Epidemiology of cardiogenic shock and cardiac arrest complicating non-ST-segment elevation myocardial infarction: 18-year US study
Abstract
Aims
This study aims to evaluate the impact of the combination of cardiogenic shock (CS) and cardiac arrest (CA) complicating non-ST-segment elevation myocardial infarction (NSTEMI).
Methods and results
Adult (>18 years) NSTEMI admissions using the National Inpatient Sample database (2000 to 2017) were stratified by the presence of CA and/or CS. Outcomes of interest included in-hospital mortality, early coronary angiography, hospitalization costs, and length of stay. Of the 7 302 447 hospitalizations due to NSTEMI, 147 795 (2.0%) had CS only, 155 522 (2.1%) had CA only, and 41 360 (0.6%) had both CS and CA. Compared with 2000, the adjusted odds ratios (ORs) and 95% confidence interval (CIs) for CS, CA, and both CS and CA in 2017 were 3.75 (3.58–3.92), 1.46 (1.42–1.50), and 4.52 (4.16–4.87), respectively (all P < 0.001). The CS + CA (61.2%) cohort had higher multiorgan failure than CS (42.3%) and CA only (32.0%) cohorts, P < 0.001. The CA only cohort had lower rates of overall (52% vs. 59–60%) and early (17% vs. 18–27%) angiography compared with the other groups (all P < 0.001). CS + CA admissions had higher in-hospital mortality compared with those with CS alone (aOR 4.12 [95% CI 4.00–4.24]), CA alone (aOR 1.69 [95% CI 1.65–1.74]), or without CS/CA (aOR 22.66 [95% CI 22.06–23.27]). The presence of CS, either alone or with CA, was associated with higher hospitalization costs and longer hospital length of stay.
Conclusions
The combination of CS and CA is associated with higher rates of acute non-cardiac organ failure and in-hospital mortality in NSTEMI admissions as compared with those with either CS or CA alone.
Introduction
Non-ST-segment elevation myocardial infarction (NSTEMI) has become the leading cause of hospitalization in patients with ischaemic heart disease and continues to be associated with high morbidity and mortality.1, 2 Increased use of revascularization and potent anti-thrombotic, anti-ischaemic and secondary prevention medications has resulted in low in-hospital mortality and shorter hospital stay in NSTEMI populations.3 About 5–7% of NSTEMI patients have concomitant cardiogenic shock (CS) and/or cardiac arrest (CA), which is associated with significantly higher in-hospital mortality and morbidity.4, 5 There are limited data on the overlap of CS and CA in NSTEMI patients,5-7 as prior studies have treated both entities in isolation.7, 8 We previously demonstrated that CA was associated with worse clinical outcomes in a national database of nearly 4.5 million admissions with acute MI and CS admissions.5 A recent statement on CS classification from the Society of Cardiovascular Angiography and Intervention emphasized the additional risk portended by CA at every CS stage.9, 10 In light of these data, it is crucial to define the epidemiology, clinical management, and outcomes of CS and CA complicating NSTEMI in the contemporary era.7, 8
In this study, we sought to assess the in-hospital mortality, resource utilization and temporal trends of CA and CS complicating NSTEMI admissions using a nationally representative database. We hypothesized that the combination of CA and CS was associated with higher in-hospital mortality than either entity alone.
Methods
Study population, variables and outcomes
The National (Nationwide) Inpatient Sample (NIS) is the largest all-payer database of hospital inpatient stays in the United States. NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the Healthcare Quality and Utilization Project (HCUP), sponsored by the Agency for Healthcare Research and Quality.11 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP-NIS does not aggregate admissions at the individual patient level but captures all information for a given admission. Institutional Review Board approval was not sought due to the publicly available nature of this de-identified database. These data are accessible to other authors via the HCUP-NIS database with the Agency for Healthcare Research and Quality.11
Using the HCUP-NIS data from 2000 to 2017, a retrospective cohort of adult admissions (≥18 years of age) with NSTEMI in the primary diagnosis field (International Classification of Diseases 9.0 Clinical Modification [ICD-9CM] 410.70–410.79 and ICD-10CM I21.4, I.22.2) was identified.12 Based on the presence or absence of CS (ICD-9CM 785.51; ICD-10CM R57.0) and CA (ICD-9CM 427.5, 427.41, 99.60 and 99.63; ICD-10CM I46.x, I49.01, I49.02; ICD-10PCS 5A12012), this NSTEMI population was classified into four cohorts—CA + CS, CS only, CA only and no CS/CA.5 The administrative codes for CS have been noted to have high positive predictive value (>90%) and specificity (>95%) but low sensitivity (>50%).13, 14 The administrative codes for CA show a high positive predictive value for the presence of CA but poor discrimination between in-hospital and out-of-hospital CA.15, 16 Demographic characteristics, hospital characteristics, coronary angiography, percutaneous coronary intervention (PCI), mechanical circulatory support (MCS), cardiac procedures, and non-cardiac organ support use were identified for all admissions using previously used methodologies from our group.4, 5, 16-24 Early coronary angiography was defined as that performed on hospital day zero, consistent with prior studies.4, 16, 19, 24 Acute non-cardiac organ failure was classified as respiratory, renal, hepatic, hematologic, and neurologic.5, 21, 22, 25 Involvement of ≥2 non-cardiac organ systems was classified as multiorgan failure.5 The Deyo's modification of the Charlson Comorbidity Index was used to identify co-morbid diseases (Supporting Information, Table S1).26
The primary outcome was in-hospital mortality. Secondary outcomes included time to angiography, performance of early coronary angiography, hospitalization costs, length of hospital stay, discharge disposition, use of do-not-resuscitate (DNR) status and palliative care referrals. The temporal trends of CS and CA, in-hospital mortality, non-cardiac organ failure, use of coronary angiography, PCI, coronary artery bypass grafting (CABG), and MCS over the study period were evaluated.
Statistical analysis
As recommended by HCUP-NIS, survey procedures using discharge weights provided with HCUP-NIS database were used to generate national estimates.27 Using the trend weights provided by the HCUP-NIS, samples from 2000–2011 were re-weighted to adjust for the 2012 HCUP-NIS re-design.27 One-way analysis of variance and t-tests were used to compare categorical and continuous variables, respectively. Multivariable logistic regression was used to analyse trends over time (with 2000 as the reference year). Univariable analysis of temporal trends and outcomes was performed and is represented as odds ratio (OR) with 95% confidence intervals (CI). Multivariable logistic regression analysis incorporating age, sex, race, socio-economic stratum, hospital characteristics, co-morbidities, acute organ failure, cardiac procedures, non-cardiac procedures, DNR status and palliative care referral was performed for in-hospital mortality. Multiple sub-group analyses stratifying by age (≤/>75 years), sex, race (white/non-white), time period (in tertiles of years), receipt of PCI, and receipt of MCS were performed to confirm the findings of the primary analysis. For the multivariable modelling, regression analysis with purposeful selection of statistically (liberal threshold of P < 0.20 in univariate analysis) and clinically relevant variables was conducted. The inherent restrictions of the HCUP-NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.27, 28 Pertinent considerations include not assessing individual hospital-level volumes (due to changes to sampling design detailed above), treating each entry as an ‘admission’ as opposed to individual patients, restricting the study details to inpatient factors because the HCUP-NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies. Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS v25.0 (IBM Corp, Armonk NY).
Results
In the period from 1 January 2000 to 31 December 2017, there were 7 302 447 admissions with a primary NSTEMI diagnosis, of which CS, CA and both were noted in 147 795 (2.0%), 155 522 (2.1%) and 41 360 (0.6%), respectively. The four cohorts had comparable age and race distributions (Table 1). Compared with the cohort without CS/CA, the CS + CA, CS only and CA only had higher rates of prior heart failure and were admitted more frequently to urban and large-sized hospitals (Table 1 and Supporting Information, Table S2). The cohort with CA, either alone or in combination with CS, was more often male and non-white, compared with the other cohorts (Table 1). During this 18-year period, there was a temporal decrease in NSTEMI admissions without CS/CA and a stable trend in the unadjusted rates of CA only (Figure 1). After adjustment for patient and hospital characteristics, there was a modest increase in the risk of CA (adjusted OR 1.46 [95% CI 1.42–1.50]), and a substantially larger increase in CS (adjusted OR 3.75 [95% CI 3.58–3.92]) and CS + CA (adjusted OR 4.52 [95% CI 4.16–4.87]) in 2017 compared with 2000 (all P < 0.001) (Figure 1).
| Characteristics | CS and CA (N = 41 360) | CS only (N = 147 795) | CA only (N = 155 522) | No CS or CA (N = 6 957 770) | P |
|---|---|---|---|---|---|
| Age (years) | 69.6 ± 12.0 | 71.9 ± 12.2 | 70.0 ± 12.9 | 69.0 ± 14.0 | <0.001 |
| Female sex | 36.2 | 40.5 | 38.3 | 42.5 | <0.001 |
| Race | |||||
| White | 63.5 | 65.8 | 63.3 | 64.1 | <0.001 |
| Black | 9.8 | 7.2 | 10.5 | 9.0 | |
| Othersa | 26.6 | 27.0 | 26.2 | 26.9 | |
| Quartile of median household income for zip code | |||||
| 0-25th | 27.1 | 26.6 | 26.4 | 25.7 | <0.001 |
| 26th–50th | 26.4 | 26.6 | 26.6 | 27.1 | |
| 51st-75th | 24.3 | 24.5 | 24.0 | 24.2 | |
| 75th–100th | 22.2 | 22.3 | 23.1 | 23.1 | |
| Primary payer | |||||
| Medicare | 66.6 | 70.8 | 66.9 | 62.5 | <0.001 |
| Medicaid | 7.2 | 5.9 | 6.3 | 6.1 | |
| Private | 19.4 | 16.9 | 20.3 | 24.3 | |
| Othersb | 6.9 | 6.3 | 6.5 | 7.1 | |
| Charlson Comorbidity Index | |||||
| 0–3 | 38.6 | 34.4 | 35.7 | 43.0 | <0.001 |
| 4–6 | 41.7 | 41.7 | 44.7 | 39.3 | |
| ≥7 | 19.7 | 23.9 | 19.6 | 17.6 | |
| Co-morbidities | |||||
| Hypertension | 41.0 | 43.3 | 50.3 | 57.2 | <0.001 |
| Hyperlipidaemia | 24.6 | 26.7 | 28.4 | 41.1 | <0.001 |
| Cancer | 5.3 | 6.0 | 6.8 | 7.7 | <0.001 |
| Heart failure | 45.6 | 52.9 | 38.7 | 27.6 | <0.001 |
| Prior CABG | 8.4 | 7.1 | 9.1 | 9.9 | <0.001 |
| Weekend admission | 26.7 | 25.3 | 26.2 | 25.2 | <0.001 |
| Hospital teaching status and location | |||||
| Rural | 4.8 | 6.6 | 8.2 | 10.7 | <0.001 |
| Urban non-teaching | 35.2 | 34.2 | 39.8 | 39.3 | |
| Urban teaching | 60.0 | 59.2 | 52.1 | 50.0 | |
| Hospital bed-size | |||||
| Small | 9.3 | 9.2 | 9.5 | 11.5 | <0.001 |
| Medium | 24.2 | 23.2 | 25.4 | 26.0 | |
| Large | 66.5 | 67.6 | 65.1 | 62.5 | |
| Hospital region | |||||
| Northeast | 17.5 | 19.8 | 19.3 | 20.7 | <0.001 |
| Midwest | 21.3 | 21.6 | 21.6 | 22.5 | |
| South | 39.9 | 39.1 | 41.4 | 40.4 | |
| West | 21.4 | 19.5 | 17.8 | 16.5 | |
| Acute non-cardiac organ failure | |||||
| Multiorgan | 61.2 | 42.3 | 32.0 | 5.1 | <0.001 |
| Respiratory | 65.2 | 45.1 | 37.6 | 6.3 | <0.001 |
| Renal | 50.9 | 46.4 | 27.8 | 12.1 | <0.001 |
| Hepatic | 15.2 | 8.2 | 4.2 | 0.4 | <0.001 |
| Hematologic | 15.2 | 14.6 | 7.6 | 3.6 | <0.001 |
| Neurologic | 27.5 | 10.1 | 20.2 | 2.0 | <0.001 |
| Coronary angiography | 59.7 | 59.9 | 51.6 | 59.5 | <0.001 |
| Percutaneous coronary intervention | 33.3 | 27.4 | 25.3 | 32.6 | <0.001 |
| Intravascular ultrasound | 1.5 | 1.5 | 1.2 | 1.5 | <0.001 |
| Coronary thrombectomy | 1.0 | 0.7 | 0.5 | 0.3 | <0.001 |
| Coronary artery bypass grafting | 17.1 | 26.0 | 14.4 | 9.4 | <0.001 |
| Invasive haemodynamic monitoringc | 18.4 | 19.8 | 7.6 | 4.3 | <0.001 |
| Mechanical circulatory support | |||||
| Total | 38.9 | 35.6 | 7.9 | 1.7 | <0.001 |
| IABP | 35.4 | 33.3 | 7.4 | 1.6 | <0.001 |
| pLVAD | 3.8 | 2.6 | 0.5 | 0.1 | <0.001 |
| ECMO | 1.6 | 0.5 | 0.2 | 0.0 | <0.001 |
| Invasive mechanical ventilation | 69.0 | 36.2 | 44.1 | 2.6 | <0.001 |
| Non-invasive ventilation | 4.1 | 6.2 | 3.3 | 1.8 | <0.001 |
| Acute haemodialysis | 4.9 | 3.6 | 2.3 | 0.5 | <0.001 |
- CA, cardiac arrest; CABG, coronary artery bypass grafting; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; NSTEMI, non-ST-segment elevation myocardial infarction; pLVAD, percutaneous left ventricular assist device.
- Represented as percentage or mean ± standard deviation.
- a Hispanic, Asian, Native American, and Others.
- b Uninsured, No charge, and Others.
- c Pulmonary artery catheterization or right heart catheterization.
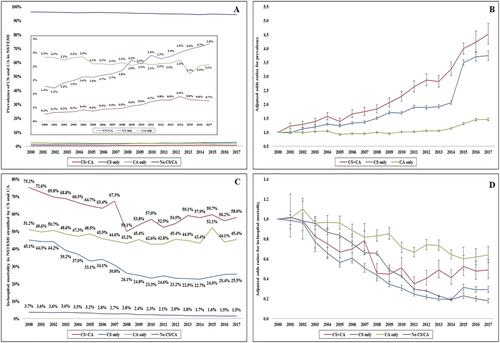
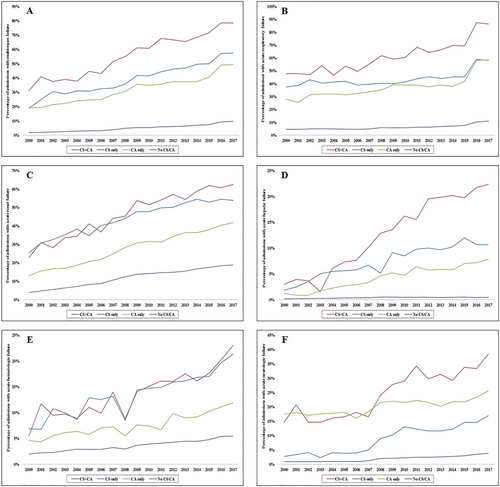
CS + CA was associated with higher rates of acute non-cardiac organ failure than either CS or CA alone (Table 1). The CS only cohort had higher rates of organ failure across all domains except neurologic compared with CA alone (Table 1). Over time, there was a steady increase in single-organ and multi-organ failure over the study period, with consistently higher rates in admissions with a combination of CS and CA (Figure 2). Coronary angiography and PCI were performed less commonly in the CA only cohort (51.6% and 25.3%, respectively) compared with the other cohorts (59–60% and 27–33%, respectively) (Table 1). The presence of CS, either alone or in combination with CA, was associated with higher rates of invasive haemodynamic monitoring, CABG, MCS, mechanical ventilation, and acute haemodialysis use (all P < 0.001) (Table 1). Over time, there was a steady increase in coronary angiography and PCI use, whereas CABG use remained relatively stable across all four cohorts (Figure 3). MCS use initially increased with a peak in 2009 and subsequently decreased in the CS + CA and CS only cohorts (Figure 3). Timing of coronary angiography was available in 3 637 205 (49.8%) admissions. The presence of CS either alone or in combination with CA was associated with higher use of early coronary angiography; however, the overall rates were <30% (Table 2).

| Outcomes | CS and CA (N = 41 360) | CS only (N = 147 795) | CA only (N = 155 522) | No CS or CA (N = 6 957 770) | P |
|---|---|---|---|---|---|
| Time to coronary angiography (days)a | 1.5 ± 3.2 | 1.5 ± 2.9 | 1.9 ± 3.2 | 1.3 ± 1.9 | <0.001 |
| Early coronary angiography (day zero)a | 27.3 | 24.6 | 17.4 | 18.4 | <0.001 |
| Do-not-resuscitate status | 11.5 | 9.4 | 6.0 | 2.7 | <0.001 |
| Palliative care referral | 9.0 | 7.3 | 4.1 | 1.0 | <0.001 |
| In-hospital mortality | 59.5 | 28.5 | 46.4 | 2.5 | <0.001 |
| Length of stay (days) | 10.7 ± 12.7 | 11.0 ± 11.0 | 8.6 ± 11.0 | 5.0 ± 5.2 | <0.001 |
| Hospitalization costs (×1000 USD) | 177 ± 227 | 157 ± 189 | 104 ± 138 | 55 ± 65 | <0.001 |
| Disposition | |||||
| Home | 25.9 | 30.7 | 44.7 | 60.0 | <0.001 |
| Transfer | 14.4 | 11.4 | 13.4 | 12.7 | |
| Skilled nursing facility | 44.4 | 36.7 | 26.6 | 14.5 | |
| Home with home health care | 14.8 | 20.8 | 14.6 | 11.9 | |
| Against medical advice | 0.5 | 0.4 | 0.6 | 0.9 |
- CA, cardiac arrest; CS, cardiogenic shock; NSTEMI, non-ST-segment elevation myocardial infarction.
- Represented as percentage or mean ± standard deviation.
- a Data available for 3 637 205 (49.8%) admissions.
Compared with NSTEMI admissions without CS or CA (in-hospital mortality 2.7%), the all-cause in-hospital mortality was higher in the other cohorts, that is, CS + CA (59.5%; OR 56.40 [95% CI 55.27–57.55]), CS only (28.5%; OR 15.34 [95% CI 15.16–15.53]), and CA only (46.4%; OR 33.24 [95% CI 32.87–33.61]) (all P < 0.001). The unadjusted in-hospital mortality in the cohort with CS + CA was higher than those with CS only (OR 3.68 [95% CI 3.59–3.76]) and CA only (OR 1.70 [95% CI 1.66–1.74] (all P < 0.001). In-hospital mortality steadily declined across all categories during the study period (Figure 1 and 1). After adjustment for potential confounders, risk of in-hospital mortality was higher in all three cohorts compared with NSTEMI without CS/CA, that is, CS + CA (adjusted OR 22.66 [95% CI 22.06–23.27]), CS only (adjusted OR 5.61 [95% CI 5.52–5.71]), and CA only (adjusted OR 24.48 [95% CI 24.13–24.85]) (all P < 0.001) (Table 3). Similar results were observed in pre-specified sub-groups stratified by age, sex, race, time period, use of PCI and use of MCS (Supporting Information, Table S3). The CS + CA cohort had higher adjusted in-hospital mortality compared with those with CS alone (adjusted OR 4.12 [95% CI 4.00–4.24]) or CA alone (adjusted OR 1.69 [95% CI 1.65–1.74]) (all P < 0.001). The CS + CA and CS only cohorts had higher use of palliative care referral and DNR status than the other groups (Table 2). The presence of CS, either alone or in combination with CA, was associated with longer hospital length of stay, higher hospitalization costs, and fewer discharges to home (all P < 0.001) (Table 2).
| NSTEMI admissions (N = 7 302 447) | Odds ratio | 95% confidence interval | ||
|---|---|---|---|---|
| Lower limit | Upper limit | P | ||
| Study cohort | ||||
| CS + CA | 22.66 | 22.06 | 23.27 | <0.001 |
| CS only | 5.61 | 5.52 | 5.71 | <0.001 |
| CA only | 24.48 | 24.13 | 24.85 | <0.001 |
| No CS/CA | Reference category | |||
| Age groups | ||||
| 19–49 years | Reference category | |||
| 50–59 years | 1.39 | 1.34 | 1.44 | <0.001 |
| 60–69 years | 1.64 | 1.58 | 1.69 | <0.001 |
| 70–79 years | 2.21 | 2.13 | 2.29 | <0.001 |
| ≥80 years | 3.43 | 3.31 | 3.56 | <0.001 |
| Female sex | 1.01 | 1.00 | 1.02 | 0.11 |
| Race | ||||
| White | Reference category | |||
| Black | 0.85 | 0.83 | 0.86 | <0.001 |
| Othersa | 1.03 | 1.02 | 1.04 | <0.001 |
| Primary payer | ||||
| Medicare | Reference category | |||
| Medicaid | 0.95 | 0.93 | 0.98 | <0.001 |
| Private | 0.79 | 0.78 | 0.81 | <0.001 |
| Othersb | 1.11 | 1.08 | 1.13 | <0.001 |
| Quartile of median household income for zip code | ||||
| 0–25th | Reference category | |||
| 26th–50th | 0.97 | 0.96 | 0.98 | <0.001 |
| 51st-75th | 0.90 | 0.89 | 0.92 | <0.001 |
| 75th–100th | 0.86 | 0.85 | 0.87 | <0.001 |
| Year of admission | ||||
| 2000 | Reference category | |||
| 2001 | 1.01 | 0.98 | 1.03 | 0.61 |
| 2002 | 0.99 | 0.97 | 1.01 | 0.41 |
| 2003 | 0.93 | 0.91 | 0.95 | <0.001 |
| 2004 | 0.85 | 0.83 | 0.87 | <0.001 |
| 2005 | 0.80 | 0.78 | 0.82 | <0.001 |
| 2006 | 0.74 | 0.72 | 0.75 | <0.001 |
| 2007 | 0.65 | 0.64 | 0.67 | <0.001 |
| 2008 | 0.63 | 0.61 | 0.65 | <0.001 |
| 2009 | 0.49 | 0.48 | 0.51 | <0.001 |
| 2010 | 0.44 | 0.43 | 0.45 | <0.001 |
| 2011 | 0.32 | 0.31 | 0.33 | <0.001 |
| 2012 | 0.31 | 0.31 | 0.32 | <0.001 |
| 2013 | 0.28 | 0.27 | 0.28 | <0.001 |
| 2014 | 0.24 | 0.24 | 0.25 | <0.001 |
| 2015 | 0.52 | 0.50 | 0.53 | <0.001 |
| 2016 | 0.41 | 0.39 | 0.42 | <0.001 |
| 2017 | 0.39 | 0.38 | 0.41 | <0.001 |
| Charlson Comorbidity Index | ||||
| 0–3 | Reference category | |||
| 4–6 | 1.92 | 1.88 | 1.97 | <0.001 |
| ≥7 | 2.38 | 2.32 | 2.44 | <0.001 |
| Hospital teaching status and location | ||||
| Rural | Reference category | |||
| Urban non-teaching | 1.01 | 0.99 | 1.02 | 0.48 |
| Urban teaching | 1.11 | 1.10 | 1.13 | <0.001 |
| Hospital bed-size | ||||
| Small | Reference category | |||
| Medium | 1.06 | 1.04 | 1.07 | <0.001 |
| Large | 1.16 | 1.14 | 1.17 | <0.001 |
| Hospital region | ||||
| Northeast | Reference category | |||
| Midwest | 0.88 | 0.87 | 0.90 | <0.001 |
| South | 0.98 | 0.96 | 0.99 | <0.001 |
| West | 0.77 | 0.76 | 0.78 | <0.001 |
| Acute organ dysfunction | ||||
| Respiratory | 2.25 | 2.23 | 2.28 | <0.001 |
| Renal | 2.08 | 2.06 | 2.10 | <0.001 |
| Hepatic | 1.94 | 1.89 | 1.99 | <0.001 |
| Haematologic | 1.29 | 1.26 | 1.31 | <0.001 |
| Neurological | 1.31 | 1.29 | 1.33 | <0.001 |
| Coronary angiography | 0.39 | 0.39 | 0.40 | <0.001 |
| Percutaneous coronary intervention | 0.56 | 0.55 | 0.57 | <0.001 |
| Coronary artery bypass grafting | 0.61 | 0.60 | 0.63 | <0.001 |
| Invasive haemodynamic monitoringc | 1.25 | 1.23 | 1.28 | <0.001 |
| Mechanical circulatory support | 2.46 | 2.42 | 2.51 | <0.001 |
| Invasive mechanical ventilation | 2.50 | 2.47 | 2.54 | <0.001 |
| Non-invasive mechanical ventilation | 1.26 | 1.24 | 1.29 | <0.001 |
| Haemodialysis | 1.51 | 1.47 | 1.56 | <0.001 |
| Palliative care referral | 7.02 | 6.89 | 7.16 | <0.001 |
| Do-not-resuscitate status | 2.86 | 2.81 | 2.91 | <0.001 |
- CA, cardiac arrest; CS, cardiogenic shock; NSTEMI, non-ST-segment elevation myocardial infarction.
- a Hispanic, Asian, Native American, and Others.
- b Uninsured, No charge, and Others.
- c Pulmonary artery catheterization or right heart catheterization.
Discussion
Among NSTEMI admissions in the United States during this 18-year study period, either CS or CA was noted in 4.7% of all admissions and 0.6% of admissions had a combination of the two conditions. There was a steady increase in CS over time with relatively stable rates in CA. The presence of CS or CA complicating NSTEMI was associated with worse outcomes, and the combination of the two diagnoses was associated with higher rates of acute organ failure and in-hospital mortality compared with either alone. The presence of CS, either alone or in combination with CA, was associated with longer length of stay and greater hospitalization costs.
Our study is consistent with population-based studies from Olmsted County, Minnesota, and Worchester, Massachusetts, that have demonstrated an increase in the prevalence of NSTEMI over time.29, 30 The use of cardiac troponin-T criteria and, more recently, high-sensitivity cardiac troponin-T criteria, in addition to an increase in the co-morbidity profile of patients, has resulted in a higher frequency of NSTEMI diagnosis in recent times.29 Using the HCUP-NIS database from 2006 to 2012, Kolte et al. demonstrated 2.5% of all NSTEMI admissions to be complicated by CS.8 CA was found to complicate 0.6–1.5% of all NSTEMI presentations in other national databases, such as NCDR-ACTION (National Cardiovascular Data Registry Acute Coronary Treatment And Intervention Outcomes Network) and NCDR-CathPCI registries.6, 7 The overlap of CS and CA in NSTEMI populations has not been as well studied as the STEMI populations.5, 7, 10, 31 We noted 11% of NSTEMI admissions with either CS or CA to have a combination of the two diagnoses. In 9682 MI patients with CA from the NCDR-ACTION registry, Kontos et al. showed concomitant CS in 38–45% of the population.6 Similarly, in 171 patients from the National Cardiogenic Shock Initiative study, 35–50% of patients had either in-hospital or out-of-hospital CA.32 These rates are significantly higher than our study because they included both STEMI and NSTEMI populations. CS and CA are more common in STEMI, which constitute nearly 70% of all MI patients with either CS or CA.6, 7, 32 In an older study from the NCDR-CathPCI registry, Gupta et al. showed CS to complicate 38% of all NSTEMI admissions with CA.7 These differences could be due to the inclusion of a longer time period in our study, the use of selective reporting (voluntary databases, PCI only databases), the use of administrative codes for the identification of CS and CA, and the nationally representative nature of this study. Furthermore, the prevalence of CA and CS is likely modulated by the use of an early invasive strategy, which remains sub-optimally utilized in this high-risk NSTEMI population.4, 8 Only one of four NSTEMI admissions with CS received early coronary angiography, although this rate was higher than for those without CS. Early coronary angiography, although recommended in high-risk NSTEMI patients, has been infrequently performed nationally.4, 33
In addition to highlighting the epidemiology of these conditions, our study highlights the clinical course and outcomes of the overlap of CS and CA in NSTEMI admissions. Our group previously demonstrated that concomitant CA was more common in admissions with multi-organ failure in CS complicating acute MI.5 CS follows a ‘haemo-metabolic cascade’ wherein the initial haemodynamic insult results in a worsening shock state characterized by systemic inflammation, metabolic injury, and end-organ damage.5, 34 This is further potentiated by the low-flow state during concomitant CA, contributing to worsening shock and organ injury. After CA, the presence of myocardial dysfunction worsens haemodynamic status and drives development of CS.35 Given the uncertainty regarding the optimal timing of coronary angiography in CA patients without ST-segment elevation on electrocardiogram, the NSTEMI population suffers from the additional vulnerability of delayed coronary revascularization.36 Furthermore, the lower rates of coronary angiography and PCI in this study are consistent with prior real-world literature that reflects reluctance to perform angiography in higher risk cohorts despite robust guideline recommendations supporting early coronary angiography in CS patients.33 The harmful effects of CA, in association with delayed coronary angiography and delayed recognition of CS, may contribute to the poor outcomes in this population.
The combination of CS and CA has been associated with worse in-hospital outcomes in acute MI.5-7 In over 400 000 acute MI-CS admissions, our group noted the presence of CA to be associated with three-fold higher in-hospital mortality.5 Using a cardiac intensive care unit patient database of 10 000 patients, our group demonstrated that CA is associated with incremental in-hospital mortality in patients with CS across the spectrum of CS severity, including in patients admitted with an acute MI.10 Using the NCDR-CathPCI registry, Gupta et al. demonstrated that the presence of CS in NSTEMI patients was associated with higher in-hospital mortality (38.5% vs. 6.6%).7 This study is of incremental value to these prior studies. In addition to in-hospital mortality, our study highlights the health care utilization—costs, hospital length of stay, and post-acute care utilization—all of which are notably higher in the cohort with CS and CA. Further studies are needed to understand the long-term implications of these disease states and their association with repeat hospitalizations and emergency department visits.
Limitations
This study has several limitations, some of which are inherent to the analysis of a large administrative database. The HCUP-NIS attempts to mitigate potential errors by using internal and external quality control measures. Given the administrative nature of this database, we cannot accurately distinguish type-1 from type-2 NSTEMIs. However, this study included admissions with a primary diagnosis of NSTEMI (i.e. the reason most likely for the admission) and, therefore, is less likely to include type-2 NSTEMI, which often have an alternate primary diagnosis. However, during the course of the study period, there was greater incorporation of troponin-T and troponin-I as the cardiac biomarker of choice, and the administrative codes changed from ICD-9CM to ICD-10CM in 2015, which may influence the selection of admissions in this database. The lack of angiographic data, such as PCI location, lesion classification, presence of multi-vessel disease, and revascularization failure, that may significantly influence outcomes, was not available in this database. Risk-stratification of NSTEMI patients, using standardized risk scoring systems, cannot be performed in the HCUP-NIS database and therefore limits our assessment of patient acuity. There is limited specific information on patients and their families wishes related to therapeutic options, which may influence the clinical outcomes in this population. It is conceivable that the increase in the organ dysfunction in this cohort may be related improvement in coding practices and earlier recognition and not the acuity of the illness. In this study, we are unable to identify the timing and causality of CS and CA precisely, and it is possible that CS was a consequence of CA (post-arrest shock). Likewise, we could not reliably discriminate in-hospital CA from out-of-hospital CA, despite potential differences in outcomes based on the specific diagnosis or procedure code used to define CA in prior administrative studies.37 Lastly, our group has previously evaluated the overlap of CS and CA in patients with STEMI38; however, outcomes of NSTEMI are typically different as compared with STEMI due to the higher co-morbidity, older age, and delayed revascularization.39, 40 Despite these limitations, this study addresses a significant knowledge gap as the first large-scale study in the contemporary era studying the interaction of CS and CA in NSTEMI admissions.
Conclusions
CS, CA, and their combination complicate nearly 5% of all NSTEMI admissions and are associated with significantly higher in-hospital mortality. Although the overall mortality from uncomplicated NSTEMI remains low (<3%), the combination of CS and CA is associated with significantly higher rates of acute non-cardiac organ failure and an in-hospital mortality rate of nearly 60%. Despite increase in use of coronary angiography and PCI in NSTEMI with/without CS and CA, there remains a crucial need to evaluate protocoled care for these patients including the timely incorporation of MCS devices. Further dedicated research related to the interactions of CS and CA in NSTEMI is crucial to improve the short-term outcomes in this acutely ill cohort.
Conflict of interest
None declared.
Funding
Dr. Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author contributions
S.V., J.C.J., A.P., L.R.S., K.K., N.D.S., and S.M.D. did the study design, literature review, and statistical analysis. S.V., J.C.J., A.P., L.R.S., K.K., N.D.S., and S.M.D. carried out the data management, data analysis, and drafting manuscript. S.V., J.C.J., A.P., L.R.S., K.K., N.D.S., and S.M.D. had access to data. J.C.J., A.P., L.R.S., K.K., N.D.S., and S.M.D. are responsible for the manuscript revision, intellectual revisions, and mentorship. All authors did the final approval of the study.




