having a clear indication for one therapy over the other. At the present time, for example, the AMARONE trial is ongoing, but only randomizing patients between 40 and 75 years of age and without any concomitant valvular diseases that necessitates surgery.6 However, several observational studies, as well as a systemic reviews and meta-analyses have shown the relative safety and efficacy of these procedures. The choice of one therapy over the other is nuanced and individualized. Surgical myectomy has been preferred in younger patients with drug-resistant obstructive HCM because its contemporary version includes resection of papillary muscles and repair of the mitral system, with very good long-term results. ASA has been refined as well and it became a reliable alternative for many patients for whom surgery is unsuitable or undesirable. A third player, mavacamten (a cardiac myosin inhibitor), could change the landscape in which the two traditional invasive therapies have faced each other in the last 20 years. The conclusion is clear: technologies are advancing and patients benefit from increasingly optimized treatments. The modern place of ASA on this stage remains to be seen. The aim of this review was to investigate the latest data on septal reduction therapies and to try to phenotype the suitable patient for each of them.
Alcohol septal ablation—The benefits
ASA is a percutaneous technique used to create a controlled infarction within the proximal portion of the septum (Figure 1). Since the first report of this new procedure in 1995,7 multiple subsequent studies have shown the effectiveness of this new procedure. When performed by skilled operators at high-volume centres, ASA results in an increase in left ventricular (LV) outflow diameter, a reduction in LVOT gradient in >80%–90% of patients, regression of LV hypertrophy, and improved diastolic function.8 Long term benefits result from the creation of a localized septal infarction and scarring, which lead to progressive increase in LVOT diameter as a result of septal thinning and LV remodelling.8 After ASA, the severity of mitral regurgitation is reduced, LV end-diastolic pressure falls, and the size of the left atrium decreases, likely contributing to secondary effects including beneficial reduction in atrial fibrillation burden and severity of pulmonary hypertension.8 However, in contrast to treatment with surgical septal myectomy, abnormalities of the mitral valve and its papillary muscles cannot be addressed at the time of septal ablation.
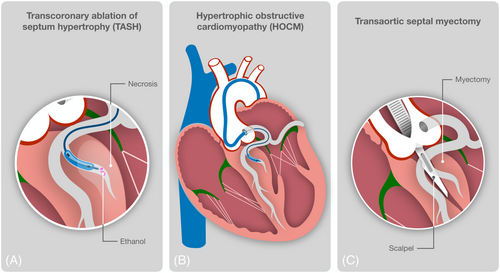
Since its advent, the procedure has undergone several modifications and improvements that have led to optimization of the results and minimization of complications, most importantly through the reduction of the effective dose of alcohol and the use of myocardial contrast echocardiography (MCE). MCE can properly identify the size of the septal vascular territory and predict the magnitude of the infarct caused by ethanol infusion.9 In one series of patients treated with ASA, the use of MCE was related with a greater percentage of acute (92% vs. 70%) and mid-term (94% vs. 64%) procedural success than patients in whom MCE was not used.10 MCE may also reduce the number of patients who require pacemaker implantation.9 The doses of injected alcohol were reduced as well and sequential ablation is now preferred, targeting smaller septals or just a branch from the major perforator, aiming for a scar ‘as small as possible and as big as necessary’. This defensive strategy is encouraged even if the gradient is not immediately massively reduced, because there is continuous gradient reduction during the first post-interventional year due cardiac remodelling and a stepped re-ASA should not be perceived as complication but integral part of the therapy.11
A rigorous individual examination of clinical symptoms, concomitant co-morbidities, and echocardiographic and angiographic data should be used to designate patients for ASA.10 The primary indications of the procedure are LVOT gradient of ≥50 mmHg at rest or with provocation, and advanced and limiting heart failure symptoms that are refractory to medical therapy should undergo septal reduction therapy. Contrary to common belief, there is no age contraindication or definitive threshold for ASA as there is no evidence indicating less safety of ASA in younger patients. The only warning coming from guidelines is that ASA is controversial in children, adolescents and young adults because there are no long-term data on the late effects of a myocardial scar in these groups, and because the technical challenges and potential hazards of the procedure in smaller children and infants are greater.12 A septal wall thickness <16 mm should be taken as a relative contraindication for ASA because of the risk of septal perforation.8 Certain anatomical criteria make ASA more favourable, such as focal ‘septal bulge’, wide angle of papillary muscles and chords to ventricular septum, absence or minimal intrinsic disease of mitral valve apparatus and papillary muscles, and favourable coronary anatomy with a single septal perforator of appropriate size supplying the targeted asymmetric hypertrophied basal septum territory. On the other hand, the presence of intrinsic disease of the mitral valve apparatus or papillary muscles clearly favours myectomy. The anatomical causes that determine the obstruction are represented by the elongation of the mitral leaflets, especially the anterior one, and by the malposition of the papillary muscles (especially the lateral one), inserted anteriorly. They change the position of the mitral valve coaptation zone, which is displaced anteriorly. As a result, in the first phase of systole, as the mitral leaflets close, the anterior leaflet is pulled anteriorly toward the septum, reaching the LVOT. The blood flow from the LV ejected to the aorta hits the anterior mitral leaflet, moves it anteriorly and presses it against the interventricular septum, the degree of obstruction being directly proportional to the duration of contact between the mitral leaflet and the septum. ASA should be performed only for dynamic LVOT obstruction and systolic anterior of the mitral valve, with predominantly posterior mitral regurgitation.13 The anterior systolic movement of the anterior mitral leaflet causes the loss of coaptation of the mitral leaflets, with the appearance of a secondary mitral insufficiency; this has a posteriorly directed jet and is usually mild or moderate in severity. When mitral insufficiency is also accompanied by the primary morphological changes of the mitral apparatus previously described, its degree can worsen, becoming severe.
Transcatheter mitral valve edge-to-edge repair (MitraClip) may be useful in lowering the LVOT gradient and easing heart failure symptoms in patients with HCM and refractory symptomatology linked to LVOT obstruction who are not appropriate for a septal reduction therapy. This is of particular importance when the gradient is mostly induced by the SAM or the mitral regurgitation is severe or perhaps most importantly, the unmet clinical need of non-obstructive HCM with very few therapeutic options at present. Various studies are reporting promising results but in small sample sizes and with limited follow-up.14-16 In a review of four studies conducted between 2010 and 2016 (15 patients, mean age of 79.5 ± 8.1 years), there was an immediate gradient reduction from 75.8 ± 39.7 to 11.0 ± 5.6 mmHg but only partial follow-up is provided, to a maximum of 6 months.14 A more recent and larger study (220 patients with HCM receiving MitraClip between 2016 and 2019) reports a low 4.5% in-hospital mortality but no data regarding symptoms or follow-up.15 Nevertheless, MitraClip has the potential to attenuate the late systolic motion of the anterior leaflet and implicitly improve symptoms in a subset of patients who are poor surgical candidates and more robust data in this field is highly needed. Finally, a recent case report showed promising results with MitraClip in non-obstructive HCM where current standard of care for this condition is only supportive.16
Although a randomized controlled trial comparing both septal reduction methods remains elusive due to practical concerns, prior retrospective comparisons focused on short-term outcomes from single centres had shown largely similar mortality, despite higher residual LVOT tract gradients and pacemaker implantation rates from ablation.8 Whether differences exist in long-term survival between myectomy and ASA has not been well defined—a pertinent issue given the relatively young age at which patients undergo these procedures (average age, early 50s). A meta-analysis by Alam et al. of five small studies with a sum of 183 ablation patients was followed for 3–28 months.17 Another meta-analysis included two longer studies comprising 87 ablation patients followed for 4 and 5 years.18 As for the common message conveyed, both studies showed similar mortality rates as well as functional status compared with surgical myectomy, with the caveat of increased conduction abnormalities and a higher post-interventional LVOT gradient.17, 18
Early mortality (occurring during or up to 30 days after the procedure) is low, with a mean value of 1.5% reported,19 which is similar to that for surgical myectomy. Causes of early mortality include left anterior descending dissection, ventricular fibrillation, cardiac tamponade, cardiogenic shock, and pulmonary embolism. Late all-cause mortality is reported at 0.5%, the most frequent causes being sudden cardiac death, pulmonary embolism, congestive heart failure, and other non-cardiac causes. Other reported adverse events include coronary dissection and spasm (1.8% and 1.4%, respectively), abrupt coronary no flow, stroke (1.1%), and pericardial effusion (0.6%). Spontaneous ventricular fibrillation in the immediate peri-procedural period is not frequent (2.2%), and sustained ventricular tachycardia is extremely rare (only three cases reported in current literature).19, 20 The most frequent complication of ASA is complete atrioventricular (AV) block requiring permanent pacemaker implantation. Occurrence of acute complete AV block during the procedure varies between 8% and 15% of patients,19 and it is highly depending on the alcohol dose. Yet there is recovery of AV conduction in most cases (41%–100%) before leaving the catheterization laboratory, and in two-thirds of patients within the first 3 days.19
A meta-analysis from 2006 of 42 observational studies (2959 patients, mean age 54) with mean follow-up of 13 months document the benefits of ASA: (1) the resting LVOT gradient had decreased from 65 to 15 mmHg, whereas the provoked LVOT gradient had decreased from 125 to 31 mmHg, (ii) the NYHA class improved from a mean of 2.9 to 1.2, peak oxygen consumption increased from 18 to 24 mL/kg/min, and mean exercise capacity increased from 325 to 438 s, and (iii) repeat alcohol septal ablation, due to incomplete reduction of outflow gradients from initial procedure, was required in 6.6% of patients.19 The 1 year mortality following ASA was 2%.19 Of note, the aforementioned numbers reflect one of the earliest experiences with ASA.19 In the largest European registry of 1275 patients with HCM (7 centres, mean age 58 years, median follow-up 5.7 years) who underwent ASA between 1996 and 2015, 30 day mortality was only 1%, with significant reductions in LVOT gradient (67 to 16 mmHg) and functional status (mean NYHA class 2.9 to 1.6) following the ablation.21 A following meta-analysis of 2013 patients who underwent ASA and were followed for an average of 6.2 years, the average reduction in LVOT gradient post-ablation was 71%, and only 7.7% of patients ultimately required repeat intervention.22 A more recent meta-analysis presenting direct comparison of both strategies (1555 cases treated with ASA vs. 2092 cases treated with surgical myectomy) found no significant difference in postoperative all-cause mortality (RR = 0.82; 95% CI: 0.65–1.04; P = 0.10), with a better LVOT gradient reduction and cardiac function improvement with surgical myectomy; unfortunately, data on any longer term outcomes was not provided.23 The meta-analysis of Liebregts et al. therefore remains the last ‘gate-keeper’ in terms of head-to-head comparison where outcomes over 5 years are offered (with a similar mortality 1.52% per year with ASA vs. 1.44% per year with surgical myectomy; P = 0.78).22 Thus, it is immediately foreseen the need for an updated and robust cohort from which the two strategies would ideally be followed for up to 10 years. This gap in evidence was recently tackled by the study of Cui et al. whose relevant results will be presented in the next section.24
Regarding certain subsets of patients, there are conflicting data regarding the efficacy of ASA markedly thickened septa; all-cause mortality may be slightly higher in patients with baseline septal thickness ≥25–30 mm but on the other hand, early complications such as permanent pacemaker implantation were higher in patients with baseline septal thickness ≤16 mm. Nevertheless, all subgroups showed markedly improved symptoms and decrease in LVOT obstruction.21, 25, 26
Some patients who do not show initial benefit can have later improvement. In a small study of 47 patients with a mean baseline LVOT gradient of 98 mmHg who underwent alcohol septal ablation the late responders had a similar LVOT gradient at 1 year compared with those who improved immediately after the procedure (27 vs. 13 mmHg, respectively).27 Of note, the residual LVOT gradient is a strong predictor of poor clinical outcome in patients who undergo alcohol septal ablation.28 In general, residual peak gradients <25 mmHg and preferably <10 mmHg are desired, prompting termination of the procedure. Repeat procedures despite initial success are required because of recurring gradient and symptoms in 7% of patients. Reported predictors of procedural failure are total peak CK < 1300 U/L and immediate residual LVOT gradient ≥25 mmHg.29
As mentioned above, data on long term survival on ASA vs. myectomy is conflicting but the latest studies show a signal toward a better outcome with myectomy. Recent meta-analyses appear to favour myectomy although on a closer look, individual studies from centres with expertise report very good outcomes with ASA. It is understood, therefore, the possible relativity of the ASA procedure which is operator- and centre-dependent. For example, in a centre in Germany, 5 year survival free of cardiovascular events with ASA was 98.6%, at 10 years 96.5% and at 15 years 92.3%; the centre injected low doses of alcohol (mean 2.1 ± 0.4 mL) and the re-ablation rate was almost a quarter of the patients.30 But myectomy is operator- and centre-dependent as well. In the next section we will discuss its course along the last 10 years, with this year's data which certifies its progress.
Surgical septal myectomy—The evolution
The experience with surgical septal myectomy has a background of 50 years31 whereas ASA has 20 years less,7 but this aspect is important only in the follow-up of patients. For surgical septal reduction, the patient receives a sternotomy and is placed on cardiopulmonary bypass; an aortotomy is performed and the proximal septum is approached through the aortic valve (Figure 1). Up to 15 g of ventricular septal muscle are excised and removed in order to widen out the LV outflow tract area. With experience, it was noticed that the dynamic obstruction is not only caused by the septum and by the time ASA was appearing, surgical myectomy has been upgraded. Messmer et al. pushed it further down the LV to the level of the mid septum and the papillary muscles.32 He proposed that the operation could be supplemented with mitral valve repair or leaflet plication, sometimes with the ‘extended myectomy’ to mid-ventricular level and with reconstruction of subvalvular apparatus. Enlarged or malpositioned papillary muscles contributing to obstruction could be ‘shaved’, incised off the ventricular wall and repositioned to the adjacent papillary muscle. This is of particular importance as abnormal anterior position of the mitral valve in the LV cavity, and elongation of mitral leaflets contribute to valve protrusion into the flow stream, causing an overlap between the inflow and outflow portions of the LV. The task of Messmer et al. was to separate the inflow and outflow portions of the LV by distal extension of the myectomy through to the base of the papillary muscles along with inspection and, if needed, revision of mitral valve leaflets and chordal structures.32 Extending the myectomy through allows for release (or realignment) of anterolateral papillary muscle, which is often apically displaced (and frequently fused to the LV wall) and as a result tethers the plane of the mitral valve towards the basal septum. These manoeuvres widen the LVOT, eliminate the Venturi effect and significantly ameliorate the mitral regurgitation.13
Recently, surgical septal myectomy has been further refined. Transesophageal echocardiography (TEE) is vital in intraoperative assessment of septal morphology and thickness, as well as for defining mitral valve and subvalvular anatomy prior to beginning surgery and to judge efficacy of the operation when complete. When the heart is on cardiopulmonary bypass and drained of blood, TEE sometimes may not provide any useful imaging. A new technique of on-pump intracardiac echocardiography (OPIE) provides online guidance to the surgeon.33 With this tool, septal thickness can be measured anywhere in proximal and mid septum that the surgeon wishes to interrogate, before resection, and after initial resection to decide if more muscle needs to be removed, or whether stopping resection would be prudent. On-pump septal thickness can be accurately assessed 90% of the time. In some cases, allowing the right ventricle to fill, better defines right ventricular endocardium.33, 34
A recent preclinical study on pigs described a novel technique called Septal Scoring Along the Midline Endocardium (SESAME) which resides actually at the intersection between the two specialties.35 By introducing two catheters via the femoral approach into the LV, portions of the septal myocardium could be lacerated using electrosurgery on a 0.014″ wire. The procedure was guided by intracardiac echocardiography and the results of this novel transcatheter myectomy technique are encouraging (mean 13.1% increase in LVOT area at 1-month follow-up with a thin fibrosis layer formed at the slice edge).35
It is therefore clear to understand the evolution of surgical myectomy and its complexity that requires centres of expertise. Here as well, the best results come with the formation of these dedicated teams. Extraordinary improvements in imaging have highlighted a high frequency of mitral abnormalities, prompting surgeons to repair the valve at the same time as index procedure. Multiple groups have shown this approach where contemporary myectomy has been reflected in good results. In the most recent of the studies and perhaps the most relevant due to its large cohort (4000 patients) and long follow-up (10 years), Cui et al. compared ASA versus myectomy in three specialized HCM centres (two American and one Chinese).24 After adjustment for age, sex, and co-morbidities, the 10 year all-cause mortality rate was substantially less in patients undergoing myectomy than those undergoing ASA (26% vs. 8%; HR, 1.68; 95% CI, 1.29–2.19; P < 0.001).24 The additional propensity score-matched analysis that included only patients with similar characteristics did not meaningfully alter the conclusions of the primary results and supports the authors' conclusions. These findings demand three main observations. First, the remarkably low postoperative mortality in myectomy group of 0.3% offers testimony to the progressive improvements in surgical technique, noteworthy considering that early experience with myectomy was associated with high mortality.36 Second, gradients were not measured in this study but we know that residual resting gradients with ASA are likely to be magnified by physiologic provocations like standing and eating, not to mention exercise. And third, in the three centres mentioned above, the mitral valve was approached in 50%–65% of the cases (in one way or another, from the chordal structures to the mitral leaflets).24 Consistently, Rastegar et al. reported routine chordal and papillary muscle release and mitral plication in 17% of the cases.37 In Cleveland, US, Hodges et al. reported additional mitral procedures in one third out of almost 1600 patients. In Beijing, China, out of 277 patients operated by a single surgeon, 46% received concomitant procedures (not only mitral repair but also CABG or tricuspid valvuloplasty).38 In a smaller cohort (121 patients), but part of a specialized centre, Patel et al. reported that half of the cases required additional nonmyectomy approach (mitral valve repair/replacement or papillary muscle reorientation).39 But these numbers should not immediately be attributed to the lower mortality of myectomy in general as isolated septal resection interventions have the same outcomes when mitral approach is not necessary. For example, in a series of 298 consecutive patients undergoing isolated septal myectomy at Mayo Clinic between 2011 and 2014, 30 day mortality was zero, with 98.7% survival at 6 years.40 Needless to say, mitral valve replacement as the primary method of relieving obstruction is now infrequently performed, given the improvements in symptoms and LVOT gradient following septal myectomy in nearly all patients and neither primary mitral valve replacement nor mitral valve plication should be performed for relief of LVOT obstruction if septal reduction therapy is an option.41
‘To ablate or operate’
The decision ‘to ablate or operate’ is not a one-procedure-fits-all situation. Based on comprehensive assessment of clinical symptoms, associated co-morbidities, echocardiographic, electrocardiographic, and angiographic features, some patients are better suited for myectomy whereas others are better suited for ASA (Figure 2). As in contemporary TAVI guidelines indications, surgery versus percutaneous treatment should follow the same principle: there are clear factors that favours one procedure over another such us age, co-morbidities, surgical risk score and certain anatomical considerations. For example, a 50-year-old patient with HCM and morphologic abnormalities such as anomalous papillary muscle, markedly elongated anterior mitral leaflet, massive ventricular septal hypertrophy, intrinsic mitral valve disease, and multivessel CAD should be addressed for surgical intervention. On the other hand, a 65 years old patient with chronic obstructive pulmonary disease with a favourable anatomy of the first septal perforator branch may benefit more from ASA. All other ‘grey-zone’ patients that do not present such clear patterns toward one procedure or another should be considered for either mode of intervention and most of the time the management is guided by the patient's preference and the local expertise. In our opinion, the need to reconstruct the mitral apparatus is the most relevant factor in this decision. The significant experience of the operator with surgical myectomy or ASA plays an obvious role in determining the safer choice of therapy for the patient. As emphasized in the latest 2020 AHA/ACC and 2014 ESC HCM guidelines,4, 5 referral to a comprehensive HCM centre would be reasonable to aid in the complex decision-making involved in patient selection for myectomy or ASA. In these guidelines, an experienced operator is defined as a person with a cumulative case volume of ≥20 procedures or one who is working in a dedicated HCM programme with a cumulative experience of ≥50 procedures.
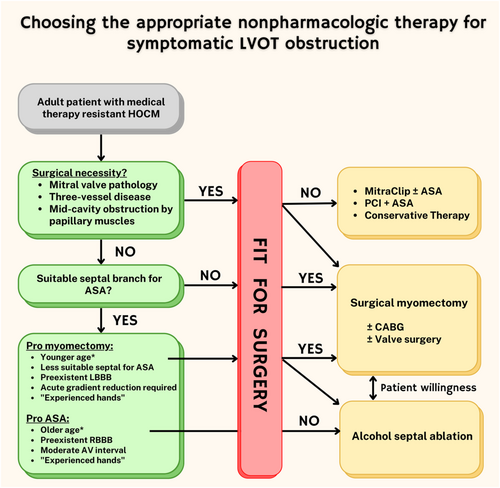
Most important factors to consider in choosing between surgical septal myectomy and ASA are: (i) given the potential concern that the induction of a transmural myocardial scar by the alcohol septal ablation procedure could increase future risk of ventricular arrhythmias, age is an important variable in deciding between procedures (ASA should not be performed in patients <21 years old and should be discouraged in younger ages unless there are significant contraindications to surgery); (ii) not only the septal bulge but other morphologic factors can contribute substantially (or can be the predominant reason) to the generation of outflow obstruction in an individual patient such as elongated mitral valve leaflet(s), anomalous insertion of the anterolateral papillary muscle directly into the mitral valve, massive LV hypertrophy, and apically displaced papillary muscles and since these features cannot be addressed with ASA, identifying one or more of these features can sway decision-making towards surgical myectomy; (iii) the presence of concomitant cardiac problems (e.g., coronary artery disease, mitral valve disease, and atrial fibrillation) may warrant a surgical approach since an adjunctive procedure will be required in addition to the surgical septal myectomy (i.e., coronary artery bypass grafting, mitral valve replacement, or MAZE procedure); (iiii) at the risk of repetition, the presence or absence of local expertise is a ‘very medical’ reason when choosing which course of treatment or when referring the patient to another centre for a particular treatment. In the US registry of all patients with HCM receiving either ASA or myectomy between 2003 and 2011 (over 11 000 patients), a retrospective analysis showed that the lowest tertile of myectomy volume among hospitals was an independent predictor of in-hospital all-cause mortality (adjusted odds ratio, 3.11; 95% CI, 1.98–4.89) and bleeding (adjusted odds ratio, 3.77; 95% CI, 2.12–6.70), whereas being in the lowest tertile of ASA by volume was not independently associated with an increased risk of adverse postprocedural events.42 This perhaps sends a signal that ‘experienced hands’ has a greater clinical impact among surgeons.
Besides these, as shown in Figure 2, myectomy is favoured in case of a pre-existing LBBB and ASA in case of a pre-existing RBBB. This may be due to the higher risk of high-degree AV block in patients with pre-existing RBBB undergoing myectomy and LBBB undergoing ASA. It is worth noting that the authors refrained from providing definitive age thresholds as in fact there is no evidence indicating more hazard with ASA in younger patients. The average age of the patients treated with ASA in most of the published studies was 54–55 years.19, 30 Thus, those trials enrolled a non-negligible proportion of patients younger than 50 years and, hence, there is indeed evidence for the safety of the procedure in younger patients. Moreover, a recently published single-centre analysis from a high-volume reference facility showed that ASA is even safe, effective and feasible in a small number of patients younger than 25 years.43 In another study, Liebregts et al found a significantly lower mortality (at 30 days and 1 year) and ICD implantation rate in young (<50 years) versus older patients (>65 years).44 Important drawbacks to discuss with young patients include the risk of permanent pacemaker implantation and the likelihood of re-intervention either via re-do ASA or the more invasive myectomy.
It should not be forgotten that treatment in HCM is not limited to septal reduction and that the prognosis and clinical course is also influenced by malignant arrhythmias and myocardial ischaemia (small vessel disease changes).45, 46 General treatment includes lifestyle modification (limit dehydration, decreased alcohol intake and decreased caffeine consumption.), pharmacological (symptomatic and pathogenetic), electrophysiological ablation, invasive septal reduction therapy and implantable devices (dual chamber pacing, ICDs). Indeed, the management of HCM is complex and requires regular visits, ideally in a specialized outpatient clinic. The disease is characterized by an enormous diversity in both phenotypic expression and clinical course. Unfortunately, some patients with HCM are asymptomatic and their first clinical manifestation of the disease may be sudden death, likely from ventricular tachycardia or fibrillation. Younger patients, particularly children, have a much higher mortality rate.47 Children have a much greater degree of ventricular hypertrophy and are much more symptomatic early on in the disease course, most likely because more malignant genotypes are present earlier in life.
Finally, time will show if mavacampten, the novel cardiac myosin inhibitor could be the missing piece for targeted HCM treatment. The EXPLORER-HCM trial showed that mavacamten was superior to placebo at improving exercise capacity and health status.48 This was evaluated by a cardiomyopathy questionnaire; the study also showed no significant long-term treatment-related adverse events (median follow-up 62 weeks) and a significant reduction in post-exercise LVOT gradient compared with placebo at 30 weeks, which was sustained to 48 weeks.48 Cardiac magnetic resonance found that mavacamten was associated with favourable remodelling compared with placebo.48 No data regarding survival is provided. Trying to find a better defined role for mavacampten, the VALOUR-HCM trial looked at whether mavacampten can replace or delay septal reduction therapy in very symptomatic patients, on maximal mediating therapy.49 The preliminary results were presented in February 2022, showing that the decision to proceed with septal reduction therapy or guideline eligible at week 16, for mavacamten vs. placebo, was 17.9% versus 76.8% (P < 0.0001).50 The improvement rate in NYHA class was 40% and the resting and provoked LVOT gradient decreased on an average of 30–40 mmHg in the mavacampten group.50 These findings show the major potential of mavacampten directly on the pathogenic mechanism of HCM. The positive signals translated into FDA approval of the drug in April 2022, with a boxed warning about the potential risk for heart failure in treated patients. The drug reduces left ventricular ejection fraction (−7% decrease in the EXPLORER-HCM population), therefore its use is not recommended in patients with a LV ejection fraction below 55%. It must be acknowledged though, that both trials had limited cohorts (100–200 enrolees) and limited follow-up (up to 13 months) and the mean age was around 60 years. The next challenge of mavacampten is to prove its efficacy in actually changing the course of the disease, even in people who do not have LVOT obstruction and in younger individuals. Larger trials with long-term data on both safety and efficacy with mavacamten, as well as any impact on clinical outcomes, are needed.
Conclusions
A randomized controlled trial comparing both septal reduction methods remains elusive due to practical concerns but there is a strong signal that the outcome of ASA is heavily dependent on appropriate patient selection, longitudinal and multidisciplinary care, and operator expertise. The procedure should only be performed in specialized centres and to older adults, due to lack of long-term data. The remaining majority of patients should be referred to a centre with surgical expertise, which adds mitral valve repair to myectomy at the time of index operation, and online imaging to ensure a tailored myectomy. However, age should not be the only factor favouring one procedure over the other as ASA remains a safe, effective and feasible option for younger patients. The excellence of the centre and the patient's choice ultimately dictates the therapeutic course. Future challenges include learning more about patients with characteristic mutations but normal phenotypes and the development of drugs that are specifically directed at the molecular abnormalities. The long-term results of new selective potent negative inotropes (i.e., mavacampten) remain to be seen.
Funding
No external financial support.
Conflicts of interest
The authors report no financial relationships or conflicts of interest regarding the content herein.




