Outcomes of coronavirus disease-2019 among veterans with pre-existing diagnosis of heart failure
Abstract
Aims
Pre-existing cardiovascular disease in general and related risk factors have been associated with poor coronavirus disease-2019 (COVID-19) outcomes. However, data on outcomes of COVID-19 among people with pre-existing diagnosis of heart failure (HF) have not been studied in sufficient detail. We aimed to perform detailed characterization of the association of pre-existing HF with COVID-19 outcomes.
Methods and results
A retrospective cohort study based on Veterans Health Administration (VHA) data comparing 30 day mortality and hospital admission rates after COVID-19 diagnosis among Veterans with and without pre-existing diagnosis of HF. Cox-regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) with adjustment for covariates. Among 31 051 veterans (97% male) with COVID-19, 6148 had pre-existing diagnosis of HF. The mean (SD) age of patients with HF was 70 (13) whereas the mean (SD) age of patients without HF was 57 (17). Within the HF group with available data on left ventricular ejection fraction (EF), 1844 patients (63.4%) had an EF of >45%, and 1063 patients (36.6%) had an EF of ≤45%. Patients in the HF cohort had higher 30 day mortality (5.4% vs. 1.5%) and admission (18.5% vs. 8.4%) rates after diagnosis of COVID-19. After adjustment for age, sex, and race, HRs (95% CIs) for 30 day mortality and for 30 day hospital admissions were 1.87 (1.61–2.17) and 1.79 (1.66–1.93), respectively. After additional adjustment for medical comorbidities, HRs for 30 day mortality and for 30 day hospital admissions were 1.37 (1.15–1.64) and 1.27 (1.16–1.38), respectively. The findings were similar among HF patients with preserved vs. reduced EF, among those taking vs. not taking angiotensin-converting enzyme inhibitors, angiotensin receptor blockers or angiotensin receptor neprilysin inhibitors, and among those taking vs. not taking anticoagulants.
Conclusions
Patients with COVID-19 and pre-existing diagnosis of HF had a higher risk of 30 day mortality and hospital admissions compared to those without history of HF. The findings were similar by EF categories and by angiotensin-converting enzyme inhibitors/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitors or anticoagulant use.
Background
Among the variable clinical manifestations of coronavirus disease-2019 (COVID-19), heart failure (HF) has been noted to be a common complication of COVID-19 associated with high mortality.1, 2 It is unclear, however, to what extent an acute exacerbation of pre-existing HF, progression of subclinical HF, or new development of HF contribute to this important complication.3 On the other hand, while poor COVID-19 outcomes have been observed in patients with pre-existing cardiovascular disease (including those with HF),4-6 the association of pre-existing HF with poor outcomes after COVID-19 diagnosis has not been explored in sufficient detail, such as accounting for comorbidities, or assessing the association within clinically relevant subgroups [e.g. type of HF or use of specific medications such angiotensin-converting enzyme inhibitors (ACEi)]. To address this knowledge gap, we used Veterans Health Administration (VHA) data to retrospectively study the association of pre-existing diagnosis of HF with 30 day mortality and with 30 day hospital admission in a large cohort of veterans who tested positive for COVID-19.
Methods
Data source and study design
This is a retrospective cohort study of VHA data on veterans who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) between 8 February 2020 (first recorded date) and 31 July 2020. We identified participants from date of first recorded test by using text searching of laboratory results containing terms consistent with SARS-CoV-2 or COVID-19. Nearly all tests utilized nasopharyngeal swabs using polymerase chain reaction technique. Testing was performed in the VA, state public health, and commercial reference laboratories using emergency use authorization approved SARS-CoV-2 assays. We did not include antibody tests in this analysis. If an individual had more than one test of SARS-CoV-2, the date of the first positive test was taken. The study received appropriate approvals from the Providence VA Medical Center Institutional Review Board.
Covariates
Presence of comorbidity was defined by the presence of at least one inpatient or outpatient International Classification of Diseases-10 code for the respective diagnosis recorded within 2 years prior to the date of COVID-19 diagnosis. The burden of comorbidity was assessed using Elixhauser comorbidity index. Haemoglobin A1c (HgbA1c) and creatinine were determined based on available laboratory data within 1 year of COVID-19 diagnosis. Glomerular filtration rate was estimated using Modification of Diet in Renal Disease formula. Veterans with HF were classified into those with preserved EF (EF > 45%, HFpEF) vs. reduced EF (EF ≤ 45%, HFrEF) based on the most recent EF information recorded within 2 years of COVID-19 diagnosis. The use of ACEi, angiotensin receptor blockers (ARB), or angiotensin receptor neprilysin inhibitors (ARNI) and the use of anticoagulants (i.e. warfarin, direct oral anticoagulants and parenteral anti-Xa inhibitors) within 3 months prior to COVID-19 was determined based on VHA-Pharmacy Benefits Management database.
Outcomes
The primary outcomes of interest were (i) 30 day all-cause mortality and (ii) 30 day all-cause hospital admissions after diagnosis date of COVID-19. Mortality data were obtained from the Beneficiary Identification Records Locator Subsystem, National Death Index, VA Vital Status File, the Social Security Administration death master file, and the VHA Medical Statistical Analysis Systems inpatient datasets.
Statistical analyses
We used Kaplan–Meier plot to depict event-free survival after COVID-19 diagnosis comparing those with and without HF. Cox-regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs), progressively adjusting for sociodemographic variables and an extensive list of comorbidity variables. Adjustment for competing risk of death was made in Cox-regression models evaluating hospital admissions. We performed secondary analyses comparing those with HF by left ventricular EF (preserved vs. reduced), by use of ACEi, ARB, ARNI, and by use of anticoagulants. Because of the significant age gap between COVID-19 patients with and without pre-existing HF, we performed a secondary analysis in an age-matched sample of HF and non-HF patients with COVID-19. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA). A two-sided P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 31 050 veterans who tested positive for SARS-CoV-2, there were 6148 patients (19.8%) with pre-existing diagnosis of HF and 24 903 patients (80.2%) without history of HF (Table 1). Among the 6148 patients, 1844 patients (30.0%) had HFpEF, and 1063 patients (17.3%) had HFrEF; the remaining 3241 patients (52.7%) did not have EF data available within 2 years of COVID-19 diagnosis (Supporting Information, Table S1). The mean (SD) age of patients with HF was 70 (13), and 6% were female, whereas the mean (SD) age of patients without HF was 57 (17), and 15% were female (Table 1). In addition to older age, the veterans with HF had higher comorbidity burden than the group without HF. Patients with HF were more likely to be smoker (29% vs. 13%), have hypertension (84% vs. 50%), diabetes (54% vs. 28%), myocardial infarction (44% vs. 10%), atrial fibrillation (28.9% vs. 4.4%), chronic kidney disease (35% vs. 9%), obesity (34% vs. 26%), and chronic obstructive pulmonary disease (38% vs. 15%), as partial list the differences between the groups (Table 1). Patients with HF were also more likely to be on an ACEI/ARB/ARNI (35.4% vs. 17.9%) and anticoagulation (23.6% vs. 9.3%) (Table 1). COVID-19 patients with HFrEF and HFpEF had broadly similar characteristics (Table S1).
| Characteristic | Overall | No HF Hx | HF history | P value |
|---|---|---|---|---|
| Mean (SD) or n (%) (n = 31 051) | Mean (SD) or n (%) (n = 24 903) | Mean (SD) or n (%) (n = 6148) | ||
| Age (mean years) | 59.6 (17.1) | 57.0 (16.9) | 69.8 (13.3) | <0.0001 |
| Gender (% female) | 4128 (13.3) | 3780 (15.2) | 348 (5.7) | <0.0001 |
| Ethnicity | <0.0001 | |||
| White | 16 112 (51.9) | 12 697 (51.0) | 3415 (55.6) | |
| Black | 10 654 (34.3) | 8402 (33.74) | 2252 (36.6) | |
| Hispanic | 2989 (9.6) | 2550 (10.2) | 439 (7.1) | |
| ECI score | 3.4 (2.9) | 2.70 (2.3) | 6.16 (3.5) | <0.0001 |
| Comorbidity | ||||
| CKD | 4282 (13.8) | 2157 (8.7) | 2125 (34.6) | <0.0001 |
| Homeless | 3460 (11.1) | 2450 (9.8) | 1010 (16.4) | <0.0001 |
| MI | 5213 (16.8) | 2479 (10.0) | 2734 (44.5) | <0.0001 |
| Smoker | 4877 (15.7) | 3104 (12.5) | 1773 (28.8) | <0.0001 |
| COPD | 5995 (19.3) | 3657 (14.7) | 2338 (38.0) | <0.0001 |
| Diabetes | 10 328 (33.3) | 7023 (28.2) | 3305 (53.8) | <0.0001 |
| Hypertension | 17 724 (57.1) | 12 570 (50.5) | 5154 (83.8) | <0.0001 |
| Obese | 8658 (27.9) | 6562 (26.4) | 2096 (34.1) | <0.0001 |
| Alcohol abuse | 3978 (12.8) | 3079 (12.4) | 899 (14.6) | <0.0001 |
| Drug abuse | 2474 (8.0) | 1814 (7.3) | 660 (10.7) | <0.0001 |
| Depression | 11 245 (36.2) | 8721 (35.0) | 2524 (41.1) | <0.0001 |
| Atrial fibrillation | 2880 (9.28) | 1103 (4.43) | 1777 (28.90) | <0.0001 |
| BMI | 25.1 (5.4) | 25.12 (5.21) | 25.04 (5.87) | 0.3647 |
| HgbA1c | 6.5 (1.5) | 6.4 (1.5) | 6.8 (1.5) | <0.0001 |
| Creatinine | 1.3 (0.9) | 1.2 (0.6) | 1.6 (1.3) | <0.0001 |
| Estimated GFR | 75.5 (28.0) | 78.9 (26.3) | 64.1 (30.7) | <0.0001 |
| Medication past 3 months | ||||
| ACEI/ARB/ARNI | 6645 (21.40) | 4467 (17.9) | 2178 (35.4) | <0.0001 |
| Anticoagulation | 2895 (9.32) | 1442 (5.79) | 1453 (23.63) | <0.0001 |
| Outcome | ||||
| Post 30 day mortality | 717 (2.3) | 383 (1.5) | 334 (5.4) | <0.0001 |
| Post 30 day readmission | 3229 (10.4) | 2091 (8.4) | 1138 (18.5) | <0.0001 |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin-converting enzyme blocker; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ECI, Elixhauser comorbidity Index; GFR, glomerular filtration rate; HgbA1c, haemoglobin A1c; HF, heart failure; Hx, history; MI, myocardial infarction.
Incidence of clinical outcomes
Compared with patients without HF, those with HF had higher mortality (5.4% vs. 1.5%) and hospital admissions (18.5% vs. 8.4%) 30 days after diagnosis of COVID-19 (Figure 1, Table 1). Among those with HF, the event rates were comparable between those with HFpEF and HFrEF with 30 day mortality of 6.6% vs. 6.3% and 30 day hospital admissions of 19.7% vs. 21.0%, after diagnosis of COVID-19 (Table S1).
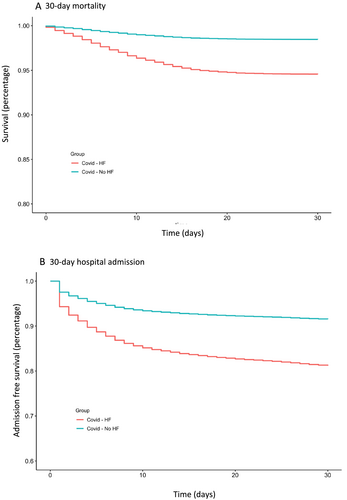
Relative risk of clinical outcomes
Compared with those without HF, patients with HF had age-, sex-, and race-adjusted HRs (95% CIs) of 1.87 (1.61–2.17) for 30 day mortality and 1.79 (1.66–1.93) for 30 day admissions (Table 2). HRs (95% CIs) adjusted for age, sex, race, and other comorbidities were 1.37 (1.15–1.64) for 30 day mortality and 1.27 (1.16–1.38) for 30 day admissions (Table 2). The association was further attenuated but remained significant after adjustment BMI, HgbA1c, and estimated glomerular filtration rate (Table S2). The age-, sex-, and race-adjusted HRs (95% CIs) comparing patients with HFpEF or HFrEF (vs. those without HF) were 2.14 (1.73–2.63) and 2.20 (1.70–2.84), respectively for 30 day mortality, and 2.01 (1.80–2.25) and 1.85 (1.60–2.14), respectively for 30 day hospital admission (Table 3). The HRs of mortality were 1.44 (1.13–1.84) and 1.45 (1.09–1.93) for those with HFpEF and HFrEF, respectively, after further adjustment for comorbidities. The corresponding HRs for 30 day hospital admission were 1.26 (1.11–1.43) and 1.15 (0.98–1.34) for those with HFpEF and HFrEF, respectively (Table 3). The findings were similar in the analyses of the aged-matched sample of COVID-19 patients with and without HF (Tables S3 and S4). The associations of pre-existing HF with COVID-19 outcomes were similar among those taking vs. not taking ACEI/ARB/ARNI (adjusted HR of mortality: 1.52 [1.19–.193] vs. 1.37 [1.12–1.68], respectively), and among those taking vs. not taking anticoagulants (1.46 [1.13–1.91] vs. 1.38 [1.14–1.67], respectively). The corresponding analyses for hospital admissions yielded comparable results (Tables S5 and S6).
| Adjustment | (a) 30 day mortality | (b) 30 day hospital admission | ||
|---|---|---|---|---|
| Hazard ratio | 95% CI | Hazard ratio | 95% CI | |
| Unadjusted | 3.49 | 3.01, 4.04 | 2.27 | 2.12, 2.45 |
| Model 1 | 1.92 | 1.66, 2.23 | 1.82 | 1.68, 1.96 |
| Model 2 | 1.87 | 1.61, 2.17 | 1.79 | 1.66, 1.93 |
| Model 3 | 1.81 | 1.55, 2.11 | 1.70 | 1.57, 1.83 |
| Model 4 | 1.76 | 1.51, 2.05 | 1.66 | 1.54, 1.79 |
| Model 5 | 1.68 | 1.44, 1.97 | 1.58 | 1.46, 1.71 |
| Model 6 | 1.64 | 1.39, 1.92 | 1.56 | 1.44, 1.69 |
| Model 7 | 1.62 | 1.37, 1.90 | 1.55 | 1.43, 1.69 |
| Model 8 | 1.57 | 1.33, 1.86 | 1.43 | 1.32, 1.56 |
| Model 9 | 1.37 | 1.15, 1.64 | 1.27 | 1.16, 1.38 |
- CI, confidence interval.
- Model 1 = age; Model 2 = Model 1 + age, sex, and race; Model 3 = Model 2 + hypertension; Model 4 = Model 3 + diabetes; Model 5 = Model 4 + chronic kidney disease; Model 6 = Model 5 + myocardial infarction; Model 7 = Model 6 + Obesity; Model 8 = Model 7 + smoking, depression, chronic obstructive pulmonary disease, alcohol abuse, and drug abuse; Model 9 = Model 8 + Elixhauser comorbidity index.
| Adjustment | (a) 30 day mortality | (b) 30 day hospital admission | |||
|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | ||
| HFpEF | Unadjusted | 4.05 | 3.29, 4.98 | 2.63 | 2.36, 2.93 |
| HFrEF | 4.26 | 3.30, 5.49 | 2.44 | 2.11, 2.81 | |
| Missing EF | 2.92 | 2.42, 3.53 | 2.03 | 1.84, 2.23 | |
| No HF | 1 | 1 | 1 | 1 | |
| HFpEF | Model 1 | 2.22 | 1.80, 2.74 | 2.05 | 1.84, 2.30 |
| HFrEF | 2.30 | 1.78, 2.98 | 1.91 | 1.66, 2.21 | |
| Missing EF | 1.62 | 1.34, 1.97 | 1.65 | 1.50, 1.82 | |
| No HF | 1 | 1 | 1 | 1 | |
| HFpEF | Model 2 | 2.14 | 1.73, 2.63 | 2.01 | 1.80, 2.25 |
| HFrEF | 2.20 | 1.70, 2.84 | 1.85 | 1.60, 2.14 | |
| Missing EF | 1.60 | 1.32, 1.94 | 1.65 | 1.49, 1.81 | |
| No HF | 1 | 1 | 1 | 1 | |
| HFpEF | Model 3 | 1.44 | 1.13, 1.84 | 1.26 | 1.11, 1.43 |
| HFrEF | 1.45 | 1.09, 1.93 | 1.15 | 0.977, 1.34 | |
| Missing EF | 1.32 | 1.08, 1.62 | 1.30 | 1.18, 1.44 | |
| No HF | 1 | 1 | 1 | 1 | |
- CI, confidence interval; EF, ejection fraction; HF, heart failure; HFpEF, HF with preserved EF; HFrEF, HF with reduced EF.
- HF+ = HF present; HF− = no HF; anticoagulation+ = on anticoagulation within 3 months of coronavirus disease-2019 diagnosis; Model 1 = age; Model 2 = Model 1 + sex and race; Model 3 = Model 2 + hypertension, diabetes, chronic kidney disease, myocardial infarction, obesity, smoking, depression, chronic obstructive pulmonary disease, alcohol abuse, drug abuse, and Elixhauser comorbidity index.
Discussion
We found that HF is associated with significantly worse clinical outcomes in patients with COVID-19 in large-scale and detailed analyses of VHA database. COVID-19 patients with HF had higher 30 day mortality and higher 30 day hospital admission rates. The association of pre-existing HF with poor COVID-19 persisted after accounting for potential confounders, and in various clinically relevant subgroups, that is, comparing HF patients with preserved vs. reduced EF; those taking vs. not taking ACEi/ARB/ARNI; and those taking vs. not taking anticoagulants.
Our findings complement and supplement the findings of prior studies6, 7 including a systematic review and meta-analysis pooling data on 21 640 COVID-19 cases from 18 studies,6 which showed that HF is associated with increased risk of hospitalization and death from COVID-19. First, we had adequate power in a single large study that allowed detailed characterization of the associations; second, we were able to adjust for extensive list of confounders in a consistent manner; third, we were able to investigate the association within clinically relevant subgroups, such as by EF or use of certain medications. Such detailed analyses were not possible in the prior study-level data meta-analysis,6 and the individual studies were mostly not adequately powered with the largest study included in the prior meta-analyses involving only 349 COVID-19 with pre-existing HF.
The interplay between COVID-19 and HF is not fully understood, but it is hypothesized that COVID-19 may lead to direct viral infiltration, inflammation, or fibrosis of the myocardium, resulting in an acute exacerbation of pre-existing HF, progression of subclinical HF, or new development of HF.3 Viral infiltration may be relevant in HF as angiotensin-converting enzyme 2 receptors, which are up-regulated in the myocardium of failing hearts, serve as receptors that allow SARS-CoV-2 virus access to host cells.8, 9 Data on the extent of inflammatory changes in those with cardiac involvement of SARS-CoV-2 is variable, as there have been autopsy cases without inflammatory cell infiltrates, but also cardiac MRIs with findings suggestive of myocardial inflammation.10, 11 The inflammatory stage of the infection may cause a massive cytokine release leading to possible stress cardiomyopathy, acute thromboembolic events such as myocardial infarction, and/or cytokine-related myocardial injury with subsequent exacerbation of HF.12 It is also important to note that there is a shared inflammatory pathophysiology and cardiometabolic risk profile of COVID-19 and HF.3
Our findings of significantly increased risk of mortality and hospital admissions in COVID-19 patients with HF require further investigation in order to help mitigate this risk, including strict institution of measures to prevent SARS-CoV-2 infection in this population. Clinicians may consider a lower threshold to test HF patients for possible SARS-CoV-2 infection if symptomatic or with recent exposure. A more intensive monitoring of those patients with pre-existing HF who develop COVID-19 may be reasonable.
Our study had a number of limitations. First, this was a retrospective observational study, and therefore, causality cannot be determined. Second, data were derived from electronic medical records using International Classification of Diseases codes for comorbidities instead of direct chart review, which can limit the accuracy of the data. Prior studies, however, have demonstrated the validity of data from the VHA electronic medical record.13 Third, we did not have data for non-VA hospital admissions for those using private insurance. Fourth, EF was not available for many patients in our HF group because not all patients had a recorded data on cardiac function assessment within 2 years prior to diagnosis of COVID-19.
Conclusions
The present large record-linkage data from the VHA demonstrated that COVID-19 patients with HF have a significantly increased risk for mortality and hospital admissions compared with COVID-19 patients without HF after accounting for confounding. The findings were similar by EF categories and by ACEi/ARB/ARNI or anticoagulant use. Further preclinical and clinical studies may uncover underlying mechanism that could make HF patients potentially more vulnerable to poor COVID-19 outcomes and help to identify strategy to improve the clinical course of HF patients with COVID-19.
Conflict of interest
All authors declared none.
Funding
The research reported/outlined here was supported by the Department of Veterans Affairs, Veterans Health Administration, VISN 1 Career Development Award to S.E. Dr S.E. is also funded by the Center for Aids Research, the Rhode Island Foundation, and Lifespan Cardiovascular Institute. This project is partially supported (statistical analyst and Investigator’s time effort and publication cost) by VA Health Service Research and Development Merit Review grant IRP 20-003 (W.-C. W.). Drs J.L.R. and W.-C.W. are funded by the VA Health Services Research and Development Center of Innovation in Long Term Services and Supports (CIN 13-4193; C19-20-213). Drs S.E., G.C., N.S., J.L.R., and W.-C.W. are employees of the Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.




