Aortic pulsatility index predicts clinical outcomes in heart failure: a sub-analysis of the ESCAPE trial
Abstract
Aims
Aortic pulsatility index (API), calculated as (systolic–diastolic blood pressure)/pulmonary capillary wedge pressure (PCWP), is a novel haemodynamic measurement representing both cardiac filling pressures and contractility. We hypothesized that API would better predict clinical outcomes than traditional haemodynamic metrics of cardiac function.
Methods and results
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial individual-level data were used. Routine haemodynamic measurements, including Fick cardiac index (CI), and the advanced haemodynamic metrics of API, cardiac power output (CPO), and pulmonary artery pulsatility index (PAPI) were calculated after final haemodynamic-monitored optimization. The primary outcome was a composite endpoint of death or need for orthotopic heart transplant (OHT) or left ventricular assist device (LVAD) at 6 months. A total of 433 participants were enrolled in the ESCAPE trial of which 145 had final haemodynamic data. Final API measurements predicted the primary outcome, OR 0.47 (95% CI 0.32–0.70, P < 0.001), while CI, CPO, and PAPI did not. Receiver operator characteristic analyses of final advanced haemodynamic measurements indicated API best predicted the primary outcome with a cutoff of 2.9 (sensitivity 76.2%, specificity 55.3%, correctly classified 61.4%, area-under-the-curve 0.71), compared with CPO, CI, and PAPI. Kaplan–Meier analyses indicated API ≥ 2.9 was associated with greater freedom from the primary outcome (83.5%), compared with API < 2.9 (58.4%), P = 0.001. While PAPI was also significantly associated, CI and CPO were not.
Conclusions
The novel haemodynamic measurement API better predicted clinical outcomes in the ESCAPE trial when compared with traditional invasive haemodynamic metrics of cardiac function.
Introduction
Heart failure affects 6.2 million people, with an expected increase to over 8 million patients by 2030.1 Heart failure is a morbid disease, and hospitalization for acute decompensation is a bell-weather for impending poor outcomes, with an almost two-fold increase in risk of re-hospitalization, as well as 28 day and 1 year mortality risks of 10.4% and 29.5%, respectively.1, 2 In 2005, the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial was conducted, in a randomized controlled fashion, to assess the utility of invasive haemodynamic monitoring with a pulmonary arterial (PA) catheter for patients with acute decompensated heart failure with reduced ejection fraction, without cardiogenic shock.3 While there was no significant benefit in the primary outcome with PA catheter use, a later sub-analysis noted that filling pressures [i.e. right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP)] at the end of the hospitalization were significantly associated with the primary outcome, but the standard assessment of cardiac function, cardiac index (CI), was not.4 This was surprising, as cardiac function, and its downstream pathophysiological effects, are central to the haemodynamic understanding of heart failure exacerbations.
While evidence of congestion is prognostically important, it is likely that an appropriate measurement of the interplay of cardiac function, along with filling pressures, would better predict clinical outcomes for this population. The aortic pulsatility index (API), calculated as (systolic – diastolic blood pressure)/PCWP, is a novel haemodynamic measurement representing both cardiac filling pressures and contractility. It was initially derived in a retrospective cohort of heart failure patients with haemodynamic evidence of cardiogenic shock requiring milrinone.5 We hypothesized that API would better predict clinical outcomes than traditional haemodynamic metrics of cardiac function in acute decompensated heart failure patients in the ESCAPE trial.
Methods
De-identified individual-level data from the ESCAPE trial were provided by the U.S. National Heart, Lung, and Blood Institute via the Biolincc repository for this analysis.6, 7 A signed data use agreement was completed prior to obtaining data, and the study was exempted from the University of Chicago Institutional Review Board approval. Data analysis was conducted between February and September 2020.
The ESCAPE trial included patients with ejection fraction less than 30%, symptoms despite 3 months of goal-directed medical therapy, systolic blood pressure less than or equal to 125 mmHg, and at least one sign or symptom of congestion. Additionally, participants had to fulfil one the following criteria: heart failure hospitalization within the prior year, urgent emergency department visit, or use of greater than 160 mg furosemide (or equivalent) daily during the prior month to indicate severity of heart failure.3 Participants were then randomized to PA catheter-guided management versus routine care. However, some patients crossed over from the control arm and had a PA catheter in place, and some participants were randomized to the PA catheter arm but did not have one placed.3 In this analysis, we included all patients that had full baseline haemodynamic data needed to calculate API (i.e. systolic and diastolic blood pressures and PCWP), regardless of randomization arm. Routine haemodynamic measurements, including Fick CI, and the advanced haemodynamic metrics of API, cardiac power output (CPO), and pulmonary artery pulsatility index (PAPI) were calculated at baseline and after final haemodynamic-monitored optimization.
The primary endpoint of the ESCAPE trial was days alive out of the hospital during the 6 months following randomization. The primary outcome in this study was a composite endpoint of death, need for orthotopic heart transplant (OHT), or need for left ventricular assist device (LVAD) at 6 months.
Statistical analysis
For baseline clinical characteristics, continuous variables were expressed as means ± standard deviations or medians with interquartile ranges and compared with either Student t tests or Mann–Whitney U (Wilcoxon) tests depending upon normality as determined by Shapiro–Wilk tests. Categorical variables were expressed as relative counts and percentages and compared with χ2 tests of association or Fisher exact tests. A multivariable logistic regression was conducted to determine which haemodynamic and baseline clinical characteristics were associated with the primary composite endpoint of death, OHT, or LVAD at 6 months. Relevant clinical parameters with a P < 0.05 in the univariable logistic regression were included in the multivariable logistic regression model, whereby results were presented as odds ratio (OR) and 95% confidence interval. Independent parameters were checked for multicollinearity using Spearman rank correlations, whereby there were no multicollinearity issues between the independent parameters. Receiver operator characteristic (ROC) curves were used to determine the cutoff point, as well as the sensitivity, specificity, correctly classified, and area under the curve (AUC) values of the haemodynamic metrics. Kaplan–Meier time-to-event analysis was generated to describe time to the primary composite endpoint, and then tested using log rank tests. Tests were two-tailed and considered statistically significant with a p-value <0.05. All statistical analyses were conducted using STATA MP version 15 (College Station, TX).
Results
A total of 433 participants were enrolled in the ESCAPE trial, of which 198 were randomized to the PA catheter arm, and 21 crossed over from the control arm and received PA catheters—a total of 219 participants. The data repository recorded 200 participants with invasive haemodynamic data available, but only 190 had baseline PCWP and blood pressure measurements. One additional participant was excluded due to presumed inaccurate data because of a recorded baseline PCWP of 0 mmHg, leaving 189 total participants with full baseline haemodynamic data. Of the 189 participants included, 24.9% were female, 59.8% were White, and 27.5% were Black. Ischaemic cardiomyopathy was the aetiology in 59.3% of participants, with an average baseline ejection fraction of 18.6%. When stratified by the final API cutoff of 2.9 (described in further detail below) there were no significant differences in baseline characteristics, but there were differences in baseline use of dobutamine, and baseline haemoglobin, haematocrit, blood urea nitrogen, and total bilirubin levels (Table 1). There were no significant differences in discharge laboratory values and medications (Supporting Information, Tables S1–S2). Finally, there were 163 participants with some recorded final haemodynamic data after haemodynamic-monitored optimization. Of the 145 participants with full, final haemodynamic data, 45 (29%) experienced the primary outcome.
| All patients (n = 189) | Final API ≥ 2.9 (n = 68) | Final API < 2.9 (n = 77) | P | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age | 58 (48–66); n = 189 | 59 (48–67.5) | 56 (48–66) | 0.43 |
| Gender | 0.16 | |||
| Male | 142 (75.1) | 45 (66.2) | 59 (76.6) | |
| Female | 47 (24.9) | 23 (33.8) | 18 (23.4) | |
| Race | 0.20 | |||
| White | 113 (59.8) | 39 (57.4) | 46 (59.7) | |
| Black | 52 (27.5) | 17 (25.0) | 23 (29.9) | |
| Asian | 19 (10.1) | 8 (11.8) | 8 (10.4) | |
| Other | 5 (2.7) | 4 (5.9) | 0 (0) | |
| Body mass index (kg/m2) | 27.57 (23.30–32.50); n = 189 | 26.42 (22.42–33.49); n = 66 | 27.69 (24.12–32.31) | 0.53 |
| Baseline ejection fraction (%) | 18.6 (12.3–26.2); n = 103 | 20.2 (14.0–26.1); n = 38 | 15.5 (10.8–25.4); n = 38 | 0.19 |
| Past medical history | ||||
| Ischaemic heart disease | 112 (59.3) | 39 (57.4) | 48 (62.3) | 0.54 |
| Percutaneous coronary intervention | 50 (26.5) | 17 (25.0) | 25 (32.5) | 0.32 |
| Coronary artery bypass graft | 59 (31.2) | 23 (33.8) | 22 (28.6) | 0.50 |
| Valvular heart disease | 28 (14.8) | 7 (10.3) | 12 (15.6) | 0.35 |
| Hypertension | 92 (48.7) | 35 (51.5) | 38 (49.4) | 0.80 |
| Diabetes | 64 (33.9) | 25 (36.8) | 21 (27.3) | 0.22 |
| Atrial fibrillation | 56 (29.6) | 16 (23.5) | 26 (33.8) | 0.18 |
| Renal insufficiency | 9 (4.8) | 3 (4.4) | 5 (6.5) | 0.72 |
| Chronic obstructive pulmonary disease | 31 (16.4) | 14 (20.6) | 11 (14.3) | 0.32 |
| Stroke | 21 (11.1) | 9 (13.2) | 7 (9.1) | 0.43 |
| Transient ischaemic attack | 11 (5.8) | 3 (4.4) | 7 (9.1) | 0.34 |
| Malignancy | 12 (6.4) | 4 (5.9) | 4 (5.2) | 1 |
| Cardiac arrest | 11 (5.8) | 2 (2.9) | 6 (7.8) | 0.28 |
| Baseline medications | ||||
| ACE inhibitor | 150 (79.4) | 59 (86.8) | 59 (76.6) | 0.12 |
| ARB | 35 (18.5) | 11 (16.2) | 17 (22.1) | 0.37 |
| Beta-blocker | 125 (66.1) | 42 (61.8) | 49 (63.6) | 0.82 |
| Digoxin | 131 (69.3) | 48 (70.6) | 55 (71.4) | 0.91 |
| Diuretics | 186 (98.4) | 66 (97.1) | 76 (98.7) | 0.60 |
| Isosorbide mononitrate | 31 (16.4) | 14 (20.6) | 10 (13.0) | 0.22 |
| Isosorbide dinitrate | 24 (12.7) | 7 (10.3) | 11 (14.3) | 0.47 |
| Dobutamine | 18 (9.5) | 9 (13.2) | 3 (3.9) | 0.042 |
| Dopamine | 5 (2.7) | 2 (2.9) | 2 (2.6) | 1 |
| Milrinone | 4 (2.1) | 2 (2.9) | 1 (1.3) | 0.60 |
| Baseline laboratory values | ||||
| White blood cell count (103/μL) | 7.3 (5.9–9.0); n = 184 | 7.6 (5.9–9.2); n = 64 | 7.1 (5.7–8.7); n = 76 | 0.62 |
| Haemoglobin (g/dL) | 12.5 (11.4–13.6); n = 183 | 12.2 (11.3–13.2); n = 64 | 12.9 (11.8–13.9); n = 76 | 0.03 |
| Haematocrit (%) | 38.1 (34.45–40.7); n = 184 | 37.6 (34.1–39.6); n = 64 | 38.6 (35.8–41.6) | 0.04 |
| Platelets (103/μL) | 210 (169.5–266); n = 180 | 237 (170–298); n = 61 | 209 (178.5–247); n = 76 | 0.14 |
| Sodium (mmol/L) | 137 (134–139); n = 187 | 137 (136–140); n = 66 | 137 (134–139) | 0.22 |
| Potassium (mmol/L) | 4.2 (3.8–4.6); n = 188 | 4.2 (3.9–4.7); n = 67 | 4.1 (3.7–4.6) | 0.15 |
| Blood urea nitrogen (mg/dL) | 29 (20–44); n = 187 | 25 (17–34); n = 66 | 32 (22–47) | 0.02 |
| Creatinine (mg/dL) | 1.4 (1.1–1.8); n = 189 | 1.3 (0.9–1.6); n = 68 | 1.5 (1.1–1.8) | 0.16 |
| Total protein (g/dL) | 7.1 (6.7–7.5); n = 125 | 7 (6.7–7.5); n = 37 | 7.1 (6.8–7.4); n = 57 | 0.97 |
| Albumin (g/dL) | 3.6 (3.3–3.9); n = 140 | 3.6 (3.3–3.8); n = 48 | 3.6 (3.1–3.9); n = 61 | 0.81 |
| Total bilirubin (mg/dL) | 0.8 (0.4–1.3); n = 150 | 0.7 (0.4–1.1); n = 49 | 1.1 (0.6–1.4); n = 65 | 0.04 |
| Aspartate transaminase (U/L) | 29 (22–39); n = 153 | 29 (21–39); n = 50 | 28 (23–36); n = 65 | 0.91 |
| Alanine transaminase (U/L) | 26 (18.5–37.5); n = 148 | 25 (18–36); n = 50 | 27.5 (18–37); n = 62 | 0.68 |
- ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker.
There were no differences in baseline ejection fraction, end-diastolic diameter, or end-systolic diameter between the high and low API groups, but patients with API < 2.9 had lower EF at baseline, 14.2% (8.6–23.5) vs. 21.8% (14.9–27.6), P = 0.02 (Supporting Information, Table S3). Baseline haemodynamics for all 190 patients with available data are listed in Table 2. When stratified by the final API cutoff of 2.9, patients with a final API < 2.9 had significantly higher baseline RAP and PA pressures, as well as lower systolic blood pressure, PAPI, and baseline API. There were no significant differences in baseline diastolic BP, mean arterial pressure, Fick cardiac output or CI, or CPO. Final haemodynamics for the participants with complete data are listed in Table 2. Similar to the baseline haemodynamics, patients with a final API < 2.9 had significantly higher final RAP and PA pressures, as well as lower systolic blood pressure and PAPI. There were no significant differences in baseline diastolic BP, mean arterial pressure, Fick cardiac output or CI, or CPO.
| All patients | Final API ≥ 2.9 | Final API < 2.9 | P | |
|---|---|---|---|---|
| Baseline haemodynamics | n = 189 | n = 68 | n = 77 | |
| RA (mmHg) | 12 (7–18); n = 187 | 10 (6–15.5) | 14 (10–20); n = 76 | 0.001 |
| PA Systolic (mmHg) | 55 (45–66); n = 181 | 50 (40–63); n = 65 | 58.5 (48–68); n = 74 | 0.01 |
| PA Diastolic (mmHg) | 25 (20–35); n = 181 | 24 (19–29); n = 65 | 27.5 (23–35); n = 74 | 0.003 |
| PCWP (mmHg) | 24 (20–30); n = 189 | 20 (16.5–26) | 26 (22–31) | <0.001 |
| Systolic BP (mmHg) | 110 (98–120) | 114 (101–129.5) | 104 (98–114) | 0.002 |
| Diastolic BP (mmHg) | 64 (57–74); n = 190 | 62 (52.5–74) | 66 (60–74) | 0.13 |
| MAP (mmHg) | 79.3 (71.7–88); n = 190 | 79.3 (68.8–91.2) | 80 (72.7–87.3) | 0.88 |
| Fick CI (L/min/m2) | 1.9 (1.6–2.3); n = 182 | 2 (1.7–2.4); n = 67 | 1.9 (1.6–2.2); n = 73 | 0.10 |
| Fick CO (L/min) | 3.8 (2.9–4.6); n = 183 | 3.7 (2.9–4.7); n = 67 | 3.7 (3.0–4.5); n = 74 | 0.81 |
| CPO (Watts) | 0.7 (0.5–0.8); n = 183 | 0.6 (0.5–0.8); n = 67 | 0.6 (0.5–0.8); n = 74 | 0.77 |
| PAPI | 2.3 (1.5–3.5); n = 180 | 2.5 (1.7–4.8); n = 65 | 2 (1.4–3); n = 74 | 0.01 |
| API | 1.8 (1.2–2.6); n = 189 | 2.6 (1.6–3.5) | 1.3 (1.0–1.9) | <0.001 |
| Final haemodynamics | ||||
| RA (mmHg) | 8 (5–12); n = 157 | 6 (4–9); n = 64 | 10 (7–15); n = 15 | <0.001 |
| PA Systolic (mmHg) | 45 (36–54); n = 157 | 38 (33–47); n = 65 | 48 (42–57); n = 74 | <0.001 |
| PA Diastolic (mmHg) | 20 (16–25); n = 157 | 17 (14–21); n = 65 | 24 (20–27); n = 74 | <0.001 |
| PCWP (mmHg) | 17.5 (13–21.5); n = 148 | 12.5 (10–15.5) | 20 (18–24) | <0.001 |
| Systolic BP (mmHg) | 100 (90–113); n = 163 | 110 (94–120) | 98 (89–106) | 0.001 |
| Diastolic BP (mmHg) | 56 (48–66); n = 163 | 55.5 (45.5–64) | 56 (48–67) | 0.45 |
| MAP (mmHg) | 70.7 (62.7–80); n = 163 | 73.3 (62.3–81.2) | 70 (62–79) | 0.42 |
| Fick CI (L/min/m2) | 2.3 (1.9–2.7); n = 154 | 2.4 (2–2.8); n = 66 | 2.3 (2.0–2.6); n = 72 | 0.21 |
| Fick CO (L/min) | 4.6 (3.6–5.4); n = 155 | 4.5 (3.6–5.6); n = 66 | 4.6 (3.7–5.3); n = 74 | 0.86 |
| CPO (Watts) | 0.7 (0.5–0.9); n = 155 | 0.7 (0.5–0.9); n = 66 | 0.7 (0.5–0.9); n = 74 | 0.85 |
| PAPI | 3 (1.9–4.4); n = 150 | 3.8 (2.3–6); n = 61 | 2.7 (1.9–3.6); n = 72 | 0.002 |
| API | 2. 8 (1.9–3.6); n = 145 | 3.7 (3.3–5.0) | 2.0 (1.6–2.5) | <0.001 |
- API, aortic pulsatility index; BP, blood pressure; CI, cardiac index; CO, cardiac output; CPO, cardiac power output; MAP, mean arterial pressure; PA, pulmonary artery; PAPI, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
On univariable analysis, multiple baseline haemodynamics were significantly associated with the primary outcome. Of the measures of cardiac function, only API, OR 0.64 (95% CI 0.47–0.87, P = 0.005) and CPO, OR 0.22 (95% CI 0.05–0.93, P = 0.04), were associated with the primary outcome (Table 3). Multiple final haemodynamics were also associated with the primary composite outcome in the univariable analysis, but of the measures of cardiac function, only API, OR 0.47 (95% CI 0.32–0.70, P < 0.001), was significantly associated with the primary outcome, while Fick cardiac output and CI, CPO, and PAPI were not (Table 3). The univariable analysis also showed that the absence of prescriptions of angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, digoxin, or diuretics on discharge were associated with the primary outcome, as were baseline sodium, blood urea nitrogen, creatinine levels and discharge haemoglobin, haematocrit, and blood urea nitrogen (Supporting Information, Tables S4–S6).
| n | Odds ratio | 95% CI | P | |
|---|---|---|---|---|
| Baseline haemodynamics | ||||
| RA (mmHg) | 187 | 1.04 | (1.00–1.08) | 0.03 |
| PA Systolic (mmHg) | 181 | 1.02 | (1.00–1.05) | 0.03 |
| PA Diastolic (mmHg) | 181 | 1.04 | (1.01–1.08) | 0.02 |
| PCWP (mmHg) | 189 | 1.05 | (1.02–1.09) | 0.01 |
| Systolic BP (mmHg) | 189 | 0.97 | (0.95–0.99) | 0.01 |
| Diastolic BP (mmHg) | 189 | 0.99 | (0.96–1.01) | 0.27 |
| MAP (mmHg) | 189 | 0.97 | (0.95–1.00) | 0.052 |
| Fick CI (L/min/m2) | 182 | 0.81 | (0.49–1.35) | 0.42 |
| Fick CO (L/min) | 183 | 0.86 | (0.68–1.10) | 0.23 |
| CPO (Watts) | 183 | 0.22 | (0.05–0.93) | 0.04 |
| PAPI | 180 | 0.85 | (0.72–1.01) | 0.061 |
| API | 189 | 0.64 | (0.47–0.87) | 0.005 |
| Final haemodynamics | ||||
| RA (mmHg) | 157 | 1.17 | (1.09–1.26) | <0.001 |
| PA Systolic (mmHg) | 157 | 1.05 | (1.02–1.08) | 0.003 |
| PA Diastolic (mmHg) | 157 | 1.11 | (1.05–1.18) | 0.001 |
| PCWP (mmHg) | 148 | 1.15 | (1.08–1.23) | <0.001 |
| Systolic BP (mmHg) | 163 | 0.99 | (0.97–1.01) | 0.43 |
| Diastolic BP (mmHg) | 163 | 0.99 | (0.96–1.01) | 0.31 |
| MAP (mmHg) | 163 | 0.99 | (0.96–1.01) | 0.27 |
| Fick CI (L/min/m2) | 154 | 0.86 | (0.50–1.48) | 0.58 |
| Fick CO (L/min) | 155 | 0.88 | (0.69–1.11) | 0.28 |
| CPO (Watts) | 155 | 0.32 | (0.08–1.29) | 0.11 |
| PAPI | 150 | 0.88 | (0.75–1.02) | 0.1 |
| API | 145 | 0.47 | (0.32–0.70) | <0.001 |
- API, aortic pulsatility index; BP, blood pressure; CI, cardiac index; CO, cardiac output; CPO, cardiac power output; MAP, mean arterial pressure; PA, pulmonary artery; PAPI, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
After conducting a univariable analysis, multivariable analyses were completed to assess for interaction between multiple significant associations with the primary outcome. We limited the multivariable analysis to six significant variables from the univariable analysis based on the 58 event outcomes in our sample. We included only significant variables associated with final, or discharge, time points, and excluded those measured at baseline (i.e. baseline sodium, creatinine, and blood urea nitrogen), since those were two different points in time. We then removed discharge prescription of digoxin and final serum laboratory values, as these were less likely to affect API directly. Two separate analyses were done to assess the interaction between API and CPO as well as API and Fick CI. CPO and Fick CI could not be included in the same analysis as they both incorporated cardiac output. In both analyses, the variables final RAP, and ACE-inhibitor, beta-blocker, and diuretic prescriptions on discharge were incorporated. In both multivariable analyses, final API, final RAP, and discharge prescriptions of ACE-inhibitors and beta-blockers remained significantly associated with the primary outcome (Table 4).
| P | OR | 95% CI | |
|---|---|---|---|
| Multivariable analysis A (n = 108) | |||
| API final | 0.03 | 0.60 | (0.39–0.94) |
| CPO final | 0.35 | 0.45 | (0.08–2.42) |
| RAP final | 0.01 | 1.13 | (1.03–1.24) |
| Discharge ACE-inhibitor prescription | 0.007 | 0.22 | (0.07–0.65) |
| Discharge Beta-blocker prescription | 0.03 | 0.33 | (0.12–0.90) |
| Discharge diuretic prescription | 0.10 | 0.19 | (0.03–1.37) |
| Multivariable analysis B (n = 107) | |||
| API final | 0.02 | 0.59 | (0.37–0.93) |
| Fick CI final | 0.86 | 1.06 | (0.54–2.10) |
| RAP final | 0.02 | 1.12 | (1.02–1.23) |
| Discharge ACE-inhibitor prescription | 0.01 | 0.23 | (0.08–0.71) |
| Discharge beta-blocker prescription | 0.02 | 0.30 | (0.11–0.80) |
| Discharge diuretic prescription | 0.09 | 0.20 | (0.03–1.28) |
- ACE, angiotensin-converting enzyme; API, aortic pulsatility index; CI, cardiac index; CPO, cardiac power output; RAP, right atrial pressure.
ROC analyses indicated final API predicted the primary outcome with a cutoff of 2.9 (AUC 0.71), which was better than CPO 0.69 (AUC 0.58) and CI 2.2 (AUC 0.52), and PAPI 2.6 (AUC 0.63) (Table 5, Figure 1). Kaplan–Meier analyses similarly showed that API (83.5% vs. 58.4%, P = 0.001) and PAPI (78.3% vs. 59.0%, P = 0.03) dichotomized the primary endpoint (Figure 2).
| Optimal cutoff value | Sensitivity (%) | Specificity (%) | Correctly classified (%) | AUC | |
|---|---|---|---|---|---|
| API | 2.9 | 76.19 | 55.34 | 61.38 | 0.71 |
| CPO | 0.69 | 57.78 | 57.27 | 57.42 | 0.58 |
| Fick CI | 2.2 | 45.45 | 58.18 | 54.55 | 0.52 |
| PAPI | 2.6 | 60.47 | 64.49 | 63.33 | 0.63 |
- API, aortic pulsatility index; CI, cardiac index; CPO, cardiac power output; PAPI, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.

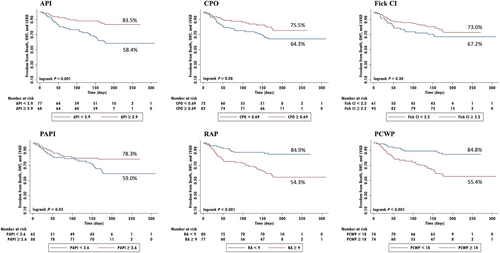
Discussion
In this sub-analysis of the ESCAPE trial, we were able to identify a novel haemodynamic variable, combining both filling pressures and cardiac function, that predicted clinical outcomes in the ESCAPE trial. API ≥ 2.9 was associated with decreased risk of death and need for LVAD or OHT at 6 months. Routine measures of cardiac function were not associated with any of these outcomes.
Not surprisingly, PAPI, the right ventricular measurement analogous to API, was also associated with the primary outcome and the secondary outcome of need for any rehospitalization. This is concordant with the previously published sub-analysis of the ESCAPE trial by Kochav et al. evaluating haemodynamic measurements of right ventricular function in which PAPI was the best predictor of adverse clinical outcomes.8 While PAPI was correlated with CI in that analysis, likely due to the complex interplay between the right and left ventricles, CI on its own has not been correlated with clinical outcomes in the ESCAPE trial.4, 8 API therefore serves as the first marker of left ventricular function from this trial data to be associated with adverse events.
Easy-to-use prognostic markers for heart failure patients are of increasing importance. The optimal timing of referral of heart failure patients for advanced options candidacy is a continuous dilemma. Additional clinical prognostic markers that could be used during this bellweather event of a heart failure patients' course are needed.9 While clinically stable outpatients may be identified by a peak oxygen consumption (VO2), there are few prognostic markers for heart failure patients admitted with an acute decompensation.10-13 We know that patients hospitalized with decompensated heart failure have almost double the increased risk of re-hospitalization, and 28 day and 1 year mortality risks of 10.4% and 29.5%, respectively.1, 2 However, as we see in this sub-analysis of the ESCAPE trial, these patients can be further delineated by their residual cardiac function and filling pressures following haemodynamic optimization. Over 40% of the participants, with API < 2.9 had the outcome of death, LVAD, or OHT at 6 months compared with 16% with an API ≥ 2.9. API could potentially be used to differentiate those patients with a higher risk of the significant clinical outcomes of death or need for OHT or LVAD by 6 months post-hospital discharge and may prompt referral for advanced options.
Cooper et al. did a similar sub-analysis of the ESCAPE trial, during which they identified that elevated filling pressures (i.e. RAP and PCWP), independent of cardiac index, were significantly associated with the primary outcome, which was survival outside the hospital 6 months following discharge, and was driven primarily by re-hospitalization rates.4 Persistently elevated filling pressures were hypothesized to have deleterious effects on cardiac physiology, as well as on renal function. Renal function was another predictor of adverse outcomes in the ESCAPE trial, independent of cardiac output, though notably in our analysis, there were no significant differences in baseline or discharge creatinine between the low and high API groups, and baseline renal insufficiency was not associated with the primary outcome.14
Even with the reported effects of filling pressures, representing preload, and renal function on their own, our common understanding of the pathophysiology of heart failure includes the intersection of preload along with afterload, contractility, and lusitropy.15 Therefore, it would make sense for a haemodynamic marker that accounts for more than one of these four factors. While no single haemodynamic parameter, to date, represents all four of these elements, API represents the effect of preload and contractility, and was associated with the significant clinical outcomes of death or need for OHT or LVAD.
The pathophysiology of heart failure is complex, and its treatment famously shifted from haemodynamic-specific to the neurohormonal blockade.16, 17 It is important to note that in our study, while the final haemodynamic measurements of API and RAP were associated with the primary outcome, so too were the use of discharge prescriptions of ACE-inhibitors and beta-blockers, even when included in multivariable analyses. This is of course not surprising as these medications have well-researched positive clinical effects in this patient population.18 Our study does not settle any debate between the haemodynamic and neurohormonal hypotheses. Rather, API may allow for additional, individualized prognostication beyond the Class I recommendation for use of ACE-inhibitors and beta-blockers in this population.
Limitations
This study is limited as it was not a pre-specified sub-analysis of the ESCAPE trial, and therefore is limited in its retrospective nature, though the data still come from a peer-reviewed randomized controlled-trial. Additionally, the data set from the ESCAPE trial is now 15 years old and does not reflect advancements in heart failure therapies, such as sacubitril/valsartan and sodium-glucose transport protein 2 inhibitors. Still, the data from the ESCAPE trial is multicentre, was prospectively collected, and all endpoints were clinically adjudicated, adding strength to the dataset. The ESCAPE trial excluded patients who clinicians felt would benefit from PA catheter-directed management and thus likely represented a less sick cohort of patients with acute decompensated heart failure. This should be taken into account when considering the appropriate API cutoff to use to help guide patient management in patient with more profound cardiogenic shock. Accordingly, our group recently showed that an API cutoff of 1.40 best predicted the need for advanced heart failure surgical therapies or death in a cohort of patients who were inotrope-dependent.5 Finally, approximately one-quarter of patients randomized to the PA catheter arm did not have full final haemodynamic data that could be used for analysis.
Conclusion
The novel haemodynamic measurement API better predicted clinical outcomes in the ESCAPE trial when compared with traditional invasive hemodynamic metrics of cardiac function.
Conflict of interest
None declared.




