Utility of left atrial and ventricular strain for diagnosis of transthyretin amyloid cardiomyopathy in aortic stenosis
Abstract
Aims
To clarify the usefulness of left atrial (LA) function and left ventricular (LV) function obtained by two-dimensional (2D) speckle tracking echocardiography to diagnose concomitant transthyretin amyloid cardiomyopathy (ATTR-CM) in patients with aortic stenosis (AS).
Methods and results
We analysed 72 consecutive patients with moderate to severe AS who underwent 99mTc-pyrophosphate (PYP) scintigraphy at Kumamoto University Hospital from January 2012 to September 2020. We divided these 72 patients into 2 groups based on their 99mTc-PYP scintigraphy positivity or negativity. Among 72 patients, 16 patients (22%) were positive, and 56 patients (78%) were negative for 99mTc-PYP scintigraphy. In clinical baseline characteristics, natural logarithm troponin T was significantly higher in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (−2.9 ± 0.5 vs. −3.5 ± 0.8 ng/mL, P < 0.05). In conventional echocardiography, the severity of AS was not significantly different between these two groups. In 2D speckle tracking echocardiography, the relative apical longitudinal strain (LS) index (RapLSI) [apical LS/ (basal LS + mid LS)] was significantly higher (1.09 ± 0.49 vs. 0.78 ± 0.23, P < 0.05) and the peak longitudinal strain rate (LSR) in LA was significantly lower in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (0.36 ± 0.14 vs. 0.55 ± 0.20 s−1, P < 0.05). Multivariable logistic analysis revealed the peak LSR in LA and RapLSI were significantly associated with 99mTc-PYP scintigraphy positivity. Receiver operating characteristic analysis showed that the area under the curve (AUC) of the peak LSR in LA for 99mTc-PYP scintigraphy positivity was 0.79 and that the best cut-off value of the peak LSR in LA was 0.47 s−1 (sensitivity: 78.6% and specificity: 72.3%). The AUC of RapLSI for 99mTc-PYP scintigraphy positivity was 0.69, and the cut-off value of RapLSI was decided as 1.00 (sensitivity: 43.8% and specificity: 87.5%) according to the previous report. The 99mTc-PYP scintigraphy positivity in patients with RapLSI ≥ 1.0 and the peak LSR in LA ≤ 0.47 s−1 was 83.3% (5/6), and the 99mTc-PYP scintigraphy negativity in patients with RapLSI < 1.0 and the peak LSR in LA > 0.47 s−1 was 96.6% (28/29).
Conclusions
Left atrial and LV strain analysis were significantly associated with 99mTc-PYP scintigraphy positivity in ATTR-CM patients with moderate to severe AS. The combination of the peak LSR in LA and RapLSI might be a useful predictor of the presence of ATTR-CM in patients with moderate to severe AS.
Introduction
Transthyretin amyloid cardiomyopathy (ATTR-CM) is being increasingly recognized because of ageing of populations, advances in diagnostic abilities, and the potential benefits of emerging therapies.1 In contrast, aortic stenosis (AS) is one of the most common valvular diseases in elderly patients.2 The incidence of both ATTR-CM and AS increases with age, and one study showed that 16% of patients with severe AS had concomitant amyloid cardiomyopathy.3 In addition, patients with amyloid cardiomyopathy appear to have significantly worse outcomes after aortic valve replacement than do patients with isolated AS.3 Therefore, it is important to diagnose concomitant cardiac amyloidosis in patients with AS to determine the most appropriate treatment plan and to predict the prognosis of these patients.
Two-dimensional (2D) speckle tracking echocardiography is a robust and sensitive echocardiographic technique for quantitative assessment of left atrial (LA) function and has been proven to play an adjunctive role in the diagnosis and prognostic stratification of amyloid cardiomyopathy.4 However, there was no report about the usefulness of LA function by using 2D speckle tracking echocardiography to diagnose concomitant ATTR-CM in patients with AS.
Although a precise diagnosis of amyloid cardiomyopathy generally requires endomyocardial biopsy, this procedure is invasive. Bone scintigraphy, including 99mTc-pyrophosphate (PYP) scintigraphy, was recently shown to be remarkably sensitive and specific for the diagnosis of ATTR-CM.5 Thus, it is considered to be the useful modality to diagnose ATTR-CM.6
In this study, we used 99mTc-PYP scintigraphy to determine concomitant ATTR-CM in patients with AS and evaluated the utility of LA and left ventricular (LV) function using 2D speckle tracking echocardiography for the diagnosis of ATTR-CM in patients with AS.
Methods
Study population
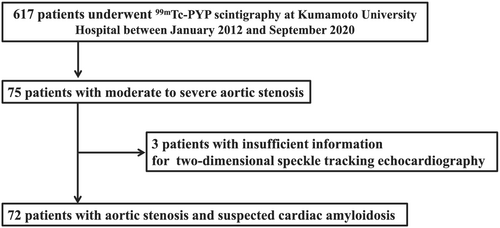
In total, 617 patients underwent 99mTc-PYP scintigraphy because of suspicion of ATTR-CM at Kumamoto University Hospital from January 2012 to September 2020. Of these 617 patients, there were 75 patients who had moderate to severe AS. Because three patients had insufficient information for evaluation by 2D speckle tracking echocardiography, the remaining 72 patients were finally enrolled in this study (Figure 1). 99mTc-PYP scintigraphy, echocardiography, and laboratory examinations were performed while the patients were in a clinically stable and non-congested condition. We divided these 72 patients into 2 groups based on their 99mTc-PYP scintigraphy positivity or negativity.
This study conformed to the principles outlined in the Declaration of Helsinki. It was approved by the institutional review board and ethics committee of Kumamoto University (No. 2334). The requirement for informed consent was waived because of the low-risk nature of this retrospective study and the inability to obtain consent directly from all participants. Instead, we extensively announced the study protocol at Kumamoto University Hospital and on our website (http://www.kumadai-junnai.com) and gave patients the opportunity to withdraw from the study.
99mTc-PYP scintigraphy protocol
99mTc-PYP scintigraphy was performed using a dual-head single-photon emission computed tomography/CT (Symbia T16; Siemens Healthcare, Erlangen, Germany) with low-energy high-resolution collimators. All patients were scanned 3 h after an intravenous injection of 370–740 MBq of 99mTc-PYP (Fujifilm RI Pharma, Tokyo, Japan). Anterior and lateral thoracic planar views were obtained over 3 min duration. The acquisition parameters used for planar imaging were 256 × 256 matrix with 1.23 zoom factor. With the same system, thoracic single-photon emission computed tomography images were acquired for each patient immediately after the planar scan. Cardiac accumulation of 99mTc-PYP in all patients was assessed by a board-certified nuclear medicine radiologist. On planar images, myocardial tracer uptake was evaluated with a visual scoring method (0 = no myocardial uptake, 1 = myocardial uptake less than rib uptake, 2 = myocardial uptake equal to rib uptake, and 3 = myocardial uptake greater than rib uptake). Myocardial uptake was also analysed quantitatively based on the heart-to-contralateral ratio of the total counts in a region of interest over the heart, which was divided by the background count of a copied and mirrored region of interest over the contralateral chest. 99mTc-PYP positivity was based on a visual grade 2 or 3 uptake and the heart-to-contralateral ratio of ≥1.3.5, 7-9
Transthoracic echocardiography analysis
Transthoracic echocardiography was performed using commercially available ultrasound equipment. The median duration from echocardiography to 99mTc-PYP scintigraphy was 26 days (interquartile range, 8–27 days). The chamber size, wall thickness, and left ventricular ejection fraction (LVEF) were evaluated using standard procedures.10 The peak early and late diastolic velocity of LV inflow (E and A velocity, respectively), the systolic mitral annular velocity (s'), the peak early diastolic velocity (e'), and the late diastolic mitral annular velocity (a') on the septal corner of the mitral annulus were measured in the apical four-chamber view, and the E/e' ratio was calculated. The LV mass index was calculated using a previously described formula.11 We defined AS in our study as moderate to severe AS. Valvular heart diseases and their severities were defined according to the American Heart Association guideline.12
Two-dimensional speckle tracking echocardiography was performed using vendor-independent software (TOMTEC Imaging Systems, Munich, Germany). LV apical views (apical four-chamber, two-chamber, and long-axis views) were acquired at maximum image quality for assessment of the LV LS. Speckles were tracked frame by frame throughout the LV myocardium over the course of one cardiac cycle; basal, mid, and apical regions of interest were then created and manually adjusted whenever needed. The LV endocardial borders were traced at the end-diastolic frame. End-diastole was defined by the QRS complex or as the frame after mitral valve closure. The software then automatically generated LV strain profiles. Segmental LS was automatically demonstrated in a bullseye 16-segment model. Global longitudinal strain (GLS) was calculated as an average peak strain from the three apical projections. The relative apical LS index (RapLSI) was defined using the following equation: [average apical LS/ (average basal LS + average mid LS)].13
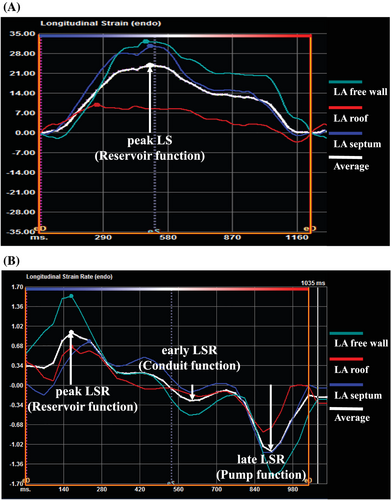
We also used the above-mentioned 2D speckle tracking software to study LA deformation. The LA LS and LS rate (LSR) was measured using strain indices calculated as the average of the three segments (septal, roof, and lateral) obtained using the apical four-chamber view.14 LA reservoir function was estimated using the peak LS and the peak LSR during ventricular systole, which represents LA filling during LV systole (Figure 2A, 2). LA conduit function was estimated using the early LSR during LV diastole (Figure 2). LA pump function was estimated using the late LSR during LV diastole15-17 (Figure 2). Strain and strain rate are described in absolute values. Echocardiography reviewers were blinded at all times to the patients' clinical history and data to minimize bias.
Analysis of intraobserver and interobserver variability among 20 reassessed patients showed good correlations for peak LSR measurements [average intraclass correlation coefficient, 0.97; 95% confidence interval (CI), 0.93–0.99 and 0.91; 95% CI, 0.79–0.96, respectively].
Statistical analysis
Continuous variables are presented as mean ± standard deviation. High-sensitivity cardiac troponin T and B-type natriuretic peptide (BNP) were not normally distributed, so we selected natural logarithm (Ln) TnT and Ln BNP. Differences in continuous variables were analysed by the unpaired t-test or Mann–Whitney U-test, as appropriate. Categorical values are presented as number (percentage) and were compared using the χ2 test. Receiver operating characteristic (ROC) curve analysis was performed to compare the diagnostic accuracy and determine the cut-off values of the peak LSR and RapLSI for 99mTc-PYP scintigraphy positivity. The independent variables associated with 99mTc-PYP scintigraphy positivity were assessed by logistic regression analysis. To evaluate the independent variables associated with 99mTc-PYP scintigraphy positivity, the following variables were initially incorporated into univariate logistic regression analysis models: age, sex, hypertension, diabetes mellitus, dyslipidaemia, smoking, Ln TnT, Ln BNP, LVEF, posterior wall thickness (PWT), LV mass index, E/e', e', s', calcium channel blocker use, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, beta-blocker use, RapLSI, EF/GLS, peak LS, peak LSR, early LSR, and late LSR. Multivariable logistic regression analysis was performed using significant factors in the univariate logistic regression analysis after excluding variables with strong internal correlation and variables which might be important clinically. Pearson's correlation coefficient was used to investigate the correlations between the peak LSR and other LA strain findings. Differences in predictability between parameters were also assessed by calculating the category-free net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) in logistic model. All statistical tests were two-sided. A P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS Version 24 (IBM Corp., Armonk, NY, USA) and R programme Version 4.0.2 (package ‘PredictABEL’).18
Results
Baseline clinical characteristics, laboratory findings, and medications
Among 72 patients with AS who underwent 99mTc-PYP scintigraphy, 16 patients (22%) were positive, and 56 patients (78%) were negative for 99mTc-PYP scintigraphy. In the 99mTc-PYP scintigraphy-positive group, 12 patients underwent tissue biopsy, of whom 9 (75.0%) had TTR amyloid deposition. Among them, 8 patients underwent endomyocardial biopsy and TTR amyloid deposition in the heart was confirmed in all cases. DNA analysis was performed for 6 patients in the 99mTc-PYP scintigraphy-positive group and all patients did not have a TTR mutation (ATTRv).
Table 1 summarizes the baseline clinical characteristics, laboratory findings, and medications between the 99mTc-PYP scintigraphy-positive and scintigraphy-negative groups. There were no significant differences in age, sex, history of cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidaemia, and smoking), Ln BNP, eGFR, or medications [beta-blocker, angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), calcium channel blocker (CCB) and loop diuretics] between these 2 groups. In contrast, Ln TnT was significantly higher in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (−2.9 ± 0.5 vs. -3.5 ± 0.8, P < 0.05).
| All (n = 72) | 99mTc-PYP scintigraphy-positive (n = 16) | 99mTc-PYP scintigraphy-negative (n = 56) | P value | |
|---|---|---|---|---|
| Age (years) | 82.6 ± 7.7 | 85.1 ± 3.2 | 81.8 ± 8.4 | 0.19 |
| Male sex, n (%) | 32 (44.4) | 7 (43.8) | 25 (44.6) | 0.95 |
| NYHA III–IV, n (%) | 12 (16.7) | 3 (18.8) | 9 (16.1) | 0.41 |
| Hypertension, n (%) | 60 (83.3) | 13 (81.3) | 47 (83.9) | 0.53 |
| DM, n (%) | 18 (25.0) | 4 (25.0) | 14 (25.0) | 0.64 |
| Dyslipidaemia, n (%) | 40 (55.6) | 9 (56.3) | 31 (55.4) | 0.95 |
| Smoking, n (%) | 27 (48.2) | 5 (31.3) | 22 (39.3) | 0.46 |
| Atrial fibrillation, n (%) | 11 (15.3) | 2 (12.5) | 9 (16.1) | 0.54 |
| Severe AS, n (%) | 51 (70.8) | 8 (50.0) | 43 (76.8) | <0.05 |
| Ln TnT | −3.3 ± 0.8 | −2.9 ± 0.5 | −3.5 ± 0.8 | <0.05 |
| Ln BNP | 5.4 ± 1.0 | 5.5 ± 0.7 | 5.3 ± 1.1 | 0.20 |
| eGFR (mL/min/1.73 m2) | 46.3 ± 20.4 | 48.1 ± 9.5 | 45.1 ± 21.8 | 0.52 |
| Haemoglobin (g/dL) | 11.5 ± 1.7 | 11.8 ± 0.9 | 11.4 ± 1.9 | 0.23 |
| Beta-blockers, n (%) | 21 (29.2) | 5 (31.3) | 16 (28.6) | 0.53 |
| ACE-I or ARB, n (%) | 34 (47.2) | 6 (37.5) | 28 (50.0) | 0.38 |
| CCB, n (%) | 38 (52.8) | 7 (43.8) | 31 (55.4) | 0.41 |
| Diuretics, n (%) | 32 (44.4) | 10 (62.5) | 22 (39.3) | 0.10 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AS, aortic stenosis; BNP, B-type natriuretic peptide; CCB, calcium channel blocker; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Ln TnT, natural logarithm troponin T; NYHA, New York Heart Association; PYP, pyrophosphate.
- Data are presented as mean ± standard deviation and number (percentage). The P values were obtained by unpaired t-test, Mann–Whitney U-test, or χ2 test.
Conventional transthoracic echocardiography findings
Table 2 summarizes the conventional transthoracic echocardiography findings between the 99mTc-PYP scintigraphy-positive and scintigraphy-negative groups. A velocity and s' were significantly lower and IVST, PWT, and RWT were significantly higher in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (A velocity: 87 ± 38 cm/s vs. 107 ± 33 cm/s, P < 0.05 and s': 3.7 ± 1.0 cm/s vs. 4.7 ± 1.2 cm/s, P < 0.05, IVST: 14.6 ± 2.6 mm vs. 13.0 ± 2.5 mm, P < 0.05, PWT: 14.6 ± 3.3 mm vs. 12.4 ± 2.1 mm, P < 0.05 and RWT: 0.70 ± 0.15 vs. 0.58 ± 0.14, P < 0.05, respectively). There were no significant differences in the severity of AS evaluated by transaortic maximum velocity (Vmax) and aortic valve area obtained by Doppler echocardiography between these two groups.
| All (n = 72) | 99mTc-PYP scintigraphy-positive (n = 16) | 99mTc-PYP scintigraphy-negative (n = 56) | P value | |
|---|---|---|---|---|
| LVEF (%) | 59.4 ± 8.9 | 57.8 ± 8.2 | 59.9 ± 9.2 | 0.22 |
| LVDd (mm) | 43.1 ± 6.1 | 42.1 ± 4.5 | 43.5 ± 6.5 | 0.43 |
| IVST (mm) | 13.3 ± 2.6 | 14.6 ± 2.6 | 13.0 ± 2.5 | <0.05 |
| PWT (mm) | 12.9 ± 2.5 | 14.6 ± 3.3 | 12.4 ± 2.1 | <0.05 |
| RWT | 0.61 ± 0.15 | 0.70 ± 0.15 | 0.58 ± 0.14 | <0.05 |
| LVMI (g/m2) | 176 ± 56 | 197 ± 68 | 170 ± 51 | 0.12 |
| LAVI (mL/m2) | 64.4 ± 20.2 | 65.8 ± 20.2 | 64.1 ± 20.4 | 0.66 |
| SV (mL) | 67.2 ± 23.9 | 59.4 ± 20.3 | 67.8 ± 25.0 | 0.10 |
| E velocity (cm/s) | 81.5 ± 27.1 | 80.9 ± 25.0 | 81.6 ± 27.9 | 0.93 |
| A velocity (cm/s) | 103 ± 34 | 87 ± 38 | 107 ± 33 | <0.05 |
| E/A | 0.89 ± 0.74 | 1.30 ± 1.35 | 0.77 ± 0.37 | 0.21 |
| E/e' | 21.3 ± 9.1 | 22.1 ± 8.9 | 21.1 ± 9.4 | 0.41 |
| e' (cm/s) | 3.9 ± 1.5 | 3.7 ± 1.2 | 4.0 ± 1.6 | 0.72 |
| a' (cm/s) | 7.2 ± 2.5 | 6.3 ± 2.9 | 7.5 ± 2.4 | 0.10 |
| s' (cm/s) | 4.5 ± 1.2 | 3.7 ± 1.0 | 4.7 ± 1.2 | <0.05 |
| Vmax (m/s) | 4.2 ± 0.7 | 3.9 ± 0.7 | 4.3 ± 0.7 | 0.06 |
| AVA (doppler) (cm2) | 0.71 ± 0.27 | 0.74 ± 0.25 | 0.71 ± 0.28 | 0.48 |
- a', tissue Doppler late diastolic mitral annular velocity; AS, aortic stenosis; ATTR-CM, transthyretin amyloid cardiomyopathy; AVA (doppler), aortic valve area obtained by Doppler echocardiography; A velocity, atrial filling velocity; E/A, ratio of E velocity to A velocity; E/e', the ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; E velocity, early diastolic mitral flow velocity; IVST interventricular septum thickness; LAVI, left atrial volume index; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PWT, posterior wall thickness; PYP, pyrophosphate; RWT, relative wall thickness; s', systolic mitral annular velocity; SV, stroke volume; Vmax, transaortic maximum velocity.
- Data are presented as mean ± standard deviation. The P values were obtained by unpaired t-test or Mann–Whitney U-test.
Two-dimensional speckle tracking echocardiography
Table 3 showed the 2D speckle tracking echocardiography between the 99mTc-PYP scintigraphy-positive and scintigraphy-negative groups. In LV strain analysis, there were no significant differences in GLS, apical LS, and LVEF/GLS ratio between these two groups. In contrast, mid LS and basal LS were significantly lower and RapLSI was significantly higher in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (mid LS: 9.6 ± 2.7% vs. 11.6 ± 3.4%, P < 0.05, basal LS: 7.0 ± 3.3% vs. 10.3 ± 3.5%, P < 0.05 and RapLSI: 1.09 ± 0.49 vs. 0.78 ± 0.23, P < 0.05). In LA strain analysis, the peak LS, the peak LSR, and the late LSR were significantly lower in the 99mTc-PYP scintigraphy-positive than scintigraphy-negative group (peak LS: 9.6 ± 4.5% vs. 15.2 ± 5.6%, P < 0.05, peak LSR: 0.36 ± 0.14 s−1 vs. 0.55 ± 0.20 s−1, P < 0.05 and late LSR: 0.26 ± 0.25 s−1 vs. 0.52 ± 0.29 s−1, P < 0.05, respectively). There was no significant difference in the early LSR between these two groups.
| All (n = 72) | 99mTc-PYP scintigraphy-positive (n = 16) | 99mTc-PYP scintigraphy-negative (n = 56) | P value | |
|---|---|---|---|---|
| GLS (%) | 12.8 ± 3.3 | 11.4 ± 2.5 | 13.3 ± 3.4 | 0.43 |
| Apical LS (%) | 16.7 ± 5.0 | 16.4 ± 3.7 | 16.9 ± 5.4 | 0.74 |
| Mid LS (%) | 11.1 ± 3.3 | 9.6 ± 2.7 | 11.6 ± 3.4 | <0.05 |
| Basal LS (%) | 9.6 ± 3.7 | 7.0 ± 3.3 | 10.3 ± 3.5 | <0.05 |
| Relative apical LS index | 0.85 ± 0.32 | 1.09 ± 0.49 | 0.78 ± 0.23 | <0.05 |
| LVEF/GLS | 4.6 ± 1.6 | 5.1 ± 1.3 | 4.5 ± 1.7 | 0.10 |
| Peak LS (%) | 14.0 ± 5.8 | 9.6 ± 4.5 | 15.2 ± 5.6 | <0.05 |
| Peak LSR (1/s) | 0.51 ± 0.20 | 0.36 ± 0.14 | 0.55 ± 0.20 | <0.05 |
| Early LSR (1/s) | 0.38 ± 0.21 | 0.39 ± 0.22 | 0.37 ± 0.21 | 0.77 |
| Late LSR (1/s) | 0.47 ± 0.30 | 0.26 ± 0.25 | 0.52 ± 0.29 | <0.05 |
- AS, aortic stenosis; ATTR-CM, transthyretin amyloid cardiomyopathy; GLS, global longitudinal strain; LS, longitudinal strain; LSR, longitudinal strain rate; LVEF/GLS, ratio of left ventricular ejection fraction to GLS; PYP, pyrophosphate; relative apical LS index, average apical LS/ (average basal LS + average mid LS).
- Data are presented as mean ± standard deviation. The P values were obtained by unpaired t-test or Mann–Whitney U-test.
Logistic regression analysis for 99mTc-PYP scintigraphy positivity
As shown in Table 4, the univariate logistic regression analysis showed that the Ln TnT, PWT, s', RapLSI, peak LS, peak LSR, and late LSR were associated with 99mTc-PYP scintigraphy positivity. Because the peak LS and late LSR had strong internal correlation with the peak LSR (peak LS: r = 0.88, P < 0.05 and late LSR: r = −0.57; P < 0.05, respectively), we incorporated the Ln TnT, PWT, s', RapLSI, peak LSR, age, sex, atrial fibrillation, hypertension, diabetes mellitus, dyslipidaemia, and smoking into the multivariable logistic regression analysis. As a result, multivariable logistic analysis using five forced inclusion models identified that peak LSR and RapLSI were independently and significantly associated with 99mTc-PYP scintigraphy positivity (Table 5).
| Variables | Univariate analysis | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Age | 1.09 | 0.98–1.21 | 0.13 |
| Sex (male) | 0.96 | 0.31–2.95 | 0.94 |
| Hypertension | 0.83 | 0.20–3.52 | 0.80 |
| DM | 1.00 | 0.28–3.61 | 1.00 |
| Dyslipidaemia | 1.04 | 0.34–3.18 | 0.95 |
| Smoking | 0.64 | 0.20–2.11 | 0.46 |
| Atrial fibrillation | 1.33 | 0.36–4.94 | 0.67 |
| Ln TnT | 2.86 | 1.23–6.67 | <0.05 |
| Ln BNP | 1.47 | 0.82–2.65 | 0.20 |
| LVEF | 0.98 | 0.92–1.04 | 0.40 |
| PWT(*100) | 1.04 | 1.01–1.06 | <0.05 |
| LVMI | 1.00 | 1.00–1.00 | 0.65 |
| E/e' | 1.01 | 0.95–1.07 | 0.71 |
| e' | 0.90 | 0.59–1.37 | 0.62 |
| s' | 0.45 | 0.25–0.81 | <0.05 |
| CCB | 0.63 | 0.21–1.92 | 0.41 |
| ACE-I or ARB | 0.60 | 0.19–1.88 | 0.38 |
| Beta-blockers | 1.14 | 0.34–3.79 | 0.84 |
| RapLSI (*100) | 1.03 | 1.01–1.05 | <0.05 |
| EF/GLS | 1.26 | 0.89–1.78 | 0.21 |
| Peak LS | 0.79 | 0.69–0.92 | <0.05 |
| Peak LSR (*100) | 0.93 | 0.89–0.97 | <0.05 |
| Early LSR (*100) | 1.00 | 0.97–1.03 | 0.98 |
| Late LSR (*100) | 1.04 | 1.01–1.06 | <0.05 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AS, aortic stenosis; ATTR-CM, transthyretin amyloid cardiomyopathy; BNP, B-type natriuretic peptide; CCB, calcium channel blocker; CI, confidence interval; DM, diabetes mellitus; E/e', the ratio of early transmitral flow velocity to tissue Doppler early diastolic mitral annular velocity; EF/GLS, the ratio of left ventricular ejection fraction to global longitudinal strain; Ln TnT, natural logarithm troponin T; LS, longitudinal strain; LSR, longitudinal strain rate; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PWT, posterior wall thickness; PYP, pyrophosphate; relative apical LS index, average apical longitudinal strain (LS)/(average basal LS + average mid LS); s', systolic mitral annular velocity.
| Models | Multivariable analysis | ||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Model 1 | |||
| Peak LSR (*100) | 0.94 | 0.89–0.98 | <0.05 |
| RapLSI (*100) | 1.03 | 1.00–1.06 | <0.05 |
| s' | 0.72 | 0.37–1.42 | 0.35 |
| Model 2 | |||
| Peak LSR (*100) | 0.93 | 0.89–0.98 | <0.05 |
| RapLSI (*100) | 1.03 | 1.00–1.06 | <0.05 |
| PWT (*100 cm) | 1.03 | 0.99–1.06 | 0.09 |
| Model 3 | |||
| Peak LSR (*100) | 0.93 | 0.89–0.98 | <0.05 |
| RapLSI (*100) | 1.03 | 1.00–1.07 | <0.05 |
| Ln TnT | 1.91 | 0.69–5.36 | 0.21 |
| Model 4 | |||
| Peak LSR (*100) | 0.92 | 0.86–0.98 | <0.05 |
| RapLSI (*100) | 1.03 | 1.00–1.07 | <0.05 |
| Age | 1.16 | 0.97–1.39 | 0.11 |
| Sex | 1.50 | 0.35–6.42 | 0.58 |
| Atrial fibrillation | 0.49 | 0.07–3.46 | 0.48 |
| Model 5 | |||
| Peak LSR (*100) | 0.89 | 0.83–0.96 | <0.05 |
| RapLSI (*100) | 1.04 | 1.01–1.08 | <0.05 |
| Hypertension | 0.15 | 0.01–1.82 | 0.14 |
| Diabetes mellitus | 2.77 | 0.41–18.6 | 0.30 |
| Dyslipidaemia | 0.60 | 0.13–2.77 | 0.51 |
| Smoking | 1.63 | 0.33–8.14 | 0.55 |
- CI, confidence interval; Ln TnT, natural logarithm troponin T; LSR, longitudinal strain rate; PWT, posterior wall thickness; PYP, pyrophosphate; RapLSI; relative apical LS index; s', systolic mitral annular velocity.
Category-free net reclassification improvement and integrated discrimination improvement in logistic model
The NRI and IDI of the peak LSR for the relative apical LS index were 0.46 (95% CI, −0.08 to 1.00; P = 0.09) and 0.05 (95% CI, −0.12 to 0.21; P = 0.56), respectively. The NRI and IDI of the peak LSR for the s' were 0.59 (95% CI, 0.07–1.11; P < 0.05) and 0.08 (95% CI, −0.019 to 0.17; P = 0.12), respectively. Considered by NRI, the peak LSR tended to improve the discrimination ability compared with s'.
Receiver operating characteristic analysis for 99mTc-PYP scintigraphy positivity
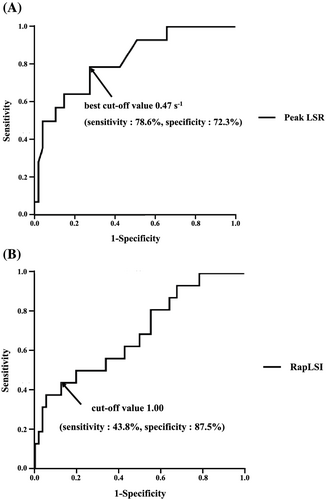
In the ROC analysis of peak LSR for 99mTc-PYP scintigraphy positivity, the area under the curve (AUC) was 0.79 (95% CI, 0.66–0.91; P < 0.05), and the best cut-off value of the peak LSR was 0.47 s−1 (sensitivity: 78.6%, specificity: 72.3%) for prediction of 99mTc-PYP scintigraphy positivity (Figure 3). In the ROC analysis of RapLSI for 99mTc-PYP scintigraphy positivity, the AUC was 0.69 (95% CI, 0.54–0.84; P < 0.05). Because it was difficult to decide the cut-off point by ROC analysis, we decided the cut-off point of RapLSI as 1.00 according to the previous report13 (Figure 3).
Predictive model for 99mTc-PYP scintigraphy positivity
Using peak LSR ≤ 0.47 s−1, we examined 99m Tc-PYP scintigraphy positivity. The 99mTc-PYP scintigraphy positivity in patients with the peak LSR in LA ≤ 0.47 s−1 was 39.4% (13/33), and the 99mTc-PYP scintigraphy negativity in patients with the peak LSR in LA > 0.47 s−1 was 92.3% (36/39).
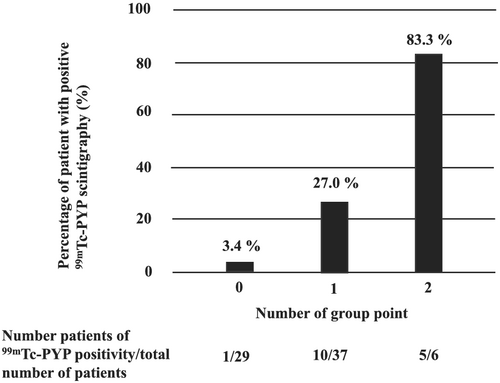
In addition to peak LSR, using RapLSI ≥ 1.0, we examined 99m Tc-PYP scintigraphy positivity. We divided the study patients into three groups according to the number of positive for each factor: 2-point group (peak LSR ≤ 0.47 s−1 and RapLSI ≥ 1.0, n = 6), 1-point group (peak LSR ≤ 0.47 s−1 and RapLSI < 1.0, or peak LSR > 0.47 s−1 and RapLSI ≥ 1.0, n = 37), and 0-point group (peak LSR > 0.47 s−1 and RapLSI < 1.0, n = 29). About 83.3% of 2-point group had a positive 99mTc-PYP scintigraphy. In contrast, 96.6% of 0-point group had a negative 99mTc-PYP scintigraphy (Figure 4).
Subgroup analysis
Next, we divided patients into severe and moderate AS group according to peak aortic valve velocity (severe AS: Vmax ≥ 4.0 m/s, moderate AS: Vmax < 4.0 m/s).
In severe AS group, the univariate logistic regression analysis showed that the peak LSR and RapLSI were significantly associated with 99mTc-PYP scintigraphy positivity (Supporting Information, Table S1a). In the ROC analysis, the AUC of peak LSR and RapLSI for 99mTc-PYP scintigraphy positivity were 0.75 (95% CI, 0.58–0.92; P < 0.05) (Figure S1a) and 0.69 (95% CI, 0.47–0.90; P = 0.09) (Figure S1b), respectively.
In moderate AS group, the univariate logistic regression analysis also showed that the peak LS and peak LSR were significantly associated with 99mTc-PYP scintigraphy positivity (Table S1b). In the ROC analysis, the AUC of peak LSR and RapLSI for 99mTc-PYP scintigraphy positivity were 0.80 (95% CI, 0.60–1.00; P < 0.05) (Figure S2a) and 0.66 (95% CI, 0.42–0.91; P = 0.22) (Figure S2b), respectively.
Discussion
The major finding of the present study is that the combination of LA and LV function evaluated by 2D speckle tracking echocardiography was useful to evaluate ATTR-CM in patients with AS.
In these days, non-biopsy diagnosis of ATTR-CM by using bone scintigraphy including 99mTc-PYP scintigraphy has been established.4 In our present study, 100% (8/8) of patients with 99mTc-PYP scintigraphy positivity had amyloid deposition in their hearts, indicating that 99mTc-PYP scintigraphy was useful to diagnose ATTR-CM even in patients with AS. However, this modality is costly and only available in specialist centres. Therefore, it is important to raise the pretest probability of 99mTc-PYP scintigraphy positivity by using inexpensive and less invasive methods. LV apical sparing, which is represented as a RapLSI in the present study, is a pattern of regional differences in deformation in which the LS in the basal and middle segments of the left ventricle is more severely impaired than that in the apical segments.13 LV apical sparing is highly specific for amyloid cardiomyopathy and has incremental diagnostic value over other echocardiographic parameters traditionally used for this purpose.19 Even in our present study, RapLSI was associated with 99mTc-PYP scintigraphy positivity. However, the AUC of RapLSI for predicting 99mTc-PYP scintigraphy positivity were relatively low, indicating that only RapLSI might not be enough to evaluate ATTR-CM in patients with AS. The stress and afterload imposed on the ventricle by a severely calcified and stenotic aortic valve might mask the LV apical sparing that is otherwise detected in a patient with ATTR-CM without AS. These might be the reason why RapLSI had low sensitivity to evaluate 99mTc-PYP scintigraphy positivity in ATTR-CM patients with AS. Thus, only RapLSI was not enough to evaluate ATTR-CM in patients with AS.
In contrast, our study revealed that LA dysfunction estimated by 2D speckle tracking echocardiography was important diagnostic marker for ATTR-CM in patients with AS. In usual, worse LA function is correlated with a greater impairment of LV systolic and diastolic function.20 LV systolic and diastolic dysfunction may contribute to LA dysfunction because the influence of downward motion of the mitral plane during ventricular systole leads to reduced systolic expansion of the LA.4, 17, 21 In addition, amyloid deposition occurs not only in the LV but also in the LA.22 Thus, LA dysfunction in patients with amyloid cardiomyopathy depend on the combination of restrictive LV function with high filling pressures and intrinsic LA function failure by direct amyloid infiltration. In patients without ATTR-CM, LA dysfunction depends on mainly restrictive LV function except for atrial fibrillation. Therefore, LA function is thought to be impaired more in AS patient with ATTR-CM than in those without ATTR-CM in our present study, which might be the reason why LA dysfunction was important diagnostic marker for ATTR-CM in our present study. Although atrial fibrillation is another important factor for LA dysfunction in ATTR-CM patients, atrial fibrillation was not significantly associated with 99mTc-PYP scintigraphy positivity in our present study. The frequency of atrial fibrillation increases according to the progression of ATTR-CM.23 In our present study, LVEF of 99mTc-PYP scintigraphy-positive group was relatively preserved compared with the previous report,24 suggesting that many ATTR-CM patients in 99mTc-PYP scintigraphy positive in this study might be diagnosed in the early phase of the disease. These might be the reason why there were only two atrial fibrillation patients in 99mTc-PYP scintigraphy-positive group and atrial fibrillation was not significantly associated with 99mTc-PYP scintigraphy positivity in our present study.
Two-dimensional speckle tracking echocardiography is a very helpful technique for investigation of advanced atrial functional components such as the reservoir phase, conduit phase, and active phase, which are otherwise difficult to investigate non-invasively.25-27 Several studies showed that LA reservoir and active function were impaired in various types of amyloid cardiomyopathy.4, 28 In our present study, the peak LSR, which represents LA reservoir function, was the strongest predictor of 99mTc-PYP scintigraphy positivity in patients with AS. In addition, late LSR, which represents LA contracting function, was also associated with 99mTc-PYP scintigraphy positivity. These indicated that both reservoir and active functions were impaired in AS patients with ATTR-CM. In contrast, LA conduit function was not significantly associated with 99mTc-PYP scintigraphy positivity. Nochioka et al. previously reported that LA conduit function did not differ significantly between patients with amyloid cardiomyopathy and control patients.4 Therefore, LA conduit function is thought to be compensatory mechanism when LA reservoir and contraction function are impaired.
Castaño et al.24 revealed that the s' is an excellent predictor for the concomitant amyloid cardiomyopathy in patients with AS. In our study, however, s' was not significantly associated with ATTR-CM after adjusting for peak LSR and RapLSI. Although Castaño et al. evaluated A velocity and LAVI in their study, they did not evaluate LA function in detail.24 Because s' is related to the absolute value of GLS, s' depends on only LV function.29 In contrast, peak LSR is affected by both LA and LV function, which might be the reason why peak LSR is more important than s' for diagnosis of ATTR-CM in our present study.
The aim of our study was to raise the pretest probability for 99mTc-PYP scintigraphy in AS patients by using 2D speckle tracking echocardiography. In our present study, we selected peak LSR and RapLSI as strongly significant factors for 99mTc-PYP scintigraphy positivity in AS patients. Of note, patients with high peak LSR and low RapLSI were rarely 99mTc-PYP scintigraphy positive, and almost all of patients with low peak LSR and high RapLSI were 99mTc-PYP scintigraphy positive. Based on these findings, we recommend performing 99mTc-PYP scintigraphy for AS patients with low peak LSR and high RapLSI on patients' selection for 99mTc-PYP scintigraphy. Because concomitant ATTR-CM is associated with a poor prognosis of AS patients,30 it is important to raise the pretest probability of 99mTc-PYP scintigraphy positivity by using combination of LA and LV strain analysis estimated by 2D speckle tracking echocardiography for patients with AS.
Study limitations
This study had several limitations. First, it included a small number of patients and was performed at a single centre. Although a similar cohort would be useful to support our results, this is not possible because there are no similar cohorts. Second, we obtained echocardiographic images using several ultrasound vendors. The 2D speckle tracking echocardiography analysis was performed with TomTec Image-Arena™ (vendor-independent) software. Although there are significant correlations in the LS values analysed using vendor-independent software for paired images obtained from different ultrasound vendors,31 inter-vendor variability still might have affected our study results. Third, there is a possibility of referral bias, although we used a standardized protocol and the 99mTc-PYP scintigraphy images were interpreted by the same independent operators. Forth, we divided patients into three subgroups to make predictive model for 99mTc-PYP scintigraphy positivity. However, there were small samples in each subgroup, affecting the possibility to get reliable results statistically. This point was another important limitation in our present study. Despite these limitations, this is the first cohort study to evaluate the usefulness of the combination of LA and LV function evaluated by 2D speckle tracking echocardiography in elderly patients with moderate to severe AS who underwent 99mTc-PYP scintigraphy for suspected amyloid cardiomyopathy. Further prospective studies involving more patients with AS are needed to validate our results.
Conclusions
Left atrial and LV strain analysis were significantly associated with 99mTc-PYP scintigraphy positivity in ATTR-CM patients with moderate to severe AS. Combination of LA and LV strain analysis might be useful to diagnose ATTR-CM in patients with moderate to severe AS.
Acknowledgements
We thank Angela Morben, DVM, ELS, from Edanz Group (https://en-author-services.edanzgroup.com/ac), for editing a draft of this manuscript.
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant 20K08476 to H.U.).




