Prognostic impact of elevated fatty acid-binding protein 1 in patients with heart failure
Abstract
Aims
Few biomarkers to evaluate pathophysiological changes in extra-cardiac tissues have been identified in patients with heart failure (HF). Fatty acid-binding protein 1 (FABP), also known as liver FABP, is predominantly expressed in the liver. Circulating FABP1 has been proposed to be a sensitive biomarker for liver injury. However, little is known about the potential role of FABP1 as a biomarker for HF.
Methods and results
Measurements of serum FABP1 and echocardiography were performed in subjects with compensated HF (n = 162) and control subjects without HF (n = 20). Patients were prospectively followed-up for a composite outcome of all-cause mortality or HF hospitalization. Compared with control subjects, levels of FABP1 were elevated in HF patients [7.9 (6.4–11.7) vs. 17.6 (10.4–28.9) ng/mL, P < 0.0001]. There were significant correlations between FABP1 levels and estimated right ventricular systolic pressure and right atrial pressure. During a median follow-up of 12.0 months, there were 55 primary composite endpoints in the HF cohort. The highest FABP1 tertile was associated with a three-fold increased risk of the composite outcome compared with the lowest tertile [95% confidence interval (1.46–6.68), P = 0.003], but other conventional hepatobiliary markers did not predict the outcome. After adjusting for age, sex, atrial fibrillation, and N-terminal pro-B-type natriuretic peptide levels, serum FABP1 remained independently associated with the outcome. Adding FABP1 to the model based on clinical factors and N-terminal pro-B-type natriuretic peptide significantly improved the prognostic value (global χ2 20.8 vs. 15.5, P = 0.01).
Conclusion
Serum FABP1 levels are elevated in compensated HF patients, and the magnitude of elevation is independently associated with pulmonary hypertension, right atrial hypertension, and worse clinical outcomes. FABP1 may serve as a new potential biomarker for the assessment of hitherto unrecognized derangement of cardio-hepatic interaction in HF.
Introduction
Heart failure (HF) is a systemic complex syndrome with the involvement of multiple organ systems. In patients with HF, a number of cardiovascular biomarkers that reflect haemodynamic stress and myocardial injury resulting from the neurohormonal and inflammatory insults to the heart have been proved to predict the risk of outcomes.1 In recent years, increasing attention has been directed towards the potential role of the distinct biomarkers reflective of interdependent mechanisms involving other organs, such as the kidney, adipose tissue, and intestine.2-4 However, there are little data about the biomarkers representing the cardio-hepatic interactions in HF.
Liver dysfunction assessed by elevations primarily in transaminases and cholestatic enzymes is common in patients with chronic HF.5, 6 Haemodynamic perturbation characterized by elevated central venous pressure and impaired hepatic perfusion has long been known as a major mechanism underlining these biochemical abnormalities of liver function in HF.7 Although prior studies demonstrated the association between elevations in these liver markers and cardiovascular outcomes,5, 6 the clinical impact of subclinical liver injury that is not detected by traditional liver function tests on HF outcome has not been reported.
Fatty acid-binding proteins (FABPs) are intracellular lipid chaperones that transport long-chain fatty acids into mitochondria and regulate of lipid metabolism.8, 9 FABP1, also known as liver FABP, is 14 kDa protein, which is predominantly expressed in the liver and to a much lesser extent in the intestine and kidney.10, 11 Previous studies have demonstrated that circulating levels of FABP1 are elevated in the setting of acute or chronic liver injury or failure.12-14 It is also reported that FABP1 may be a more sensitive marker to detect hepatocyte damage than conventional hepatic makers such as alanine aminotransferase (ALT).12 Besides, FABP1 is associated with systemic hypertension and elevated natriuretic peptide levels, suggesting a potential role of FABP1 in cardiovascular diseases.15 While other subtypes of FABPs, including heart FABP (FABP3) and adipocyte FABP (FABP4), have been shown to be associated with poor prognosis in patients with HF,16, 17 no study has examined the prognostic significance of serum FABP1.
Accordingly, the aims of this study were (i) to assess whether serum FABP1 levels would be elevated in HF patients compared with control subjects without HF; (ii) to evaluate the association between serum FABP1 levels and cardiac remodelling and dysfunction; (iii) to determine whether FABP1 levels would predict adverse outcomes; and (iv) to elucidate whether they had independent and incremental prognostic value over natriuretic peptide levels.
Methods
Subjects
Subjects with HF who admitted to the Gunma University Hospital between 2015 and 2018 were enrolled in this prospective study. All patients were required to have recent hospitalization for HF treated with intravenous diuretics to ensure the unequivocal presence of HF. Subjects with unstable coronary disease, recent revascularization, constrictive pericarditis, myocarditis, or significant liver diseases were excluded. Subjects underwent clinical history, blood sampling, and resting echocardiography in a compensated state.
Subjects free of HF who were referred to coronary angiography were included as a comparator group (controls, n = 20). Written informed consent was provided by all subjects before participation. The study was approved by the Gunma University Hospital Clinical Research Review Board. The authors had full access to the data and take responsibility for its integrity.
Biomarker measurements
In HF patients, venous blood samples were obtained after an overnight fast in a compensated state. In control subjects, blood sampling was performed 1 day before the indexed coronary angiography. Serum haemoglobin, creatinine, glucose, hepatobiliary enzymes, lipid profiles, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were measured by routine automated laboratory procedures. Troponin I levels were collected from the medical chart. Serum FABP1 levels were measured using a commercially available enzyme-linked immunosorbent assay kit (Abcam, Cambridge, UK). As specified by the manufacturer, the lower limits of detection of serum FABP1 were 9.4 pg/mL.
Assessment of cardiac structure and function
Two-dimensional and Doppler echocardiography was performed within 2 days of the blood sampling. Echocardiographic measurements were performed according to the current guidelines.18 Left ventricular (LV) systolic function was assessed by ejection fraction (EF) and systolic mitral annular tissue velocity (mitral s′ velocity). LV diastolic function was assessed using the transmitral velocities [early and late diastolic inflow velocities (E and A) and deceleration time of the E], early diastolic septal mitral annular tissue velocity (e′), and the ratio of E/e′. Left atrial (LA) volume was calculated with the method of discs. LA volume and LV mass were then indexed to body surface area. Right atrial pressure (RAP) was estimated from the diameter of the inferior vena cava and its respiratory change.18 Right ventricular systolic pressure was then calculated as (4 × peak tricuspid regurgitation velocity)2 + estimated RAP.
Outcome assessment
Patient follow-up was initiated on the day of blood sampling. The primary endpoint was a composite of all-cause death or HF hospitalization. The secondary endpoint was HF hospitalization, which was defined as dyspnoea and pulmonary oedema on chest X-ray requiring intravenous diuretics treatment. Investigators obtained follow-up data from the patient's medical records, telephone interviews, and notices of death from other hospitals.
Statistical analysis
Data are reported as mean (standard deviation), median (inter-quartile range), or number (%) unless otherwise specified. Between-group differences were compared by unpaired t-test, Wilcoxon rank-sum test, or χ2 test, as appropriate. Pearson's or Spearman's correlation coefficients were used to assess relationships between two variables of interest, as appropriate. Kaplan–Meier curve analysis was used to assess event-free rates, and univariable and multivariable Cox proportional hazard models were then applied to evaluate the independent prognostic power. The incremental prognostic value was assessed by comparing −2 log-likelihood values with and without the parameter to χ2 distribution at degree of freedom of 1. All tests were two sided, with a P value of <0.05 considered significant. All analyses were performed by JMP 14.0.0 (SAS Institute, Cary, NC, USA).
Results
Subject characteristics
Age, gender, body mass index, and prevalence of diabetes mellitus and hypertension were similar between HF patients and controls (Table 1). As compared with control subjects, dyslipidaemia was less prevalent, but atrial fibrillation (AF) was more prevalent in the patients. Systolic and diastolic blood pressures and heart rates were similar between the groups. As expected, HF patients were more treated with loop diuretics and mineralocorticoid receptor antagonists, but the use of other medications was similar between groups. Patients with HF had lower haemoglobin and estimated glomerular filtration rate and higher γ-glutamyl transpeptidase (γGT), alkaline phosphatase (ALP), and NT-proBNP levels than control subjects. Troponin I levels were on average modestly elevated in a subset of HF patients with obtainable data [0.09 (0.03–0.28) ng/mL]. Troponin I levels were obtainable in three control subjects, all of which were below the detection sensitivity (0.03 ng/mL).
| Controls (n = 20) | Heart failure (n = 162) | P value | |
|---|---|---|---|
| Age (years) | 70 ± 8 | 71 ± 14 | 0.72 |
| Male, n (%) | 14 (70%) | 89 (55%) | 0.20 |
| Body mass index (kg/m2) | 22.7 ± 3.5 | 22.3 ± 4.4 | 0.73 |
| HFrEF/HFmrEF/HFpEF (%) | — | 33%/15%/52% | — |
| Co-morbidities | |||
| Diabetes mellitus, n (%) | 7 (35%) | 45 (28%) | 0.51 |
| Hypertension, n (%) | 14 (70%) | 99 (61%) | 0.45 |
| Dyslipidaemia, n (%) | 13 (65%) | 61 (38%) | 0.02 |
| Atrial fibrillation, n (%) | 2 (10%) | 69 (44%) | 0.003 |
| Vital signs | |||
| Systolic BP (mmHg) | 121 ± 16 | 122 ± 21 | 0.80 |
| Diastolic BP (mmHg) | 67 ± 8 | 67 ± 13 | 0.97 |
| Heart rate (b.p.m.) | 70 ± 13 | 72 ± 13 | 0.53 |
| Medications | |||
| ACEI or ARB, n (%) | 9 (45%) | 84 (52%) | 0.54 |
| Beta-blocker, n (%) | 7 (35%) | 90 (57%) | 0.07 |
| Loop diuretic, n (%) | 0 (0%) | 127 (84%) | <0.0001 |
| MRA, n (%) | 0 (0%) | 85 (56%) | <0.0001 |
| Laboratories | |||
| Haemoglobin (g/dL) | 13.2 ± 1.7 | 12.2 ± 2.2 | 0.04 |
| Glucose (mg/dL) | 106 (88–138) | 108 (94–147) | 0.70 |
| eGFR (mL/min/1.73 m2) | 69 ± 15 | 55 ± 28 | 0.002 |
| AST (U/L) | 22 (20–26) | 23 (18–28) | 0.94 |
| ALT (U/L) | 16 (12–24) | 16 (11–24) | 0.57 |
| γGT (U/L) | 19 (12–30) | 32 (18–59) | 0.005 |
| ALP (U/mL) | 176 (139–239) | 229 (186–299) | 0.02 |
| Total bilirubin (mg/dL) | 0.65 (0.6–0.8) | 0.7 (0.5–0.8) | 0.75 |
| NT-proBNP (pg/mL) | 194 (103–303) | 1615 (830–3615) | <0.0001 |
- ACEI, angiotensin-converting enzyme inhibitor; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BP, blood pressure; eGFR, estimated glomerular filtration rate; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; γGT, γ-glutamyl transpeptidase.
- Data are mean ± standard deviation, median (inter-quartile range), or n (%).
Cardiac structure and function
Compared with control subjects, HF patients had larger LV end-diastolic volume and mass (Table 2). LV systolic function was depressed in patients compared with controls, with lower EF and mitral s′ tissue velocity. As compared with control subjects, HF patients displayed higher mitral E wave and E/e′ ratio, shorter deceleration time, and larger LA volume index. Patients with HF also had elevated estimated right ventricular systolic pressure (eRVSP) compared with controls.
| Controls (n = 20) | Heart failure (n = 162) | P value | |
|---|---|---|---|
| LV structure | |||
| LV end-diastolic volume (mL) | 71 ± 29 | 121 ± 54 | <0.0001 |
| LV mass index (g/m2) | 89 ± 16 | 122 ± 37 | <0.0001 |
| LV ejection fraction (%) | 66 ± 7 | 48 ± 15 | <0.0001 |
| Diastolic function and PH | |||
| Mitral E wave (cm/s) | 63 ± 16 | 86 ± 29 | <0.0001 |
| Deceleration time (s) | 259 ± 65 | 206 ± 78 | 0.005 |
| Mitral A wave (cm/s) | 83 ± 20 | 76 ± 26 | 0.27 |
| Mitral annular e′ (cm/s) | 5.2 ± 1.1 | 4.7 ± 1.6 | 0.07 |
| Mitral annular s′ (cm/s) | 6.7 ± 1.3 | 4.7 ± 1.7 | <0.0001 |
| E/e′ ratio | 12.3 ± 3.4 | 19.9 ± 8.2 | <0.0001 |
| LA volume index (mL/m2) | 25 (18–28) | 52 (38–68) | <0.0001 |
| eRVSP (mmHg) | 22 ± 6 | 31 ± 11 | <0.0001 |
| eRAP, 3/8/15 mmHg (%) | 100%/0%/0% | 76%/19%/5% | 0.25 |
- A wave, late diastolic mitral inflow velocity; E wave, early diastolic mitral inflow velocity; e′, early diastolic mitral annular tissue velocity; eRAP, estimated right atrial pressure; eRVSP, estimated right ventricular systolic pressure; LA, left atrial; LV, left ventricular; PH, pulmonary hypertension; s′, systolic mitral annular tissue velocity.
- Data are mean ± standard deviation or median (inter-quartile range).
Biomarker levels
As compared with control subjects, serum levels of FABP1 were elevated in HF patients [7.9 (6.4–11.7) vs. 17.6 (10.4–28.9) ng/mL, P < 0.0001] (Figure 1). Levels of FABP1 were correlated with lower haemoglobin (r = −0.24, P = 0.001) and estimated glomerular filtration rate (r = −0.37, P < 0.0001), but it remained higher in HF patients than controls after adjusting for them (P < 0.0001). Levels of FABP1 were not correlated with aspartate aminotransferase (r = −0.03, P = 0.68), ALT (r = −0.09, P = 0.22), γGT (r = 0.04, P = 0.58), ALP (r = 0.03, P = 0.71), bilirubin (r = 0.01, P = 0.91), and troponin I (r = 0.03, P = 0.76). While serum levels of FABP1 were unrelated to indices of LV structure and function (end-diastolic volume, LV mass index, EF, E and A waves, mitral e′ and s′ velocities, and E/e′ ratio; all |r| < 0.2), there was modest but significant correlation between FABP1 levels and eRVSP (r = 0.26, P = 0.002). Furthermore, levels of FABP1 were higher in subjects with elevated RAP (≥8 mmHg) than those with normal RAP (<8 mmHg) [23.9 (12.4–35.1) vs. 14.4 (8.6–24.2) ng/mL, P = 0.03].
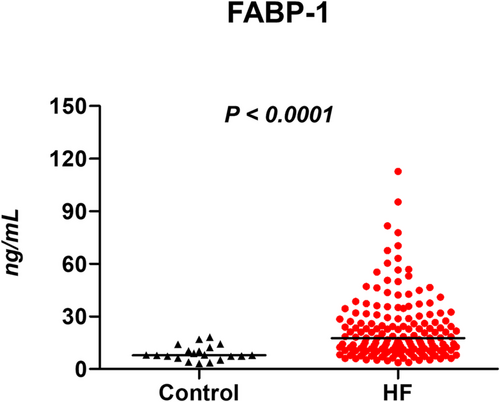
Prognostic impact of fatty acid-binding protein 1 in heart failure
Over a median follow-up of 12.0 months (inter-quartile range 11.8–30.6), there were 55 composite endpoints (13 all-cause deaths and 42 HF hospitalizations) in the HF cohort (n = 164). As expected, higher levels of NT-proBNP were associated with the composite outcome (Table 3). However, no hepatobiliary enzymes predicted the adverse outcome in HF (Table 3). In contrast, rates of the primary composite outcome monotonically increased from 115 per 1000 person years in the lowest FABP1 tertile to 398 per 1000 person years in the highest tertile (Table 4). Kaplan–Meier analysis showed a dose-dependent worsening of event-free survival among FABP1 tertiles (Figure 2). In an unadjusted Cox model, patients in the highest FABP1 tertile (T3) had a three-fold increased risk of adverse outcomes compared with those in the lowest tertile [T1; hazard ratio (HR), 3.02; 95% confidence interval (CI) (1.46–6.68); P = 0.003; Table 4]. After adjusting for age, sex, and the presence of AF, risk estimates for the composite outcome associated with FABP1 tertiles remained significant [T3 vs. T1; HR, 3.07; 95% CI (1.39–7.51); P = 0.005]. Even after further adjusting for NT-proBNP levels, the association remained significant [T3 vs. T1; HR, 3.05; 95% CI (1.36–7.49); P = 0.006]. Sequential Cox hazard models revealed that the addition of NT-proBNP levels significantly improved the model based on age, gender, and AF (Figure 3, global χ2 15.5 vs. 6.7, P = 0.004). Further incremental prognostic value was observed by adding FABP1 levels to the previous model (20.8 vs. 15.5, P = 0.01).
| HR (95% CI) | P | |
|---|---|---|
| Ln NT-proBNP, per 1 unit | 1.31 (1.09–1.55) | 0.006 |
| AST, per 1 U/L | 1.00 (0.97–1.04) | 0.84 |
| ALT, per 1 U/L | 0.99 (0.96–1.01) | 0.28 |
| Ln γGT, per 1 unit | 1.00 (0.69–1.42) | 1.00 |
| Ln ALP, per 1 unit | 0.90 (0.39–1.95) | 0.80 |
| Total bilirubin, per 1 mg/dL | 1.10 (0.47–2.19) | 0.81 |
- CI, confidence interval; HR, hazard ratio; and other abbreviations as in Table 1.
| Tertiles of serum levels of FABP1 (ng/mL) | |||
|---|---|---|---|
| T1 | T2 | T3 | |
| Subjects (n) | 53 | 54 | 55 |
| FABP-1 levels (ng/mL) | 8.6 (6.5–10.4) | 17.2 (14.2–20.9) | 35.7 (28.7–49.8) |
| Events, n (%) | 10 (19%) | 23 (43%) | 22 (40%) |
| Incident rate (per 1000 person years) | 115 | 265 | 398 |
| Models | — | HR (95% CI) | HR (95% CI) |
| 1. Unadjusted | 1 (ref) | 2.34 (1.15–5.15)* | 3.02 (1.46–6.68)* |
| 2. Age + sex | 1 (ref) | 2.15 (1.04–4.75)* | 2.91 (1.38–6.58)* |
| 3. Age + sex + AF | 1 (ref) | 2.26 (1.04–5.40)* | 3.07 (1.39–7.51)* |
| 4. Age + sex + AF + NT-proBNP | 1 (ref) | 2.24 (1.04–5.39)* | 3.05 (1.36–7.49)* |
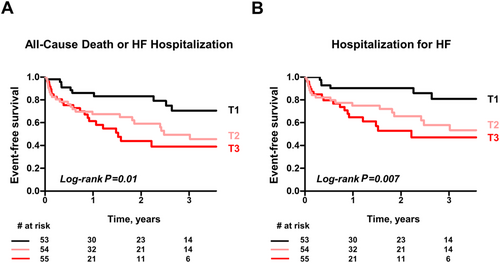
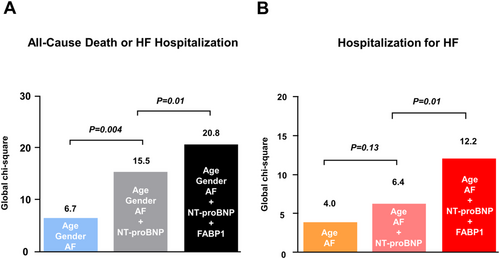
Incident rates of HF hospitalization also monotonically increased from 69 per 1000 person years in the lowest FABP1 tertile to 326 per 1000 person years in the highest tertile (Supporting Information, Table S1). Kaplan–Meier analysis showed a dose-dependent decrease of event-free survival among FABP1 tertiles (Figure 2). In an unadjusted Cox model, patients in the highest FABP1 tertile (T3) had a four-fold increased risk of HF hospitalization compared with those in the lowest tertile [T1; HR, 4.09; 95% CI (1.71–11.3); P = 0.003; Supporting Information, Table S1]. After adjusting for age and the presence of AF, risk estimates for the secondary endpoint remained significant [T3 vs. T1; HR, 3.45; 95% CI (1.43–9.63); P = 0.005]. After further adjusting for NT-proBNP levels, the association remained significant [T3 vs. T1; HR, 3.51; 95% CI (1.44–9.82); P = 0.005]. Similar to the result obtained for the primary composite outcome, the addition of FABP1 levels to the model based on age, AF, and NT-proBNP significantly improved the risk stratification for predicting incident HF hospitalization (Figure 3, global χ2 12.2 vs. 6.4, P = 0.01).
Discussion
To our knowledge, we for the first time demonstrated significant relationships between serum FABP1 and adverse outcomes in patients with HF. As compared with control subjects without HF, FABP1 levels were significantly elevated in HF patients. The magnitude of elevation in FABP1 was associated with NT-proBNP levels, pulmonary hypertension, and elevated RAP. Serum FABP1 levels were independently associated with adverse outcomes of HF, but other conventional hepatobiliary markers such as transaminases, cholestatic enzyme, and total bilirubin were not. Furthermore, FABP1 had an incremental prognostic value over clinical factors and NT-proBNP levels. These results highlight the importance of FABP1 as a potential biomarker that reflects cardio-hepatic interactions in HF.
Elevation in fatty acid-binding protein 1 levels in heart failure
One of the key findings in the current study is the elevation of FABP1 in the absence of the increases in established biomarkers for hepatic injury or congestion. We demonstrated that patients with HF had higher γGT and ALP than controls, while levels of other hepatobiliary enzymes were similar between groups. In contrast, the difference in FABP1 levels between HF and controls was substantial (Figure 1). Ischaemia–reperfusion, hypoxia, and congestion are common pathophysiology in the liver dysfunction in patients with HF, and therefore, one of the plausible mechanisms behind the higher FABP1 levels in HF seems to be linked to subclinical liver damage.
How should we interpret the release of FABP1 from subtle liver damage? Taking it into account that FABP1 is a highly abundant and small soluble cytoplasmic protein (14 kDa) in the hepatocytes, FABP1 diffuses more rapidly than large proteins such as ALT (96 kDa) and aspartate aminotransferase (AST) (90 kDa) through the interstitial space and the endothelial clefts to the vascular system even in the condition where conventional liver markers are not released.19 Furthermore, as our results demonstrate FABP1 to be associated with elevated right-sided cardiac pressures such as eRVSP and estimated RAP, which seem mainly to be secondary to chronically elevated left-sided filling pressure, we propose that this mechanism at least partly contributes to the elevation of FABP1 in HF patients without clear evidence of liver injury.
Another plausible and perhaps more intriguing mechanism is the release of FABP1 from the liver in response to adrenergic overdrive in HF patients. In our separate study, we examined the serum FABP1 in healthy volunteers who underwent a cardiopulmonary exercise test on a cycle ergometer. We found that FABP1 rapidly increased during exercise, and such an increase was significantly correlated with that of norepinephrine and epinephrine, but not with ALT or AST, suggesting that catecholamines are the physiologically relevant regulator for FABP1 induction (manuscript in preparation). Given that prolonged and excessive sympathetic nervous system activation is a hallmark of HF, FABP1 may be secreted into circulation secondary to an increased sympathetic nervous system activity. This assumption also explains the strong association between FABP1 and HF prognosis. In line with this consideration, we and others have recently reported that adipocyte FABP (FABP4) is actively released from adipocytes by catecholamines despite the lack of a known secretory signal sequence.20, 21 Clearly, further studies are needed to determine the mechanisms underlying elevation in FABP1 in HF patients.
Prognostic impact of fatty acid-binding protein 1 in heart failure
The most salient feature of the present study is that circulating FABP1 has the prognostic value in the patients with HF, even after adjusting for age, sex, presence of AF, and NT-proBNP levels. It has long been known that the heart and the liver are in close relation with each other, and abnormal liver function test is associated with poor outcome in HF.5-7 However, our study revealed that FABP1, but not AST, ALT, γGT, ALP, and total bilirubin, was significantly associated with outcome in HF patients. Although the mechanistic basis for this association remains to be determined, it is intriguing to speculate that the interaction between the heart and the liver is more complicated than we have previously thought. The present study highlights the role of FABP1 as a potential marker for the assessment of the cardio-hepatic interaction that may not be otherwise detected by traditional markers in patients with chronic HF.
We should emphasize that FABP 1 had an incremental prognostic value over NT-proBNP for the primary composite outcome of all-cause death or HF hospitalization. Consistent with this, the addition of FABP1 to the model based on clinical factors and NT-proBNP also improved the risk stratification for predicting incident HF hospitalization. These findings may be attributed to the distinct pathophysiological basis underlying an increase in the expression and/or the secretion of these tissue-restricted proteins. NT-proBNP is released specifically from cardiomyocytes under conditions of pressure and volume overload, while FABP1 is released mainly, but not exclusively, from the hepatocytes through the process independent of cardiac overload. For this reason, FABP1 and NT-proBNP might provide complementary information regarding risk stratification. Despite evidence-based treatment, many patients with HF experience adverse events.22 A strategy of natriuretic peptide-guided therapy may not be sufficient to improve the outcome in HF patients.23 The current data suggest that FABP1 in addition to NT-proBNP measurements may be helpful in clinical practice to identify patients who are at increased risk.
Limitations
This is a single-centre study from a tertiary referral centre and as such has inherent flaws related to selection and referral bias. The control group was not normal in that they were referred to coronary angiography. However, the fact that the control population is more diseased than a truly normal healthy control population only biases our data towards the null. Although patients with liver disorders were excluded from the analysis, unrecognized diseases could bias the results. Lastly, our results should be confirmed in an independent external cohort.
Conclusions
Serum FABP1 levels are elevated in HF patients, and the magnitude of elevation is associated with pulmonary hypertension, right atrial hypertension, and worse clinical outcomes. In addition, FABP1 measurements have incremental prognostic information over conventional clinical risk factors and NT-proBNP levels. These data suggest that FABP1 may be a new potential biomarker in the complex syndrome of HF.
Conflict of interest
The authors have declared that no conflict of interest exists.
Funding
This work was supported by MSD Life Science Foundation, Public Interest Incorporated Foundation (to H.S.) and Japan Heart Foundation Research Grant (to H.S.). M.O. received research grants from the Fukuda Foundation for Medical Technology, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Nippon Shinyaku, and the Japanese Circulation Society.




