Impact of readmissions on octogenarians with heart failure with preserved ejection fraction: PURSUIT-HFpEF registry
Abstract
Aims
Heart failure (HF) readmissions with preserved ejection fraction (HFpEF) are increasing in the elderly, which is a major socioeconomic problem. We investigated the clinical impact of HF readmissions (HFR) on octogenarians with HFpEF.
Methods and results
We enrolled consecutive octogenarians (≥80 years old) from June 2016 to February 2020 in PURSUIT-HFpEF registry. We divided them into HFR group readmitted for HF during the follow-up period and non-HF readmission (non-HFR) group. We evaluated the impact of HFR on all-cause mortality, cardiac death, and quality of life (QOL). Additionally, we evaluated the factors at discharge correlated with HFR. HFR group comprised 116 patients (21.4%). Among all-cause deaths, 40 patients suffered cardiac deaths (48.2%). The Kaplan–Meier analysis revealed a similar prognosis between HFR and non-HFR groups as well as similar incidences of HF deaths. The QOL scores had significantly deteriorated by 1 year later in the HFR group (0.71 ± 0.19 vs. 0.59 ± 0.21, P < 0.001), while it was similar at 1 year in the non-HFR group. In the multivariate analysis, diabetes mellitus (DM) (P = 0.019), N-terminal pro-B-type natriuretic peptide (NT-pro BNP) levels ≥ 1611 pg/mL (P < 0.001), and serum albumin level ≤ 3.7 g/dL (P = 0.011) were useful markers for HFR in octogenarians.
Conclusions
In octogenarians with HFpEF, HF readmission was not directly correlated with the prognosis but was well correlated with the QOL. Close follow-up is essential to decrease HFR of octogenarians with HFpEF with DM, high NT-pro BNP (≥1611 pg/mL) and low albumin (≤3.7 g/dL) levels at discharge.
Introduction
Heart failure (HF) is a pathological entity with an increasing incidence due to an increased longevity.1, 2 Elderly patients tend to be hospitalized for acute decompensated heart failure (ADHF).1, 3 Thus, recently, the management of HF in elderly patients is clinically important. On the other hand, HF with preserved ejection fraction (HFpEF) is an important clinical condition observed mainly in elderly patients.3, 4 Although HFpEF has an incidence that is comparable with that of HF with reduced ejection fraction (HFrEF), unlike HFrEF, there have been no significant improvements in the prognosis of HFpEF and no particular treatments for this entity.5 The number of hospitalizations for HF is increasing, especially in the elderly. This exerts a major social and economic impact.3 The concept of the deterioration of HF due to repeated episodes of ADHF was established over 10 years ago,6 and HF readmissions (HFR) are a strong predictor of mortality in community HF patients.7-9 Therefore, it is important to decrease HFR, but the clinical impact of readmissions for HFpEF on the elderly patients, especially octogenarians, has not been well investigated. It has been reported that the HFR rate for HFpEF is comparable with that for HFrEF.4 In general, octogenarians actually have several comorbidities that may have more influence on the prognosis than HF. Accordingly, in octogenarians with HFpEF, the effect on prognosis of decreasing HFR might be weak. On the other hand, for octogenarians, the quality of life (QOL) is also crucial in the consideration of longevity.
In this study, we focused on octogenarians with HFpEF. Our aim was to investigate the clinical impact of HFR and evaluate which factors at discharge were correlated with HFR in octogenarians with HFpEF.
Methods
PURSUIT-HFpEF registry
We performed a prospective, multicentre, observational cohort study in consecutive hospitalized patients with HFpEF defined as a left ventricular ejection fraction (LVEF) of ≥50%. Briefly, the PURSUIT-HFpEF (Prospective, mUlticenteR, obServational stUdy of patIenTs with Heart Failure with Preserved Ejection Fraction) registry is being conducted at the Osaka University Hospital, in collaboration with 32 hospitals in the Kansai area of Japan (UMIN-CTR ID: UMIN000021831).10 The aim of this large-scale registry is to collect and record a comprehensive range of patient data including demographic, laboratory, echocardiographic, and therapeutic data on admission, at discharge, and at each annual follow-up time, to analyse the various clinical questions and determine the prognostic factors in patients with HFpEF. Research cardiologists and specialized research nurses at each collaborating hospital were encouraged to enrol patients in the registry and record relevant patient data on admission, at discharge, and at designated follow-up visits. The obtained data were transferred to the data centre of Osaka University Hospital for processing and analysis. Acute decompensated HFpEF was diagnosed if the patients fulfilled the Framingham HF diagnostic criteria and the following criteria: (i) LVEF ≥ 50% and (ii) N-terminal pro-B-type natriuretic peptide (NT-pro BNP) level ≥ 400 pg/mL or B-type natriuretic peptide (BNP) level ≥ 100 pg/mL on admission.11 The exclusion criteria were as follows: (i) severe aortic stenosis, aortic regurgitation, mitral stenosis, or mitral regurgitation due to structural changes in the valve detected by transthoracic echocardiography; (ii) age < 20 years old; (iii) acute coronary syndrome on admission; (iv) a prognosis ≤ 6 months due to non-cardiac diseases; (v) status post-heart transplantation; and (vi) considered as otherwise not appropriate for the study by the attending physician.
Written informed consent was received from each participating patient. This study complied with the Declaration of Helsinki and has been approved by the Institutional Review Board of each participating site.
Study patients
In this study, we focused on ADHF in octogenarians with HFpEF. We extracted consecutive octogenarians (age ≥ 80 years old) with HFpEF from June 2016 to February 2020 in the PURSUIT-HFpEF registry. We excluded those patients who died during the hospitalization. Finally, we divided our study patients into the following two groups: an HFR group who were readmitted due to HF during the follow-up period and a non-HF readmission (non-HFR) group who were not readmitted due to HF after discharge. We evaluated the impact of the HFR on the all-cause mortality, cardiac death, and QOL. In addition, we evaluated the factors at discharge correlating with HFR.
Cause of death
In this study, we evaluated the causes of cardiac and non-cardiac death in the HFR and non-HFR groups. We classified cardiac deaths into those due to HF, myocardial infarctions, arrhythmias/sudden cardiac deaths, and others; and non-cardiac deaths into malignancy, infections, renal failure, cerebrovascular disease, and others. The cause of death was classified by the death certificate and/or the interview to the attending physician by research cardiologists or specialized research nurses at each collaborating hospital.
Evaluation of quality of life
In this study, we evaluated the QOL using the EQ-5D, an instrument for obtaining QOL score that is used to calculate quality-adjusted life years. The EQ-5D originally consisted of five items with three levels (EQ-5D-3L). Because the sensitivity of the EQ-5D-3L was insufficient and a ceiling effect existed, the EQ-5D-5L was redeveloped by changing the three-level system into a five-level system.12 In this study, we used the EQ-5D-5L to objectively evaluate the HFR and non-HFR patients' QOL.
Data collection at discharge
Investigative cardiologists and trained research nurses recorded the patients' data such as the past medical history including HF admissions, length of stay, comorbidities, therapeutic procedures, and clinical events from the medical records and by direct interviews of the patients and their family members during their hospital stay and after discharge. They also obtained the physical findings, vital signs, echocardiographic data, laboratory data, and medications at discharge. We evaluated the clinical frailty score13 as the physical frailty component and whether the patients were living alone or were nursing home residents for the social frailty component. In addition, the investigative cardiologists and trained research nurses followed all the study patients carefully to avoid lost to follow-up.
Laboratory measurements at discharge
Blood samples were collected at discharge. Laboratory measurements, including the sodium, chloride, potassium, creatinine, white blood cell count, haemoglobin, creatinine, estimated glomerular filtration rate, NT-pro BNP, uric acid, total cholesterol, triglycerides, cholinesterase, albumin, and haemoglobin A1c levels, were performed by the standard methods in the clinical laboratory of the participating hospital.
Echocardiographic data at discharge
A comprehensive echocardiographic examination was performed at discharge by trained physicians at each institution. The left ventricular diastolic diameter, left ventricular systolic diameter, left atrial diameter at end-systole, right ventricular diastolic diameter, tricuspid annular plane excursion, and inferior vena cava diameter (IVCD) were measured as previously described.14, 15 The LVEF was measured by the modified Simpson method.14 The E/e' was the mean of the septal and lateral E/e' values. Moderate mitral regurgitation and tricuspid regurgitation were also evaluated. The tricuspid regurgitation pressure gradient (TRPG) was measured by a simplified Bernoulli equation.14
Medications at discharge
We evaluated the medications at discharge to determine if the following medications were prescribed: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β blockers, diuretics, mineralocorticoid receptor antagonists, and statins. We also evaluated sodium-glucose co-transporter 2 inhibitor and insulin as medication for diabetes mellitus (DM).
Statistical analysis
JMP 15 statistical software (SAS Institute Inc., Cary, North Carolina, USA) was used for statistical analysis. Normally distributed data are expressed as the mean ± standard deviation, non-parametric data as the median (interquartile range), and categorical data as the number (percentage). Continuous variables were compared with the Mann–Whitney U test, and categorical variables with Fisher's exact test. Comparisons between the HFR and non-HFR groups for all-cause mortality and cardiac death were estimated using Kaplan–Meier curves, and statistical significance was determined using the log-rank test. A univariate analysis with a Cox proportional hazards regression model for HFR was performed. Multivariate analyses with a Cox proportional hazards regression model for HFR was performed using the factors that had a P of <0.05 as a result of the univariate analysis with a Cox proportional hazards regression model. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated. A receiver operating characteristic curve analysis was performed to define the cut-off value for predicting HFR. Values of P < 0.05 were considered statistically significant.
Results
Study patients
We enrolled 871 patients from the PURSUIT-HFpEF registry between June 2016 and February 2020. We excluded 116 patients whose age was <80 years old. We also excluded 13 patients who died in hospital. Our study patients consisted of 541 octogenarians (≥80 years old) with HFpEF (Figure 1). The HFR group consisted of 116 patients (21.4%) and the non-HFR group of 425 patients. In HFR group, 22 patients (19.0%) readmitted within 1 month, 43 patients (37.0%) readmitted within 3 months, and 64 patients (55.2%) readmitted within 6 months after discharge.
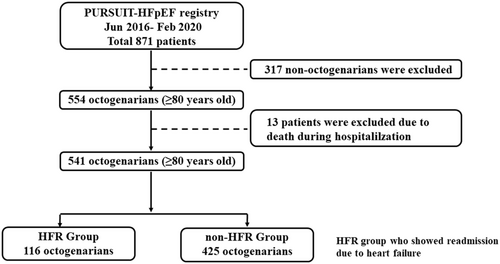
The incidence of hyperuricemia and the history of previous HF admissions were significantly higher in the HFR than the non-HFR group. The age, gender, body mass index, frailty parameters, and other comorbidities did not differ between the HFR and non-HFR groups (Table 1). Among the laboratory data, the HFR group had a lower haemoglobin, creatinine, and estimated glomerular filtration rate levels and higher NT-pro BNP and uric acid levels than the non-HFR group. Among the echocardiographic data, IVCD, and TRPG were larger in the HFR group than non-HFR group. The incidences of β blocker and diuretics use were higher in the HFR group than in the non-HFR group.
| Clinical data at discharge | HFR group (N = 116) | non-HFR group (N = 425) | P value |
|---|---|---|---|
| Age, years | 86.1 ± 4.2 | 86.2 ± 4.4 | 0.793 |
| Male, n (%) | 48 (41.4) | 177 (41.4) | 0.996 |
| Body mass index (kg/m2) | 23.4 (20.5–26.5) | 23.2 (20.7–26.4) | 0.720 |
| Clinical frailty score | 4 (3–6) | 4 (3–6) | 0.127 |
| Living alone, n (%) | 52 (44.8) | 156 (36.4) | 0.107 |
| Nursing home resident, n (%) | 17 (14.7) | 40 (9.3) | 0.122 |
| History of HF admission, n (%) | 45 (38.8) | 95 (22.1) | <0.001 |
| Length of stay (days) | 17 (13–24) | 17 (13–24) | 0.931 |
| Systolic blood pressure (mmHg) | 116 (107–129) | 118 (106–129) | 0.719 |
| Pulse pressure (mmHg) | 63 (42–63) | 42 (52–64) | 0.913 |
| Diastolic pressure (mmHg) | 62 (56–72) | 64 (57–72) | 0.629 |
| Heart rate (bpm) | 72 (64–80) | 71 (61–80) | 0.478 |
| Atrial fibrillation, n (%) | 49 (42.2) | 166 (38.8) | 0.522 |
| Hypertension, n (%) | 98 (84.5) | 363 (84.8) | 0.930 |
| Diabetes mellitus, n (%) | 40 (34.5) | 122 (28.5) | 0.211 |
| Dyslipidaemia, n (%) | 46 (39.7) | 159 (37.1) | 0.666 |
| Hyperuricemia | 50 (43.1) | 135 (31.8) | 0.027 |
- HF, heart failure; HFR, heart failure readmission.
Impact of heart failure readmissions on all-cause mortality and cardiac death
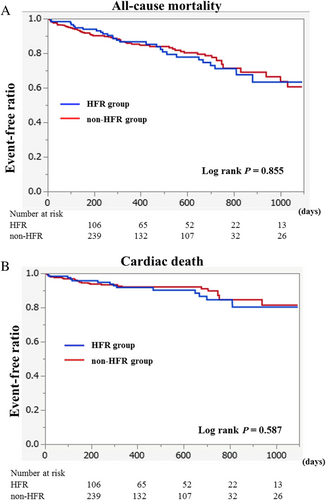
The mean follow-up duration was 503 ± 284 days. There were no patients with lost to follow-up. All-cause deaths occurred in 83 patients, with cardiac deaths in 40 (48.2% of all-cause deaths). The Kaplan–Meier analysis showed that the HFR and non-HFR groups had similar prognoses regarding all-cause mortality and cardiac deaths as shown in Figure 2.
Causes of cardiac and non-cardiac death
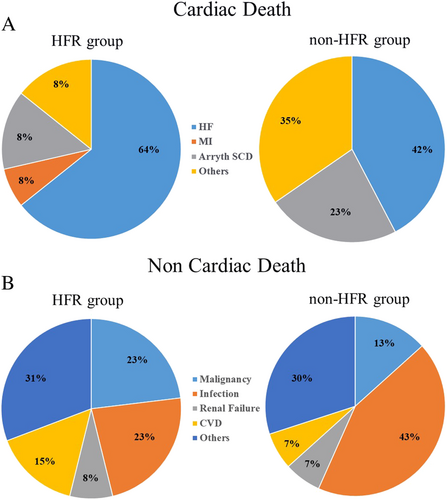
In the HFR group, cardiac deaths occurred in 14 patients and non-cardiac deaths in 13. In the non-HFR group, cardiac deaths occurred in 26 patients and non-cardiac deaths in 30. The incidence of cardiac deaths among the all-cause deaths in the HFR and non-HFR groups was similar (52% vs. 46%, P = 0.815). A detailed classification of the causes of cardiac deaths and non-cardiac deaths between the two groups is shown in Figure 3. The incidence of HF causing cardiac death was similar between the HFR group and non-HFR group (64% vs. 42%, P = 0.320) (Figure 3). In addition, the incidence of infections, which was a major cause of non-cardiac deaths, was also similar between the HFR group and non-HFR group (23% vs. 43%, P = 0.737) (Figure 3).
Impact of heart failure readmissions on quality of life
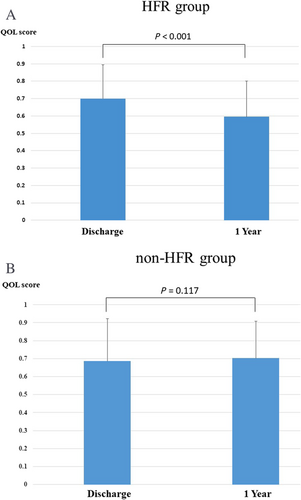
We evaluated the QOL using the EQ-5D in 66 patients from the HFR group and 142 from the non-HFR group in whom the EQ-5D scoring data at both discharge and 1 year later could be obtained. As shown in Figure 4, the baseline QOL scores were similar between the HFR and non-HFR groups (0.71 ± 0.19 vs. 0.69 ± 0.23, P = 0.519). The QOL scores had significantly deteriorated by 1 year later in the HFR group (0.71 ± 0.19 vs. 0.59 ± 0.21, P < 0.001) (Figure 4), whereas there were no significant differences in the QOL scores at 1 year in the non-HFR group (0.69 ± 0.23 vs. 0.71 ± 0.21, P = 0.117) (Figure 4). In addition, there were no significant difference of the number of readmission due to any other reason except HF between the HFR group and the non-HFR group (2.6% vs. 3.6%, P = 0.09).
Factors at discharge correlated with heart failure readmissions
A univariate Cox proportional hazards analysis showed that the clinical frailty score, living alone, DM, left atrial diameter at end-systoles, NT-pro BNP, albumin, and cholinesterase were significantly associated with HFR (Table 2). A multivariate Cox proportional hazards analysis showed that DM (HR 1.49, 95% CI; 1.07–2.09, P = 0.0191) and the NT-pro BNP (HR 1.00, 95% CI; 1.00–1.00, P < 0.001) and albumin (HR 0.62, 95% CI; 0.43–0.90, P = 0.011) levels were independently and significantly correlated with HFR (Table 2). An receiver operating characteristic curve analysis revealed cut-off values of NT-pro BNP ≥ 11 611 pg/mL (area under curve: 0.641, sensitivity: 67.6%, specificity: 58.3%) and albumin levels ≤3.7 g/dL (area under curve: 0.506, sensitivity: 79.6%, specificity: 26.1%), respectively, for predicting HFR.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.01 | 0.99–1.04 | 0.300 | |||
| Clinical frailty score | 1.10 | 1.03–1.18 | 0.006 | 1.00 | 0.92–1.10 | 0.894 |
| Living alone | 1.42 | 1.07–1.87 | 0.013 | 1.17 | 0.84–1.64 | 0.347 |
| History of HF admission | 1.10 | 0.69–1.20 | 0.487 | |||
| Hypertension | 1.15 | 0.80–1.56 | 0.525 | |||
| Diabetes mellitus | 1.47 | 1.13–1.91 | 0.003 | 1.49 | 1.07–2.09 | 0.019 |
| Hyperuricemia | 1.10 | 0.85–1.41 | 0.464 | |||
| Atrial fibrillation | 1.23 | 0.97–1.58 | 0.088 | |||
| LADs | 0.98 | 0.97–1.00 | 0.012 | 0.98 | 0.97–1.00 | 0.068 |
| E/e' | 1.00 | 0.99–1.03 | 0.353 | |||
| TRPG | 1.00 | 0.99–1.02 | 0.427 | |||
| IVCD | 1.00 | 0.97–1.03 | 0.811 | |||
| TAPSE | 0.98 | 0.95–1.01 | 0.125 | |||
| Mitral regurgitation | 0.99 | 0.71–1.40 | 0.976 | |||
| Tricuspid regurgitation | 0.92 | 0.69–1.24 | 0.590 | |||
| Haemoglobin | 1.01 | 0.95–1.08 | 0.777 | |||
| Creatinine | 1.07 | 0.93–1.20 | 0.338 | |||
| eGFR | 1.00 | 0.99–1.01 | 0.591 | |||
| NT-pro BNP | 1.00 | 1.00–1.01 | <0.001 | 1.00 | 1.00–1.00 | <0.001 |
| Uric acid | 0.96 | 0.91–1.02 | 0.300 | |||
| Albumin | 0.59 | 0.47–0.76 | <0.001 | 0.62 | 0.43–0.90 | 0.011 |
| Choline esterase | 1.00 | 0.99–1.00 | 0.002 | 1.00 | 1.00–1.00 | 0.403 |
| β blocker | 1.08 | 0.86–1.37 | 0.503 | |||
| Diuretics | 1.05 | 0.78–1.44 | 0.782 | |||
- CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; HDL-C, high density lipoprotein cholesterol; IVCD, intra vena cava diameter; LAD, left atrial diameter; NT-pro BNP, N-terminal pro-brain natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid pressure gradient.
Discussion
Our study demonstrated that in octogenarians with HFpEF, (i) HFR were not correlated with the prognosis, but were well correlated with the QOL; (ii) the incidence of non-cardiac death and cardiac deaths were almost equal and HFR might not be directly related to HF death; and (iii) the presence of a history of DM, elevated NT-pro BNP level, and hypoalbuminemia were independently and significantly associated with HFR.
Clinical impact of heart failure readmissions on prognosis in octogenarians with heart failure with preserved ejection fraction
It has been reported that HFR are a strong predictor of mortality in the HFpEF patient population,7-9 but for octogenarians, the impact of HFR on the prognosis may be weak because of other comorbidities, which may correlate more closely with the prognosis than HFR.16 We found that HFR were not associated with all-cause mortality or cardiac deaths in octogenarians with HFpEF, partially because elderly patients with HFpEF usually have more comorbidities including coronary artery disease, atrial fibrillation, chronic kidney disease, and/or malignancies,17 which are directly connected to the prognosis more than HF.18 Accordingly, in octogenarians with HFpEF, the effect of HFR on the prognosis was weak.
Little is known about the specific modes of death in HFpEF. The PARADIGM-HF trial, one of the large HFrEF trials, showed that 81% were deemed to have suffered a cardiac death.19 As we have stated, compared with patients with HFrEF, patients with HFpEF are usually older, with higher rates of non-cardiac comorbidities.20 Accordingly, similar to HFrEF, cardiac causes are the predominant mode of death in HFpEF, but the HFpEF trials suggest that 60–70% of deaths are cardiovascular in nature, which is lower than that of HFrEF.21 Conversely, non-cardiac deaths make up a higher proportion of deaths in HFpEF than in HFrEF.22 In the present study, the incidence of cardiac and non-cardiac deaths was similar (cardiac death: 48.2% and non-cardiac death: 51.8%), because our study patients were octogenarians with HFpEF who usually had further non-cardiac comorbidities. The age-associated non-cardiac comorbidity burden may lead to more non-cardiac deaths in octogenarians with HFpEF. Worsening chronic HF has been recognized as an important endpoint in HFrEF, but HFpEF tends to feature dysfunction involving multiple organ systems and does not demonstrate the classic ‘pump failure’ during the end-stage.21 To improve the prognosis of HFpEF, attention should be paid to managing non-cardiac comorbidities, especially in octogenarians.
On the other hand, for octogenarians, the QOL is also crucial for the consideration of the longevity. Our data demonstrated that HFR had a worse impact on the QOL in octogenarians with HFpEF. From the viewpoint of the QOL, it is important to pay particular attention to HFR in octogenarians with HFpEF.
Diabetes mellitus
Our data showed that DM was one of the significant and independent factors for HFR in octogenarians with HFpEF. DM is one of the key risk factors for HFpEF and correlates with a higher risk of hospitalization.23 It has been reported that a partial reason why DM contributes to HFpEF is increased arterial stiffness.24 The mechanism underlying the arterial stiffness in DM may not be fully understood. It has been proposed to result from accelerated advanced glycation end product-mediated collagen cross-linking,25 aortic wall calcification,26 endothelial dysfunction,27 chronic low-grade inflammation, increased oxidant stress, and an increased sympathetic tone.28 The increased atrial stiffness due to DM can adversely impact the myocardium, given that an increased and/or abnormal pulsatile load favours left ventricular hypertrophy, fibrosis, and diastolic dysfunction,29 which may be important for the development of HFpEF. In addition, arterial stiffness is an age-related process.30 We recently reported that the increased arterial stiffness in elderly patients may be correlated with weak tolerance of an acute afterload elevation, which is a trigger of ADHF in HFpEF.31 Thus, in the octogenarians with HFpEF, DM can be one of the major risk factors for HFR.
N-terminal pro-B-type natriuretic peptide
Our study revealed that the NT-pro BNP level at discharge was one of the useful predictors for HFR in octogenarians with HFpEF. Because it has been reported that the NT-pro BNP level at discharge has better prognostic information for predicting readmissions or deaths than that on admission,32 we used the NT-pro BNP level at discharge in this study. A recent large population-based study has also shown that the BNP level may aid in predicting HFR, but not in predicting readmissions for other causes.33 Januzzi et al. reported that in elderly HF patients (≥75 years old), the optimal cut-off point for the NT-pro BNP level for acute HF is 1800 pg/mL.34 Thus, NT-pro BNP level of ≥1611 pg/mL may represent a sign of a mild fluid overload at discharge, which can be a reliable predictor of an HFR.35 The data of higher IVCD and TRPG in the HFR group as compared with non-HFR group (Table 3) may support mild fluid overload at discharge in the HFR group while the length of stay was similar between the two groups. Based on these results, in octogenarians with HFpEF, an NT-pro BNP level ≥ 1611 pg/mL may represent incompletely treated ADHF, leading clinicians to consider a later discharge or closer post-discharge follow-up including more intensive care measures (e.g. telemonitoring of weight).
| HFR group (N = 116) | non-HFR group (N = 425) | P value | |
|---|---|---|---|
| Laboratory data | |||
| White blood cell (× 103/μL) | 5.3 (4.3–6.4) | 5.4 (4.5–6.7) | 0.351 |
| Haemoglobin (g/dL) | 10.8 (9.6–17.1) | 11.2 (10.0–12.4) | 0.004 |
| Sodium (mEq/L) | 139 (137–141) | 140 (137–141)7 | 0.786 |
| Chloride (mEq/L) | 102 (99–105) | 103 (100–106) | 0.071 |
| Potassium (mEq/L) | 4.4 (4.0–4.6) | 4.3 (3.9–4.6) | 0.529 |
| Creatinine (mg/dL) | 1.0 (1.0–1.6) | 1.1 (0.9–1.5) | 0.011 |
| eGFR (mL/min/1.73 m2) | 35.1 (27.4–45.0) | 41.1 (30.3–52.9) | 0.005 |
| NT-pro BNP (pg/mL) | 1980 (961–3520) | 1160 (527–2362) | <0.001 |
| Uric acid (mg/dL) | 7.0 (5.9–8.1) | 6.4 (5.3–7.8) | 0.027 |
| Total cholesterol (mg/dL) | 154 (131–181) | 157 (137–180) | 0.781 |
| Triglyceride (mg/dL) | 92 (68–113) | 94 (71–124) | 0.583 |
| Cholinesterase (U/l) | 188 (149–228) | 200 (165–235) | 0.153 |
| Albumin (g/dL) | 3.4 (3.1–3.8) | 3.4 (3.1–3.7) | 0.838 |
| Haemoglobin A1c (%) | 5.9 (5.6–6.4) | 6.0 (5.6–6.5) | 0.691 |
| Echocardiographic data | |||
| LVDd (mm) | 45 (41–43) | 44 (41–48) | 0.225 |
| LVDs (mm) | 29 (26–32) | 29 (25–32) | 0.141 |
| IVSTd (mm) | 10 (9–11) | 10 (9–11) | 0.411 |
| LVPWTd (mm) | 10 (9–11) | 10 (9–11) | 0.499 |
| LADs (mm) | 47 (43–54) | 46 (41–51) | 0.097 |
| LVEF (%) | 60 (54–68) | 61 (56–66) | 0.306 |
| Mitral regurgitation (≥Moderate), n (%) | 21 (18.8) | 64 (15.1) | 0.474 |
| Tricuspid regurgitation (≥Moderate), n (%) | 16 (14.3) | 90 (22.2) | 0.066 |
| Mean E/e' | 13.6 (10.3–17.0) | 12.7 (10.0–17.3) | 0.730 |
| RVDd (mm) | 31 (27–36) | 32 (28–36) | 0.803 |
| TAPSE (mm) | 17.4 (13.4–19.5) | 17.0 (14.0–19.8) | 0.921 |
| Inferior vena cava diameter (mm) | 7.0 (4.2–10.0) | 5.9 (4.0–7.1) | 0.014 |
| TRPG (mmHg) | 28.3 (24.5–34.2) | 26.3 (22.0–32.7) | 0.003 |
| Medication | |||
| ACEI, n (%) | 21 (18.1) | 76 (17.8) | 0.931 |
| ARB, n (%) | 44 (37.9) | 148 (34.6) | 0.513 |
| β blocker, n (%) | 76 (65.5) | 220 (51.4) | 0.008 |
| Diuretics, n (%) | 105 (90.5) | 351 (82.0) | 0.032 |
| MRA, n (%) | 47 (40.5) | 164 (38.3) | 0.669 |
| Statin, n (%) | 45 (38.8) | 141 (32.9) | 0.270 |
| SGLT2 inhibitor, n (%) | 6 (5.2) | 19 (4.5) | 0.803 |
| Insulin, n (%) | 6 (5.2) | 13 (3.1) | 0.802 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CRP, C reactive protein; eGFR, estimated glomerular filtration rate; HFR, heart failure readmission; IVSTd, interventricular septum thickness at end-diastole; LADs, left atrial diameter at end-systole; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; LVPWTd: left ventricular posterior wall thickness at end-diastole; MRA, mineralocorticoid receptor antagonist, NT-pro BNP, N-terminal pro-brain natriuretic peptide; RVDd, right ventricular diastolic diameter; SGLT2, sodium-glucose co-transporter 2; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid pressure gradient.
Albumin
Similarly, hypoalbuminemia at discharge was another significant and independent factor correlated with HFR in this study. Several reports have shown hypoalbuminemia in HF patients, reflecting malnutrition and inflammation, which are associated with worse HF outcomes, especially in elderly patients.36 In addition, it has been proposed that hypoalbuminemia impairs the vasodilatory response to nitric oxide37 and antioxidant capacities, contributing to additional protection from oxidative stress,38 which renders potential implications for the prognosis of HF. We previously showed that hypoalbuminemia (serum albumin ≤ 3.4 g/dL) on admission is a useful marker for predicting long stays in elderly ADHF patients (>75 years old).31 The cut-off value of serum albumin (3.7 g/dL) in the present study was similar to those in several previous reports (3.4–3.5 g/dL).31, 39 Again, a finding of hypoalbuminemia may indicate incompletely treated ADHF and indicate the need for more treatment prior to discharge or more intense post-discharge follow-up.
Clinical implications
According to our results, a history of DM, discharge NT-pro BNP levels ≥ 1611 pg/mL, and discharge serum albumin levels ≤ 3.7 g/dL are predictive of the need for eventual HFR in octogenarians with HFpEF. Even if the HFR were not correlated with the prognosis, to maintain the QOL is also valuable for octogenarians. It has been reported that intensive follow-up reduces readmissions by 56.2% and also improves the QOL scores.40 Actually, a meta-analysis of interventions for elderly HF patients found that comprehensive discharge planning including close follow-up with post-discharge support reduces HFR and improves the outcome without increasing the cost.41 Our data showed 19.0%, 37.0%, and 55.2% of HFR group readmitted due to HF within 1, 3, and 6 months after discharge, respectively. Accordingly, it is better to follow-up the octogenarian with HFpEF at least 1 month after discharge at outpatient clinic and subsequently every 3 months of follow-up visits are recommended for a while. The follow-up visits included a clinical interview, physical examination and blood examination including NT-pro BNP and albumin. Therefore, closer follow-up may be necessary to decrease HFR if octogenarians with HFpEF have DM, a high NT-pro BNP level (≥1611 pg/mL), and low albumin level (≤3.7 g/dL) at discharge.
The management of HFpEF in octogenarians faces many difficulties due to the lack of robust evidence. Thereby, we believe that our results supply valuable information to remind clinicians that non-cardiac comorbidities can be directly associated with the prognosis, and the QOL is also crucial in octogenarians with HFpEF. In addition, our proposed factors for preventing HFR in octogenarians with HFpEF and negative prognostic factors, which are a present history of DM and the abnormal NT-pro BNP and albumin levels, are clinically useful because we can easily obtain these data in routine clinical practice.
Limitations
This study had several limitations. First, the education level and economic status are also important parameters that are correlated with HF. Unfortunately, we could not collect these data in this registry. Second, we tried to evaluate the Scoring for EQ-5D-5L in all study patients, but we could obtain the scores in approximately 40% of the study patients due to various causes including incomplete answer from the patients and dementia. Therefore, our data may not perfectly reflect the QOL in all the study patients with octogenarians. Third, we analysed the past history of HFR, but we could not analyse the number of readmission in this study. Finally, our study was an observational cohort study, and despite covariate adjustment by multivariate analysis, unmeasured or unknown variables may influence the clinical outcome. Therefore, further prospective studies are required to confirm our findings.
Conclusions
In ADHF octogenarians with HFpEF, readmissions for HF did not have a direct effect on the prognosis but had a worse effect on QOL. The incidences of non-cardiac and cardiac deaths were almost the same, and HF deaths were not high even in patients with HFR. If ADHF octogenarians with HFpEF have a present history of DM, NT-pro BNP level of ≥1611 pg/mL, and serum albumin level of ≤3.7 g/dL, additional treatment before discharge or closer follow-up after discharge should be performed to decrease HFR.
Acknowledgements
The authors thank all the investigators, clinical research coordinators, and data managers involved in the PURSUIT-HFpEF registry for their dedicated contributions. The authors thank Mr John Martin for his linguistic assistance with this manuscript. We thank Libby Cone, MD, MA, from DMC Corp. (www.dmed.co.jp <http://www.dmed.co.jp/>) for editing a draft of this manuscript.
Conflict of interest
Daisaku Nakatani has received honoraria from Roche Diagnostics. Shungo Hikoso has received personal fees from the Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals, and Boehringer Ingelheim Japan and received grants from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Yasushi Sakata received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Actelion Pharmaceuticals and received grants from Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. The other authors have no conflicts of interest to disclose.
Funding
This work is funded by Roche Diagnostics K.K. and Fuji Film Toyama Chemical Co. Ltd.
Appendix A: The OCVC-heart failure investigators
Chair: Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Suita 565-0871, Japan.
Secretariat: Shungo Hikoso (Chief), Daisaku Nakatani, Hiroya Mizuno, Shinichiro Suna, Katsuki Okada, Tomoharu Dohi, Takayuki Kojima, Akihiro Sunaga, Hirota Kida, Bolrathanak Oeun, and Taiki Sato; Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Suita, Japan.
Investigators: Shunsuke Tamaki, Tetsuya Watanabe, and Takahisa Yamada, Osaka General Medical Center, Osaka, Japan; Takaharu Hayashi and Yoshiharu Higuchi, Osaka Police Hospital, Osaka, Japan; Masaharu Masuda, Mitsutoshi Asai, and Toshiaki Mano, Kansai Rosai Hospital, Amagasaki, Japan; Hisakazu Fuji, Kobe Ekisaikai Hospital, Kobe, Japan; Daisaku Masuda, Yoshihiro Takeda, Yoshiyuki Nagai, and Shizuya Yamashita, Rinku General Medical Center, Izumisano, Japan; Masami Sairyo, Yusuke Nakagawa and Shuichi Nozaki, Kawanishi City Hospital, Kawanishi, Japan; Haruhiko Abe, Yasunori Ueda, Masaaki Uematsu, and Yukihiro Koretsune, National Hospital Organization Osaka National Hospital, Osaka, Japan; Kunihiko Nagai, Ikeda Municipal Hospital, Ikeda, Japan; Masamichi Yano, Masami Nishino, and Jun Tanouchi, Osaka Rosai Hospital, Sakai, Japan; Yoh Arita and Shinji Hasegawa, Japan Community Health Care Organization Osaka Hospital, Osaka, Japan; Takamaru Ishizu, Minoru Ichikawa and Yuzuru Takano Higashiosaka City Medical Center, Higashiosaka, Japan; Eisai Rin, Kawachi General Hospital, Higashiosaka, Japan; Yukinori Shinoda and Shiro Hoshida, Yao Municipal Hospital, Yao, Japan; Masahiro Izumi, Kinki Central Hospital, Itami, Japan; Hiroyoshi Yamamoto and Hiroyasu Kato, Japan Community Health Care Organization, Osaka Minato Central Hospital, Osaka, Japan; Kazuhiro Nakatani and Hisatoyo Hiraoka, Sumitomo Hospital, Osaka, Japan; Mayu Nishio and Keiji Hirooka, Saiseikai Senri Hospital, Suita, Japan; Takahiro Yoshimura and Yoshinori Yasuoka, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Japan; Akihiro Tani, Kano General Hospital, Osaka, Japan; Yasushi Okumoto and Hideharu Akagi, Kinan Hospital, Tanabe, Japan; Yasunaka Makino, Hyogo Prefectural Nishinomiya Hospital, Nishinomiya, Japan; Toshinari Onishi and Katsuomi Iwakura, Sakurabashi Watanabe Hospital, Osaka, Japan; Nagahiro Nishikawa and Yoshiyuki Kijima, Japan Community Health Care Organization, Hoshigaoka Medical Center, Hirakata, Japan; Takashi Kitao and Hideyuki Kanai, Minoh City Hospital, Minoh, Japan; Wataru Shioyama and Masashi Fujita, Osaka International Cancer Institute, Osaka, Japan; Koichiro Harada, Suita Municipal Hospital, Suita, Japan; Masahiro Kumada and Osamu Nakagawa, Toyonaka Municipal Hospital, Toyonaka, Japan; Ryo Araki and Takayuki Yamada, Otemae Hospital, Osaka, Japan; Akito Nakagawa and Yoshio Yasumura, Amagasaki Chuo Hospital, Amagasaki, Japan; and Taiki Sato, Akihiro Sunaga, Bolrathanak Oeun, Hirota Kida, Takayuki Kojima, Yohei Sotomi, Tomoharu Dohi, Kei Nakamoto, Katsuki Okada, Fusako Sera, Shinichiro Suna, Hidetaka Kioka, Tomohito Ohtani, Toshihiro Takeda, Daisaku Nakatani, Hiroya Mizuno, Shungo Hikoso, Yasushi Matsumura and Yasushi Sakata, Osaka University Graduate School of Medicine, Suita, Japan.




