Metabolomics and cardiovascular imaging: a combined approach for cardiovascular ageing
Abstract
The purpose of this review is to explore how metabolomics can help uncover new biomarkers and mechanisms for cardiovascular ageing. Cardiovascular ageing refers to cardiovascular structural and functional alterations that occur with chronological ageing and that can lead to the development of cardiovascular disease. These alterations, which were previously only detectable on tissue histology or corroborated on blood samples, are now detectable with modern imaging techniques. Despite the emergence of powerful new imaging tools, clinical investigation into cardiovascular ageing is challenging because ageing is a life course phenomenon involving known and unknown risk factors that play out in a dynamic fashion. Metabolomic profiling measures large numbers of metabolites with diverse chemical properties. Metabolomics has the potential to capture changes in biochemistry brought about by pathophysiologic processes as well as by normal ageing. When combined with non-invasive cardiovascular imaging tools, metabolomics can be used to understand pathological consequences of cardiovascular ageing. This review will summarize previous metabolomics and imaging studies in cardiovascular ageing. These methods may be a clinically relevant and novel approach to identify mechanisms of cardiovascular ageing and formulate or personalize treatment strategies.
Introduction
Metabolomics and cardiovascular imaging have been used as a strategy to study various disease states. However, combining metabolomics and imaging to better understand cardiovascular ageing is still in its infancy, and there are minimal published data. The purpose of this review is to consider the clinical manifestations and pathologic mechanisms for cardiovascular ageing and explore how metabolomics and cardiovascular imaging can help uncover new biomarkers and mechanisms for cardiovascular ageing.
Metabolomics
Metabolomic profiling is a systems biology tool that measures large numbers of metabolites with diverse chemical properties. The metabolome differs from the genome/transcriptome/proteome because its net output is influenced by genomic, transcriptomic, and proteomic variability. Metabolomics thus provides an integrated profile of an individual's biological status: ‘the genome defines what may happen, the metabolome defines what has happened’.1 Metabolomics profiles are also influenced by environmental exposure.2-8 There are two main analytical approaches used in metabolomics research: targeted and untargeted. Untargeted approaches involve comprehensive analysis of all the measurable analytes in a sample. The advantage to untargeted approaches is broad coverage of potentially important analytes and/or unbiased detection of biomarkers. The disadvantage to untargeted approaches includes a workflow that makes analysing large sample sets difficult, relative quantitation of compounds, a bias towards identifying compounds with high abundance, and frequent inability to identify peaks of interest. Targeted approaches involve measuring pre-defined metabolites. Advantages to targeted approaches include use of internal standards that allows identification and absolute quantitation of analytes, including low abundance compounds as well as relatively fast workflow. The disadvantage of targeted metabolomics is that clinically important analytes can be overlooked. Metabolomics techniques have been applied to the study of human disease with novel findings that have helped our understanding of insulin resistance and diabetes,9, 10 chronic kidney disease,11, 12 depression,13, 14 and cardiovascular disease (CVD).15-21
Cardiovascular ageing
Description
By the year 2030, approximately 20% of the world population will be aged 65 years or older.22 CVD is a leading cause of death in older adults,23 which underscores the importance of gaining a better understanding of the risk factors for CVD in older patients. The ageing process can lead to pathologic effects on the cardiovascular system, which can contribute to CVD.23 Cardiovascular ageing refers to cardiovascular structural and functional alterations that may occur with chronological ageing and that can lead to the development of CVD. A prominent age-related change is increased stiffness of central arteries referred to as vascular ageing.24 This results from loss of elastic fibres and increase in collagen in the large elastic arteries.25 Arterial stiffness exposes the endothelium to greater haemodynamic loading, thus promoting endothelial activation, inflammation, and damage.26 Increased arterial stiffness can affect ventricular relaxation by altering afterload.27, 28 Altered relaxation is associated with cellular hyperplasia and fibrosis that can ultimately worsen left ventricular diastolic function.29 Impairments in left ventricular relaxation cause the left atrium to compensate with increases in left atrial pressure to fill the left ventricle.30, 31 Elevated left atrial pressure results in left atrial enlargement secondary to pressure and volume overload.31 With progressive worsening of diastolic function, left atrial volume increases, giving rise to complications including atrial fibrillation and stroke.32, 33 Large-scale cohort studies have found increased incidences of chronic heart failure, atrial fibrillation, and left ventricular hypertrophy among ageing older adults.33-39 Several mechanisms have been proposed to be involved in the pathogenesis of cardiovascular ageing including inflammation, redox stress, and endothelial dysfunction.
Inflammation
Inflammation is a well-established driver of ageing and ageing-related diseases.40, 41 The inflammatory process evolved as an immunologic defence system.42 Acute inflammation acts as a response to noxious agents such as pathogens, allergens, and toxic substances.42 The response includes activation of immune cells to eliminate pathogens and tissue remodelling processes to repair damage. However, when the acute inflammatory response fails to resolve, more components are activated to generate a continued immune activation that leads to longer-term chronic inflammation.43-45 Ageing is linked to dysregulation of the immune response with release of inflammatory mediators and cytokines, giving rise to a concept of age-related chronic inflammation known as ‘senescent inflammation’.46-48 This chronic low-grade inflammatory state is characterized by increased levels of circulating cytokines such as interleukin-1, interleukin-6, and tumour necrosis factor.47, 48 In addition, age-related changes in adipose tissue content and function enhance release of pro-inflammatory cytokines.49, 50 Inflammation is associated with changes in metabolism pathways including fatty-acid-derived lipid signalling molecules.51, 52 Dietary and gut-microbiome-derived metabolites have also been implicated in human chronic inflammatory diseases.53 These processes promote recruitment of macrophages and T cells into tissues like myocardium and vascular walls, altering vascular structure and function, leading to arterial stiffening, atherosclerosis, and hypertension.54 Cardiac myofibroblasts respond to cytokines with resulting changes in cell proliferation, increased expression, and activity of extracellular matrix degrading metalloproteinases.55, 56 Over time, these lead to fibrotic cardiac remodelling and myocardial dysfunction.57 Furthermore, cardiac fibrosis and modification contribute to increased vulnerability to cardiac arrhythmias.58
Redox stress
The ageing heart shows changes in mitochondrial function, particularly in electron transport chain activity.59, 60 Mitochondrial oxidative stress is a molecular hallmark of cardiovascular ageing. Overproduction of reactive oxygen species (ROS) in the mitochondrial electron transport chain leads to formation of highly reactive products such as peroxide that promotes cellular damage including DNA mutations. Redox stress can cause inflammation and activation of cell death pathways and may eventually contribute to cellular senescence.61, 62 A rodent model of ageing has shown increased vascular endothelium redox stress in older animals. In this study, elevated levels of a major class of systemic bioactive lipids known as lysophosphatidylcholines (LPCs) contribute to the build-up of redox stress.63 LPCs stimulate oxidative stress through interaction with overactive 5-lipoxygenase pathways in ageing endothelial cells. LPCs can also stimulate ROS production in human monocyte-derived macrophages found in atherosclerotic arterial walls.64 These ROS-stimulated macrophages go on to activate expression of urokinase-type plasminogen activator and its cell surface receptor. These observations at the cellular level may account for clinical findings. Levels of soluble urokinase-type plasminogen activator receptor have been useful for predicting risks of myocardial dysfunction in aged adults.65 LPC has also been shown to be predictive of ageing phenotypes such as cognitive impairment and gait speed in older adults.66, 67
Endothelial function
Ageing alters the endothelium and leads to reductions in vasodilatory and antithrombotic properties while also promoting atherogenesis and thrombosis.68, 69 The vascular changes that occur with ageing result in large artery thickening and stiffness in otherwise healthy older persons who are prone to increases in systolic and pulse pressures. These functional changes in vessel haemodynamics precede clinical disease and are associated with higher risks for developing atherosclerosis, hypertension, and stroke.23, 70-72 Brachial artery flow-mediated dilation is a standard method for assessing endothelial function in a clinical research setting. Nitric oxide supplementation has been shown to improve brachial artery flow-mediated dilation.73-75 Among middle-aged and older adults, a 10 week trial of sodium nitrite supplementation improved endothelial function and carotid artery elasticity.73 Metabolites such as glycerophospholipids and fatty acyls predicted improved vascular function with nitrite.76 Interestingly, improved endothelial function occurred independently of well-known risk factors such as blood lipids, glucose, blood pressure, and body mass. These findings suggest that circulating metabolites may be helpful in gaining mechanistic insight into therapies that target endothelial function.76-78
Dietary supplements have been shown to alter markers of redox stress, improve endothelial function, and reduce risks of chronic disease and premature ageing.79, 80 Circulating metabolites derived from dietary polyphenols (such as blueberries) have been linked to more robust vascular flow-mediated dilatation, attenuated lipotoxicity-induced endothelial dysfunction, and may complement therapies to reduce vascular complications.81, 82 All these suggest an important role for antioxidant and antioxidant defence for proper maintenance of endothelial cell function.
These mechanistic and interventional studies have contributed to our understanding of the pathogenesis of cardiovascular ageing. Circulating metabolites have been figured prominently in many of these studies, highlighting the potential role for metabolomics in elucidating the underlying mechanisms through which ageing exerts pathological changes in the cardiovascular system.
Metabolism, ageing, and the heart
Mitochondrial function is altered in the ageing heart.59, 60 Changes in mitochondrial function have a knock-on effect on central carbon and related pathways. Fatty acid oxidation and ketone use declines with ageing in mouse hearts.83, 84 Reprogramming of cardiac metabolism away from fatty acid oxidation is a prominent feature of heart failure85, 86 and may partly explain changes in heart function with ageing. The sphingolipids are a major class of lipids that play an important role in tissue signalling and are active players in diseases such as insulin resistance87 and CVD.88 Sphingolipid physiology changes with ageing89 and may contribute to ageing-related decline in heart function. Declining mitochondrial function with ageing has been linked to redox state, nicotinamide adenine dinucleotide levels, and the activity of nicotinamide adenine dinucleotide-dependent deacylases known as the sirtuins.90 The sirtuins can be activated by caloric restriction, a well-described and potent stimulus for longevity in animal models,91 as well as resveratrol, a component of red wine.92 Sirtuins can modulate tissue metabolic activity93 and have been linked directly to CVD.94 Alpha-ketoglutarate is an important central carbon metabolite and intermediate in the tricarboxylic acid cycle. Alpha-ketoglutarate has been shown to extend lifespan in worms,95 flies,96 and mice97 likely through interactions with mammalian target of rapamycin and adenosine monophosphate-activated protein kinase pathways. The related tricarboxylic acid cycle intermediate succinate has been shown to accumulate during ischaemia and contributes to ROS production and damage during reperfusions.98
Metabolomics and cardiovascular ageing
Challenges
Many biomarkers are in use for detecting risk of CVD as well as for monitoring response to therapy. In contrast, few biomarkers have been identified for evaluating cardiovascular ageing. Because metabolomics provides an integrated profile of an individual's biological status, it may serve as a useful tool to help us understand the complexities that surround the mechanisms of pathologic ageing. The challenge going forward will be to design methods and studies to help identify new biomarkers that can predict risk of disease and/or follow disease progression.
Metabolomics and environmental exposure
Because ageing is a life course phenomenon, environmental exposure is an important consideration. Dynamic lifestyle factors such as physical activity,99 alcohol use, and food intake can alter the course of physical ageing.100, 101 Dietary assessment in research studies is challenging. Dietary exposures are hard to measure, and established methods such as dietary recall and food frequency questionnaires have their limitations. Similar challenges exist for quantifying other important environmental exposures including lifetime exercise patterns, bouts of acute and chronic illness, medication and other drug use, and exposure to toxins from air pollution. Given that these dynamic factors all converge upon the human metabolome, metabolomics might provide a comprehensive and integrated picture of these lifelong environmental exposures. An example of how metabolomics can detect differences in environmental exposure comes from studies of host–gut microbiome interactions. The INTERMAP study performed nuclear magnetic resonance-based urinary metabolome analyses among African–American compared with non-Hispanic white Americans, where they found higher blood pressure levels among African–American men and women.102 They found significant differences in metabolites between the two groups that were related to differing food intakes between the groups, as well as differences in gut microbiota. Another series of studies identified trimethylamine N-oxide, a diet-derived gut microbial metabolite, as an environment-linked factor associated with increased cardiovascular and mortality risk.103-105 Another lifestyle exposure such as exercise may also alter metabolomics profiles.106, 107 Exercise training may produce widespread changes in energy metabolism, owing to increases in lipolysis, fatty acid oxidation, or ketogenesis.106 Differences in exercise intensity may also result in different profiles. Under moderate-intensity exercise, for example, medium chain acylcarnitines appear to dominate, to support fat oxidation.108
These studies highlight the importance of considering environmental exposure such as diet on cardiovascular health and strengthen the evidence base for applying metabolomics profiling onto life course exposures such as ageing.
Metabolomics and cardiovascular disease in the elderly
While there are community-based studies that have looked at the association between metabolomics signatures, CVD, and cardiovascular function,109, 110 few have studied the elderly. A study by Rizza et al.111 looked at a high-risk cohort of elderly subjects in which over half of the participants had documented coronary artery disease or stroke. Rizza et al. found a distinct signature comprising medium-chain and long-chain acylcarnitines that predicted major adverse cardiac events in this high-risk elderly population. Because these acylcarnitines are derived from fatty acid oxidation,112 this finding suggests that mitochondrial beta-oxidation pathways are linked to increased cardiovascular risk. In an interventional study that investigated the effect of Mediterranean diet on incident CVD, participants with higher baseline concentrations of short-chain, medium-chain, and long-chain acylcarnitines had higher risk of CVD and stroke.113 In both of these studies, metabolomics has been able to uncover new associations between disease and altered fuel metabolism pathways. The studies also show how metabolomics can be complementary to other omics technologies that have helped to unravel disease mechanisms in complex phenotypes such as heart failure, yielding biomarkers for diagnosis, prognosis, or identifying new therapeutic targets.114
Table 1 contains a summary of human studies (selected for older age groups) that have used metabolomics to study CVD among older adults.
| aHuman studies, publication year | Study population | Results | Inferences | Details/limitations |
|---|---|---|---|---|
| Rizza et al., 2014111 |
N = 67 Mean age 85 ± 3 years High rate of prior CVD (85%) |
Medium-chain and long-chain acylcarnitines were associated with major adverse cardiac events (MACE) |
Ageing mitochondrial dysfunction associated with MACE |
Small sample size; high-risk cohort |
| Ganna et al., 2014115 |
N = 1028 Average age 70 years |
Lipid-related metabolites lysophosphatidylcholine, monoglyceride, and sphingomyelin were associated with incident coronary heart disease over 3.9 to 10 years of median follow-up |
Potential causal role in coronary heart disease development |
Population-based, longitudinal cohorts; integrated genetic and metabolomic analyses |
| Cheng et al., 2015116 |
N = 515 Average age 55 to 64 years across groups |
Metabolite panel consisting of methylarginine/arginine ratio, butyrylcarnitine, spermidine, total essential amino acids, and prognosticated endpoints of death or heart failure-related hospitalization over 6 and 12 months |
Metabolite panel provided better prognostic value over B-type natriuretic peptide | Targeted metabolomics; participants were in heart failure stages A, B, and C |
| Zordoky et al., 201519 |
Total N = 82 Heart failure with preserved ejection fraction (N = 24) Heart failure with reduced ejection fraction (N = 20) Age-matched controls (N = 38) Average age 61 to 67 years across groups |
Short-chain acylcarnitines were higher in both HFpEF and HFrEF than in controls Medium-chain and long-chain acylcarnitines were higher in HFpEF than both HFrEF and controls |
Metabolomics fingerprint of HFpEF is distinct from that of HFrEF and controls | Small sample size; 181 metabolites; other heterogeneous factors involved such as background coronary artery disease and medication usage |
| Hunter et al., 201616 |
CATHGEN study of sequential patients who underwent cardiac catheterization Comparison between HFpEF cases (N = 282) and HFrEF (N = 279) and controls (N = 191) Average age 55 to 66 years across groups |
Long-chain acylcarnitines were higher in HFrEF than HFpEF, increasing linearly with declining ejection fraction |
Possible shared mechanism in HF regardless of ejection fraction | Replication cohort data unavailable; cardiac catheterization cohort could have over-represented ischaemic phenotypes; clinically obtained data; targeted metabolite profiling |
| Ahmad et al., 201615 |
N = 453 chronic systolic heart failure patients (HF-ACTION cohort) Median age 59 years |
Long-chain acylcarnitines were associated with increased risk of all-cause mortality, all-cause hospitalization, cardiovascular death, and cardiovascular hospitalization |
Greater circulating levels of long-chain acylcarnitines predicted functional status and mortality in patients with chronic systolic HF |
Subset study from HF-ACTION cohort |
| Bedi Jr et al., 2016117 |
N = 15 patients with chronic dilated nonischaemic cardiomyopathy N = 20 controls Transmural sampling of the left ventricular myocardium obtained during left ventricular assist device implantation or heart transplantation |
Increased abundance of ketogenic β-hydroxybutyryl-CoA, decreased levels of myocardial β-OH-butyrate, increased circulating levels of ketones | Increased ketone utilization in the end-stage failing heart | End-stage heart failure; male gender predominance in the failing heart group |
| Wang et al., 2017118 |
PREDIMED trial N = 230 incident CVD cases N = 787 random participants Patients were randomized to Mediterranean diets or control diet Average age 67 to 69 years across groups |
Plasma ceramide concentrations associated with elevated risk of composite CVD outcome (acute myocardial infarction, stroke, and cardiovascular death) | Mediterranean diet may have the potential to mitigate detrimental effect associated with elevated baseline plasma ceramide concentrations on CVD risk | Participants were European Caucasians, limits generalizability to other populations; high CVD risk profiles |
| Menni et al., 2018119 |
N = 617 middle-aged women Average age 61 years TwinsUK cohort |
Pulse wave velocity correlated negatively with gut microbiome alpha diversity, adjusted for levels of gut-derived metabolites (indolepropionate, trimethylamine oxide, and phenylacetylglutamine) | Gut microbiome diversity is inversely associated with arterial stiffness in women, only minimally mediated by metabolic syndrome |
Analyses limited to middle-aged white female twins; faecal sampling not necessarily taken at time of arterial stiffness assessment |
- CVD, cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
- a Studies selected based on human studies, older age groups, and cardiovascular endpoints/surrogate endpoints.
Imaging as a tool to detect and manage cardiovascular ageing
Modern cardiovascular imaging has evolved to detect underlying disease that was previously only detectable on tissue histology or corroborated on blood samples. Disease processes commonly involved in ageing, such as inflammation, may be characterized by cardiovascular imaging (Figure 1). For example, in the setting of acute myocardial infarction, imaging of the myocardium via absolute native T1 values on magnetic resonance or fluorodeoxyglucose positron emission tomography correctly determined level of myocardial injury as well as systemic inflammation response.120 Mitochondrial dysfunction has been implicated as a mechanism involved in cardiovascular ageing and can be assessed with imaging tools such as hyperpolarized carbon-13 magnetic resonance spectroscopy in animals121, 122 and phosphorus-31 spectroscopy in humans.123 Endothelial dysfunction has traditionally been characterized by reactive hyperaemia-peripheral arterial tonometry.124, 125 In recent times, coronary endothelial dysfunction, which leads to coronary microvascular dysfunction, has been quantitatively measured in humans by positron emission tomography.126-128 Effects of environmental exposures such as smoking and infective agents on the cardiovascular system have also been imaged by cardiovascular imaging techniques.129-131
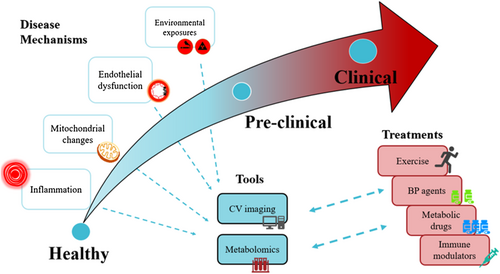
Quantifying subtle functional cardiovascular changes through imaging is important in the setting of preclinical disease and may be relevant in ageing. In a large community study from MESA (Multi-Ethnic Study of Atherosclerosis), older age was associated with presence of myocardial fibrosis measured by T1 mapping on cardiac magnetic resonance imaging.132 Cardiovascular imaging has also refined clinical phenotyping. Most cohorts define the presence of clinical disease solely by development of cardiovascular events such as acute myocardial infarction. This clinical event-driven approach may underestimate actual disease among apparently healthy controls who may have substantial asymptomatic coronary atherosclerosis.133 The use of non-invasive imaging techniques to identify and better classify preclinical disease will enhance potential of novel omics technologies to discover early biomarkers.
Another key concept is that changes should occur as a result of ageing and be viewed distinctly from concomitant risk factors that frequently accompany ageing. Pre-specified cohorts that study cardiovascular ageing independently of traditional risk factors might be necessary. Analysis of community and disease cohorts that include samples acquired prior to disease development may help to uncover novel biomarkers and pathways associated with ageing-related diseases, CVD.
When used in conjunction with metabolomics techniques, non-invasive cardiovascular imaging represents a way to understand pathological consequences of ageing-related cardiovascular changes in preclinical cohorts.134, 135 In a community-based study of older adults without clinical CVD, left atrial function assessed by cardiac magnetic resonance was used as a marker of cardiovascular ageing.135 Left atrial function as represented by left atrial reservoir and conduit phases is known to decrease with age.136 This study highlighted the impact of age on left atrial function, independent of traditional risk factors. Importantly, these specific left atrial functions were associated with a metabolic signature comprising medium-chain and long-chain dicarboxyl carnitines, serine, citrulline, and valine molecules,135 highlighting the potential role of mitochondrial fuel metabolism on pathogenesis of atrial ageing. In another similar analysis, an imaging marker of arterial stiffness, known as pulse wave velocity assessed by applanation tonometry, was independently associated with a similar signature of medium-chain and long-chain dicarboxyl carnitines, independent of blood pressure.137 These results demonstrate how preclinical imaging using established and new imaging markers, when used in conjunction with metabolomics, may be a clinically relevant and novel approach to help identify mechanisms of cardiovascular ageing. In a study that integrated whole-genome sequencing, comprehensive metabolomics, and advanced human body imaging (by echocardiography, electrocardiography, computed tomography, and magnetic resonance imaging), genomics and metabolomics association analysis identified over 5% of heterozygotes with phenotypic manifestations affecting serum metabolite levels.138 In the near future, precision medicine strategies may require integrated methodologies to identify and predict risk of disease prior to disease manifestation.
Risk stratification for cardiovascular ageing
For metabolomics approaches to be clinically relevant for future applications in cardiovascular ageing, we must move beyond mere cross-sectional type analyses. Examples of how to proceed can be found in the literature. In the area of cardiovascular risk stratification, metabolomics has complemented well-established risk scoring systems to provide finer cardiovascular risk stratification,139 efficiently predicting CVD event risk in CVD cohorts, such as patients with coronary artery disease.88, 140 In clinical trials that target established risk factors such as hypertension and dyslipidaemia, metabolomics has been used before and after intervention to study the effects of salt-lowering141 and novel statin therapies.142 Lifestyle factors such as diet factors have also used metabolomics to quantify effect of diet interventions on CVD incidence.143, 144 Physical activity, a key lifestyle factor that influences cardiovascular health, has also been shown to be associated with wide spectrum of acylcarnitines and amino acids.145 These studies point to the role that metabolomics can play in a range of clinical settings, from observational studies, to risk stratification, to clinical trial interventions, to lifestyle-type evaluations. All of these settings are pertinent to the entire life course phenomenon of ageing. What remains to be done is for more similar type study designs to be applied onto ageing cohorts, deeply focused on measuring age as a key exposure of interest, and intervening on specific mechanisms of cardiovascular ageing.
In summary, as cardiovascular ageing progresses, tools such as cardiovascular imaging and biological tools such as metabolomics may be useful for detecting early changes and also study mechanisms of progression. As cardiovascular ageing becomes clinically apparent, insights from these mechanisms may assist in formulating and/or personalizing treatment strategies (Figure 1).
Conclusions and implications
Cardiovascular ageing is a pathologic process that likely involves inflammation, redox stress, and endothelial dysfunction as well as other undefined mechanisms. Metabolomics has the potential to be a powerful tool to help unravel mechanisms of cardiovascular ageing and to identify new biomarkers that predict risk and/or monitor disease progression. Reaching these milestones will require more large-scale studies with robust cross-validation across cohorts.
Acknowledgement
We thank Dr Yen How Tan for his artistic contributions.
Conflict of interest
None declared.
Funding
A.K. received grant support from the National Medical Research Council of Singapore (NMRC/TA/0031/2015, MOH-000153), Hong Leong Foundation, Duke-NUS Medical School, Estate of Tan Sri Khoo Teck Puat, and SingHealth Foundation.
Author contributions
A.K. and J.P.K.: conceptualization and methodology. A.K. and J.P.K.: writing—original draft preparation and writing—reviewing and editing.
Impact statement
Cardiovascular ageing is a life course phenomenon that involves known and unknown risk factors that play out in a dynamic fashion. As metabolomics provides an integrated profile of biological ageing that sums up complexities of cardiovascular ageing, metabolomics may represent a novel way to detect early changes as detected by cardiovascular imaging. A combined approach involving both metabolomics and cardiovascular imaging would expand mechanistic understanding about progressive cardiovascular ageing and provide targeted treatment strategies.




