Patient factors associated with titration of medical therapy in patients with heart failure with reduced ejection fraction: data from the QUALIFY international registry
Abstract
Aims
Failure to prescribe key medicines at evidence-based doses is associated with increased mortality and hospitalization for patients with Heart Failure with reduced Ejection Fraction (HFrEF). We assessed titration patterns of guideline-recommended HFrEF medicines internationally and explored associations with patient characteristics in the global, prospective, observational, longitudinal registry.
Methods and results
Data were collected from September 2013 through December 2014, with 7095 patients from 36 countries [>18 years, previous HF hospitalization within 1–15 months, left ventricular ejection fraction (LVEF) ≤ 40%] enrolled, with dosage data at baseline and up to 18 months from 4368 patients. In 4368 patients (mean age 63 ± 17 years, 75% male) ≥ 100% target doses at baseline: 30.6% (ACEIs), 2.9% (ARBs), 13.9% (BBs), 53.8% (MRAs), 26.2% (ivabradine). At final follow-up, ≥100% target doses achieved in more patients for ACEI (34.8%), BB (18.0%), and ivabradine (30.5%) but unchanged for ARBs (3.2%) and MRAs (53.7%). Adjusting for baseline dosage, uptitration during follow-up was more likely with younger age, higher systolic blood pressure, and in absence of chronic kidney disease or diabetes for ACEIs/ARBs; younger age, higher body mass index, higher heart rate, lower LVEF, and absence of coronary artery disease for BBs. For ivabradine, uptitration was more likely with higher resting heart rate.
Conclusions
The international QUALIFY Registry suggests that few patients with HFrEF achieve target doses of disease-modifying medication, especially older patients and those with co-morbidity. Quality improvement initiatives are urgently required.
Introduction
Recent evidence shows that although heart failure (HF) survival has improved by approximately 20% since 1970, 1 and 5 year mortality rates remain high at 11% and 40%, respectively.1 A recent study of temporal trends in 1 year HF mortality in the UK showed a modest decline from 13% in 2002–2004 to 10% in 2011–2013, with a 6% reduction in hospital admissions.2 Almost half of the patients were over 80 years at diagnosis with multiple co-morbidities, and in this group, there was less change in survival and hospitalizations, suggesting an urgent need to address the growing HF burden in our ageing population.2
Prescription of guideline recommended therapies for heart failure with reduced ejection fraction (HFrEF),3, 4 and uptitration to evidence-based dosages, remains one of the most effective ways to ensure that patients receive optimal care targeted at reducing the risk of recurrent HF hospitalization and cardiovascular mortality. For example, the composite of all-cause mortality or HF hospitalization is significantly improved with higher doses of angiotensin converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) compared with lower doses.5
Patients with HFrEF who are treated with <50% of recommended doses of ACE inhibitors/ARBs and beta-blockers (BBs) have been shown to be at greater risk of death and/or heart failure hospitalization than patients treated with ≥100% of recommended doses.6, 7
US and European registries have shown that many patients do not receive recommended doses of HF medication,8, 9 but little is known about longer-term dose uptitration and the clinical factors that may affect HF prescribing in routine practice globally. In this context, the QUALIFY (QUality of Adherence to guideline recommendations for LIFe-saving treatment in heart failure surveY) registry provides a novel opportunity to describe the patterns of longitudinal titration of recommended therapies in real world practice across many countries.
QUALIFY was established to address the need for a longer-term, global perspective on physician adherence to five classes of medications recommended for HFrEF in the 2012 European Society of Cardiology (ESC) guidelines: ACEIs/ARBs, BBs, mineralocorticoid receptor antagonists (MRAs), and ivabradine10 in a large population recruited in Europe, the Middle East, Asia, Australia, and the Americas. Previous reports have presented baseline characteristics and guideline adherence scores (good, moderate, and poor) for the study population at enrolment11 and shown the beneficial impact of physicians' adherence to target doses of five guideline-recommended classes of HF medication on clinical outcomes.12, 13
The current analysis investigates dosage patterns of these five classes of HF medications during 18 months' follow up.
Methods
QUALIFY is a global, prospective, observational, longitudinal survey of outpatients with chronic HFrEF [LVEF ≤40%, age >18 years, HF hospitalization (minimum of one overnight stay) within one to 15 months prior to enrolment] conducted in 36 countries. Details of the study design, baseline evaluation, and data management have been published previously.11 QUALIFY was carried out in accordance with the Declaration of Helsinki and was approved by relevant ethics committees and/or regulatory bodies in participating countries. All patients gave written informed consent to participate.
The QUALIFY survey is registered in the ISRCTN registry of clinical trials under the number ISRCTN87465420. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data were collected from September 2013 through December 2014, with 7095 patients enrolled and fulfilling the inclusion criteria. Patients were recruited in Africa, Asia, Australia, Europe, the Middle East, and North, Central, and South America.11 Follow-up examinations were conducted at 6, 12, and 18 months after baseline. Medication data were available for 4368 patients who attended all four visits.
For this analysis, we investigated dosage at baseline and subsequent titration to target doses of ACEIs, ARBs, BBs, MRAs, and ivabradine at 18 months. Target doses were as defined by the ESC guidelines relevant at the time of data collections.10 We investigated associations between titration patterns and patient factors, including age, sex, body mass index (BMI), presence of co-morbidity [coronary artery disease (CAD), diabetes mellitus (DM), chronic kidney disease (CKD), asthma, chronic obstructive pulmonary disease (COPD), hypertension], resting heart rate, systolic blood pressure (SBP), left ventricular ejection fraction (LVEF), and time since HF diagnosis.
Statistical analysis
Study participants with no follow-up data or with incomplete data for model fitting were excluded from the analysis. Baseline characteristics of the study population are expressed as absolute and relative frequencies for categorical variables, mean ± standard deviation for approximately normally distributed continuous variables, and median (interquartile range) for non-normally distributed continuous variables. Comparisons concerning these characteristics between included and excluded cases were conducted using independent Student's t-test, Mann–Whitney U-test, χ2 test, and the Fisher's exact test. Individual changes in doses for ACEIs, ARBs, BBs, MRAs, and ivabradine during the 18 month follow-up were plotted using alluvial diagrams. Associations between patient characteristics at baseline and uptitration at 18 months compared with baseline were assessed using log-binomial regression. Patients on an optimal dose of ACEI, ARB, BB, MRA, or ivabradine at baseline and patients who were not treated with a drug class due to documented contraindications, intolerances, or other clinical reasons were excluded from relevant analyses. Univariable analyses were conducted for age, sex, BMI, CAD, DM, CKD, asthma, COPD, resting heart rate, hypertension, SBP, LVEF, and time since HF diagnosis (Model 1), after adjustment for initial dose at baseline (Model 2), and after adjustment for age, sex, and initial dose at baseline (Model 3).
Significance level was set at 5% and the reported two-sided P-values were not adjusted for multiple comparisons due to the explorative nature of the investigation. All analyses were conducted using the BSW, ggplot2, ggalluvial, and stats packages in R version 4.0.0.14, 15
Results
A total of 7095 patients from 549 centres in 36 countries who were enrolled in the QUALIFY survey between September 2013 and December 2014 met eligibility criteria. The final analysis dataset with at least 12 (maximum 18 month) follow-up with drug dosage information included 4368 patients. Figure 1 shows the patient flow and disposition.
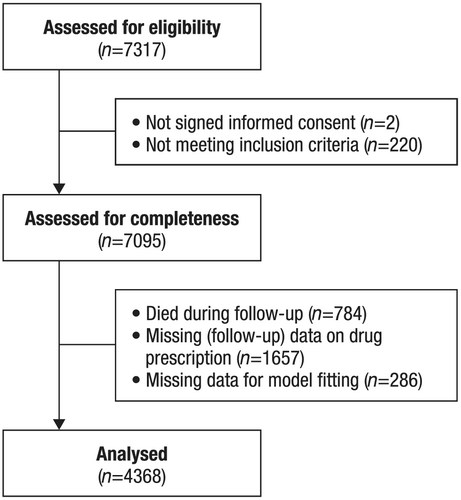
Characteristics of the patients with complete data, with a comparison of data for patients with incomplete data and for the total QUALIFY cohort, are shown in Supporting Information, Table S1. Mean age of the study cohort was 63 years, and 75.3% were men. Data showed that 58.9% were Caucasian, 28.4% were Asian, 0.7% were Black/African, and 12.0% were ‘Other’. Forty-two per cent were in New York Heart Association class III–IV, and mean left ventricular ejection fraction (LVEF) was 34% (10%). Mean blood pressure was 125/78 mmHg, and mean resting heart rate was 74 bpm. Hypertension was reported in 65% of patients, CAD in 58%, atrial fibrillation in 28%, dyslipidaemia in 58%, DM in 34%, and CKD in 16%. Nine per cent had an implantable cardioverter-defibrillator, 8% had undergone cardiac resynchronization therapy, and 6% had a non-CRT pacemaker.
At baseline, 69.6% of patients were prescribed ACEIs, 20.8% were prescribed ARBs (thus 90.3% on either ACEI or ARB), 87.9% BBs, 70.6% MRAs, and 30.8% ivabradine. Data concerning the number of patients who did not receive treatment owing to contraindications, tolerability issues, lack of indications, or other clinically stated reasons are summarized in Table 1.
| n = 4368 | Treated | Contraindication | Not tolerated | Not indicated | Other reason |
|---|---|---|---|---|---|
| Angiotensin converting enzyme inhibitor | 3038 | 275 | 878 | 0 | 177 |
| Angiotensin receptor blocker | 908 | 162 | 203 | 2822 | 273 |
| Beta-blocker | 3838 | 120 | 188 | 204 | 18 |
| Mineralocorticoid receptor antagonist | 3084 | 208 | 173 | 839 | 64 |
| Ivabradine | 1346 | 574 | 91 | 1877 | 480 |
Full baseline patient characteristics by achieved dose of medication, by drug class, are shown in Supporting Information, Table S2, and similarly for data at 18 months in Supporting Information, Table S3.
Despite a high proportion of patients being prescribed the individual drug classes, the majority of patients did not receive target doses of recommended therapies at any point during the 18 month follow up (Figure 2).
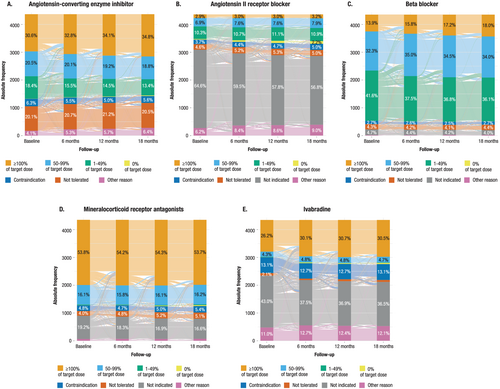
At baseline, patients achieving at least 100% target doses were 30.6% (1337/4368) for ACEIs, 2.9% (128/4368) for ARBs, 13.9% (607/4368) for BBs, 53.8% (2352/4368) for MRAs, and 26.2% (1145/4368) for ivabradine. In general, there was a trend showing that patients who were on target dose of one drug at baseline were more likely to be on target doses of other drugs at baseline (Supporting Information, Table S4).
At 12–18 months, these dosages were achieved in 34.8% (1520/4368), 3.2% (141/4368), 18% (787/4368), 53.7% (2346/4368), and 30.5% (1332/4368) for patients prescribed with ACEIs, ARBs, BBs, MRAs, and ivabradine, respectively.
The clinical features associated with target drug usage at baseline are shown in Table 2. In brief, higher BMI, a history of hypertension or a higher systolic blood pressure, longer duration of HF, and a higher LVEF were associated with a higher likelihood of being on target dose of ACEI/ARB at baseline; for BBs, target dose at baseline was more likely in those who were younger, had a higher BMI, DM (49% more likely), a history of hypertension or a higher systolic blood pressure, or a lower LVEF. For MRAs, target dose at baseline was more likely if the patient did not have CKD, had a higher resting heart rate, a history of hypertension or a higher systolic blood pressure, or a higher EF. For ivabradine, this was more likely in younger patients, those with asthma, a higher resting heart rate, a higher EF, or a higher systolic blood pressure.
| Model I | ||
|---|---|---|
| RR [95% CI] | P | |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker (n = 3893) | ||
| Age (years) | 1.000 [0.997; 1.003] | 0.917 |
| Asthma | 1.061 [0.854; 1.318] | 0.593 |
| Body mass index (kg/m2) | 1.023 [1.016; 1.029] | <0.001 |
| Chronic kidney disease | 0.838 [0.739; 0.949] | 0.006 |
| Chronic obstructive pulmonary disease | 1.040 [0.918; 1.180] | 0.536 |
| Coronary artery disease | 1.094 [1.007; 1.188] | 0.034 |
| Diabetes mellitus | 1.113 [1.024; 1.210] | 0.012 |
| Resting heart rate (bpm) | 1.003 [1.001; 1.006] | 0.017 |
| Hypertension | 1.412 [1.283; 1.554] | <0.001 |
| Time since last hospitalization (months) | 1.023 [1.009; 1.037] | 0.002 |
| Left ventricular ejection fraction (%) | 1.018 [1.012; 1.024] | <0.001 |
| Female | 1.026 [0.935; 1.126] | 0.584 |
| Systolic blood pressure (mmHg) | 1.011 [1.010; 1.012] | <0.001 |
| Time since heart failure diagnosis (years) | 1.007 [0.999; 1.015] | 0.076 |
| Beta-blocker (n = 3838) | ||
| Age (years) | 0.990 [0.984; 0.995] | <0.001 |
| Asthma | 1.223 [0.819; 1.826] | 0.324 |
| Body mass index (kg/m2) | 1.042 [1.030; 1.055] | <0.001 |
| Chronic kidney disease | 1.225 [1.021; 1.471] | 0.029 |
| Chronic obstructive pulmonary disease | 1.183 [0.947; 1.478] | 0.139 |
| Coronary artery disease | 0.887 [0.766; 1.026] | 0.107 |
| Diabetes mellitus | 1.494 [1.291; 1.729] | <0.001 |
| Resting heart rate (bpm) | 0.997 [0.991; 1.002] | 0.230 |
| Hypertension | 1.278 [1.087; 1.503] | 0.003 |
| Time since last hospitalization (months) | 1.013 [0.987; 1.039] | 0.338 |
| Left ventricular ejection fraction (%) | 0.983 [0.973; 0.994] | 0.002 |
| Female | 0.928 [0.780; 1.103] | 0.396 |
| Systolic blood pressure (mmHg) | 1.004 [1.000; 1.008] | 0.032 |
| Time since heart failure diagnosis (years) | 1.010 [0.996; 1.025] | 0.155 |
| Mineralocorticoid receptor antagonist (n = 3080) | ||
| Age (years) | 0.999 [0.998; 1.001] | 0.275 |
| Asthma | 1.031 [0.923; 1.151] | 0.588 |
| Body mass index (kg/m2) | NA [NA; NA] | NA |
| Chronic kidney disease | 0.933 [0.876; 0.993] | 0.030 |
| Chronic obstructive pulmonary disease | 1.033 [0.974; 1.094] | 0.279 |
| Coronary artery disease | 1.107 [1.063; 1.153] | <0.001 |
| Diabetes mellitus | 1.019 [0.978; 1.061] | 0.374 |
| Resting heart rate (bpm) | 1.002 [1.001; 1.003] | <0.001 |
| Hypertension | 1.111 [1.063; 1.161] | <0.001 |
| Time since last hospitalization (months) | 1.007 [1.000; 1.014] | 0.049 |
| Left ventricular ejection fraction (%) | 1.004 [1.001; 1.007] | 0.009 |
| Female | 1.032 [0.987; 1.078] | 0.166 |
| Systolic blood pressure (mmHg) | 1.003 [1.002; 1.003] | <0.001 |
| Time since heart failure diagnosis (years) | 1.003 [0.999; 1.006] | 0.159 |
| Ivabradine (n = 1343) | ||
| Age (years) | 0.997 [0.996; 0.999] | 0.003 |
| Asthma | 1.111 [1.036; 1.192] | 0.003 |
| Body mass index (kg/m2) | 1.005 [1.001; 1.009] | 0.006 |
| Chronic kidney disease | 0.929 [0.856; 1.009] | 0.081 |
| Chronic obstructive pulmonary disease | 0.983 [0.914; 1.057] | 0.643 |
| Coronary artery disease | 1.042 [0.993; 1.093] | 0.096 |
| Diabetes mellitus | 1.011 [0.967; 1.058] | 0.624 |
| Resting heart rate (bpm) | 1.002 [1.001; 1.003] | 0.004 |
| Hypertension | 1.008 [0.962; 1.056] | 0.739 |
| Time since last hospitalization (months) | 1.002 [0.994; 1.011] | 0.557 |
| Left ventricular ejection fraction (%) | 1.004 [1.000; 1.007] | 0.044 |
| Female | 1.011 [0.961; 1.062] | 0.681 |
| Systolic blood pressure (mmHg) | 1.001 [1.000; 1.002] | 0.038 |
| Time since heart failure diagnosis (years) | 0.997 [0.992; 1.003] | 0.361 |
Associations between patient characteristics at baseline and likelihood of uptitration over 18 months are summarized in Table 3. After adjustment for baseline dosage (Model II), older patients were less likely to be uptitrated for ACEI/ARB therapy (P = 0.01) as were those with CKD (P = 0.004), T2DM (P = 0.004), or a lower SBP (P < 0.001). For BBs, uptitration was less likely in older patients (P < 0.001) and those with a lower BMI (P < 0.001), underlying CAD (P < 0.001), a lower resting heart rate (P < 0.001) or a higher LVEF (P < 0.001). For ivabradine, patients with a lower resting HR were less likely to be uptitrated (P < 0.001). The associations described earlier were unaltered after further adjustment for age and sex (Model III).
| Model I | Model II | Model III | ||||
|---|---|---|---|---|---|---|
| RR [95% CI] | P | RR [95% CI] | P | RR [95% CI] | P | |
| Angiotensin converting enzyme inhibitor/angiotensin receptor blocker (n = 2428) | ||||||
| Age (years) | 0.993 [0.990; 0.997] | 0.001 | 0.995 [0.991; 0.999] | 0.011 | 0.995 [0.991; 0.999] | 0.010 |
| Asthma | 0.814 [0.582; 1.139] | 0.230 | 0.994 [0.719; 1.375] | 0.972 | 1.001 [0.725; 1.383] | 0.995 |
| Body mass index (kg/m2) | 1.004 [0.994; 1.014] | 0.451 | 1.009 [0.999; 1.019] | 0.066 | 1.008 [0.998; 1.017] | 0.113 |
| Chronic kidney disease | 0.778 [0.664; 0.911] | 0.002 | 0.799 [0.685; 0.932] | 0.004 | 0.824 [0.704; 0.964] | 0.015 |
| Chronic obstructive pulmonary disease | 1.078 [0.923; 1.260] | 0.344 | 1.108 [0.954; 1.286] | 0.178 | 1.138 [0.980; 1.323] | 0.091 |
| Coronary artery disease | 1.034 [0.934; 1.146] | 0.518 | 1.026 [0.929; 1.133] | 0.614 | 1.061 [0.957; 1.175] | 0.260 |
| Diabetes mellitus | 0.820 [0.731; 0.920] | <0.001 | 0.850 [0.760; 0.950] | 0.004 | 0.859 [0.768; 0.961] | 0.008 |
| Resting heart rate (bpm) | 1.003 [0.999; 1.006] | 0.151 | 1.002 [0.999; 1.006] | 0.206 | 1.001 [0.998; 1.005] | 0.434 |
| Hypertension | 0.994 [0.895; 1.104] | 0.917 | 1.078 [0.974; 1.194] | 0.147 | 1.115 [1.004; 1.237] | 0.041 |
| Time since last hospitalization (months) | 0.989 [0.971; 1.007] | 0.237 | 0.987 [0.969; 1.005] | 0.150 | 0.988 [0.970; 1.006] | 0.179 |
| Left ventricular ejection fraction (%) | 0.994 [0.987; 1.001] | 0.076 | 0.999 [0.992; 1.006] | 0.744 | 0.999 [0.992; 1.006] | 0.874 |
| Female | 0.960 [0.851; 1.082] | 0.504 | 1.009 [0.898; 1.133] | 0.885 | 1.028 [0.915; 1.156] | 0.642 |
| Systolic blood pressure (mmHg) | 1.003 [1.000; 1.006] | 0.032 | 1.005 [1.003; 1.008] | <0.001 | NA [NA; NA] | NA |
| Time since heart failure diagnosis (years) | 0.986 [0.974; 0.999] | 0.033 | 0.987 [0.975; 0.999] | 0.038 | 0.990 [0.978; 1.002] | 0.103 |
| New York Heart Association classification | ||||||
| II vs. I | 1.031 [0.882; 1.205] | 0.703 | 1.001 [0.862; 1.161] | 0.994 | 1.016 [0.875; 1.179] | 0.837 |
| III vs. I | 0.878 [0.744; 1.037] | 0.125 | 0.859 [0.733; 1.008] | 0.062 | 0.870 [0.742; 1.021] | 0.088 |
| IV vs. I | 0.859 [0.642; 1.149] | 0.306 | 0.820 [0.619; 1.086] | 0.166 | 0.805 [0.608; 1.067] | 0.131 |
| Atrial fibrillation | 1.099 [0.986; 1.225] | 0.088 | 1.116 [1.005; 1.239] | 0.039 | 1.154 [1.038; 1.283] | 0.008 |
| Beta-blocker (n = 3231) | ||||||
| Age (years) | 0.992 [0.988; 0.996] | <0.001 | 0.993 [0.989; 0.996] | <0.001 | 0.992 [0.989; 0.996] | <0.001 |
| Asthma | 0.817 [0.575; 1.161] | 0.259 | 0.854 [0.605; 1.205] | 0.369 | 0.877 [0.622; 1.237] | 0.454 |
| Body mass index (kg/m2) | 1.016 [1.006; 1.025] | 0.001 | 1.022 [1.013; 1.031] | <0.001 | 1.014 [1.005; 1.023] | 0.004 |
| Chronic kidney disease | 0.851 [0.736; 0.984] | 0.030 | 0.878 [0.761; 1.012] | 0.073 | 0.925 [0.800; 1.070] | 0.296 |
| Chronic obstructive pulmonary disease | 0.819 [0.681; 0.984] | 0.033 | 0.865 [0.722; 1.037] | 0.118 | 0.891 [0.743; 1.068] | 0.212 |
| Coronary artery disease | 0.769 [0.698; 0.847] | <0.001 | 0.782 [0.712; 0.860] | <0.001 | 0.809 [0.734; 0.893] | <0.001 |
| Diabetes mellitus | 0.898 [0.807; 1.001] | 0.051 | 0.917 [0.825; 1.019] | 0.106 | 0.927 [0.835; 1.030] | 0.160 |
| Resting heart rate (bpm) | 1.008 [1.005; 1.011] | <0.001 | 1.006 [1.003; 1.009] | <0.001 | 1.005 [1.002; 1.008] | 0.003 |
| Hypertension | 0.892 [0.807; 0.985] | 0.024 | 0.933 [0.846; 1.028] | 0.162 | 0.968 [0.877; 1.070] | 0.528 |
| Time since last hospitalization (months) | 0.984 [0.967; 1.002] | 0.077 | 0.982 [0.965; 0.999] | 0.041 | 0.983 [0.967; 1.001] | 0.061 |
| Left ventricular ejection fraction (%) | 0.984 [0.977; 0.991] | <0.001 | 0.986 [0.980; 0.993] | <0.001 | 0.988 [0.981; 0.995] | <0.001 |
| Female | 1.036 [0.926; 1.157] | 0.539 | 1.042 [0.935; 1.162] | 0.455 | 1.080 [0.967; 1.205] | 0.172 |
| Systolic blood pressure (mmHg) | 1.001 [0.998; 1.003] | 0.469 | 1.002 [0.999; 1.004] | 0.181 | NA [NA; NA] | NA |
| Time since heart failure diagnosis (years) | 0.991 [0.980; 1.002] | 0.125 | 0.994 [0.983; 1.005] | 0.270 | 0.999 [0.988; 1.010] | 0.834 |
| New York Heart Association classification | ||||||
| II vs. I | 0.894 [0.772; 1.034] | 0.131 | 0.953 [0.827; 1.098] | 0.506 | 0.973 [0.844; 1.120] | 0.700 |
| III vs. I | 0.825 [0.709; 0.962] | 0.014 | 0.830 [0.715; 0.962] | 0.014 | 0.848 [0.731; 0.983] | 0.029 |
| IV vs. I | 0.659 [0.490; 0.887] | 0.006 | 0.635 [0.474; 0.850] | 0.002 | 0.628 [0.470; 0.840] | 0.002 |
| Atrial fibrillation | 1.178 [1.063; 1.307] | 0.002 | 1.212 [1.096; 1.340] | <0.001 | 1.278 [1.153; 1.415] | <0.001 |
| Mineralocorticoid receptor antagonist (n = 728) | ||||||
| Age (years) | 1.009 [0.998; 1.020] | 0.099 | 1.000 [0.989; 1.012] | 1.000 | 1.000 [0.989; 1.012] | 1.000 |
| Asthma | 0.995 [0.412; 2.404] | 0.991 | 1.000 [0.388; 2.579] | 1.000 | 1.000 [0.387; 2.585] | 1.000 |
| Body mass index (kg/m2) | 1.058 [1.030; 1.085] | <0.001 | 1.001 [0.974; 1.030] | 0.918 | 1.001 [0.973; 1.030] | 0.919 |
| Chronic kidney disease | 1.490 [1.080; 2.056] | 0.015 | 0.988 [0.750; 1.300] | 0.929 | 0.988 [0.743; 1.312] | 0.931 |
| Chronic obstructive pulmonary disease | 2.045 [1.483; 2.821] | <0.001 | 1.098 [0.787; 1.532] | 0.583 | 1.098 [0.760; 1.587] | 0.619 |
| Coronary artery disease (Asian) | 1.908 [0.969; 3.759] | 0.062 | 1.000 [0.505; 1.981] | 1.000 | 1.000 [0.501; 1.998] | 1.000 |
| Coronary artery disease (non-Asian) | 0.865 [0.654; 1.145] | 0.311 | 0.924 [0.684; 1.247] | 0.604 | 0.928 [0.682; 1.262] | 0.632 |
| Diabetes mellitus | 1.158 [0.866; 1.549] | 0.322 | 0.998 [0.780; 1.276] | 0.986 | 0.998 [0.776; 1.283] | 0.986 |
| Resting heart rate (bpm) | 0.995 [0.984; 1.005] | 0.331 | 1.000 [0.989; 1.011] | 1.000 | 1.000 [0.989; 1.011] | 1.000 |
| Hypertension | 1.703 [1.252; 2.317] | <0.001 | 1.244 [0.932; 1.659] | 0.138 | 1.244 [0.924; 1.674] | 0.150 |
| Time since last hospitalization (months) (Asian) | 1.077 [0.951; 1.220] | 0.240 | 1.017 [0.893; 1.159] | 0.796 | 1.016 [0.891; 1.158] | 0.812 |
| Time since last hospitalization (months) (non-Asian) | 1.016 [0.969; 1.066] | 0.509 | 1.017 [0.968; 1.069] | 0.496 | 1.016 [0.966; 1.068] | 0.534 |
| Left ventricular ejection fraction (%) | 0.998 [0.977; 1.019] | 0.823 | 1.000 [0.983; 1.018] | 0.970 | 1.000 [0.983; 1.018] | 0.971 |
| Female | 0.967 [0.689; 1.357] | 0.845 | 1.000 [0.748; 1.336] | 1.000 | 1.000 [0.748; 1.337] | 1.000 |
| Systolic blood pressure (mmHg) | 0.999 [0.992; 1.007] | 0.852 | 1.000 [0.994; 1.006] | 1.000 | 1.000 [0.993; 1.007] | 1.000 |
| Time since heart failure diagnosis (years) | 1.023 [0.998; 1.049] | 0.075 | 1.000 [0.972; 1.029] | 1.000 | 1.000 [0.971; 1.030] | 1.000 |
| New York Heart Association classification | ||||||
| II vs. I | 1.361 [0.872; 2.125] | 0.175 | 1.000 [0.672; 1.488] | 1.000 | 1.000 [0.672; 1.488] | 1.000 |
| III vs. I | 1.378 [0.859; 2.212] | 0.184 | 1.023 [0.671; 1.561] | 0.915 | 1.023 [0.668; 1.568] | 0.916 |
| IV vs. I | 1.109 [0.527; 2.334] | 0.785 | 0.981 [0.557; 1.730] | 0.948 | 0.981 [0.555; 1.735] | 0.948 |
| Atrial fibrillation | 1.509 [1.132; 2.013] | 0.005 | 1.083 [0.830; 1.413] | 0.558 | 1.083 [0.822; 1.427] | 0.572 |
| Ivabradine (n = 198) | ||||||
| Age (years) | 1.006 [0.991; 1.020] | 0.436 | 1.006 [0.992; 1.021] | 0.407 | 1.007 [0.992; 1.022] | 0.387 |
| Asthma | 2.437 [2.060; 2.884] | <0.001 | 1.630 [0.719; 3.693] | 0.242 | 1.611 [0.664; 3.906] | 0.292 |
| Body mass index (kg/m2) | 1.013 [0.982; 1.043] | 0.419 | 1.014 [0.983; 1.045] | 0.382 | NA [NA; NA] | NA |
| Chronic kidney disease | 0.924 [0.573; 1.489] | 0.744 | 0.926 [0.574; 1.494] | 0.754 | 0.899 [0.556; 1.454] | 0.664 |
| Chronic obstructive pulmonary disease | 0.853 [0.492; 1.480] | 0.572 | 0.839 [0.482; 1.463] | 0.537 | 0.823 [0.472; 1.433] | 0.490 |
| Coronary artery disease | 1.187 [0.840; 1.678] | 0.330 | 1.187 [0.840; 1.677] | 0.331 | 1.161 [0.816; 1.653] | 0.407 |
| Diabetes mellitus | 1.340 [0.966; 1.858] | 0.079 | 1.339 [0.966; 1.856] | 0.080 | 1.330 [0.959; 1.844] | 0.088 |
| Resting heart rate (bpm) | 1.022 [1.009; 1.034] | <0.001 | 1.022 [1.009; 1.035] | <0.001 | 1.021 [1.009; 1.035] | 0.001 |
| Hypertension | 1.067 [0.753; 1.513] | 0.714 | 1.072 [0.756; 1.521] | 0.695 | 1.026 [0.712; 1.480] | 0.890 |
| Time since last hospitalization (months) | 0.980 [0.922; 1.041] | 0.514 | 0.980 [0.923; 1.040] | 0.504 | 0.978 [0.920; 1.040] | 0.476 |
| Left ventricular ejection fraction (%) | 0.981 [0.959; 1.003] | 0.085 | 0.981 [0.959; 1.003] | 0.092 | 0.979 [0.957; 1.002] | 0.067 |
| Female | 0.981 [0.666; 1.445] | 0.922 | 0.986 [0.669; 1.454] | 0.945 | 0.949 [0.638; 1.410] | 0.794 |
| Systolic blood pressure (mmHg) | 0.984 [0.981; 0.987] | <0.001 | NA [NA; NA] | NA | NA [NA; NA] | NA |
| Time since heart failure diagnosis (years) | 0.970 [0.929; 1.012] | 0.162 | 0.970 [0.929; 1.013] | 0.172 | 0.962 [0.921; 1.006] | 0.092 |
| New York Heart Association classification | ||||||
| II vs. I | 1.042 [0.537; 2.022] | 0.904 | 1.089 [0.547; 2.168] | 0.808 | 1.054 [0.533; 2.084] | 0.879 |
| III vs. I | 0.933 [0.470; 1.853] | 0.844 | 0.972 [0.478; 1.973] | 0.937 | 0.931 [0.462; 1.877] | 0.842 |
| IV vs. I | 1.667 [0.797; 3.485] | 0.175 | 1.740 [0.812; 3.729] | 0.154 | 1.764 [0.818; 3.803] | 0.147 |
| Atrial fibrillation | 0.865 [0.485; 1.544] | 0.623 | 0.860 [0.482; 1.536] | 0.611 | 0.797 [0.440; 1.443] | 0.454 |
- Model I is unadjusted, Model II is adjusted for dosage level at baseline, Model III is adjusted for age, sex and dosage level at baseline. Patients on target dose of drug at baseline are not included in the analyses.
Discussion
In a contemporary population of patients with HFrEF who had recently been hospitalized in 36 countries, a high proportion of patients did receive guideline-directed medical therapy at baseline and throughout follow-up. At baseline, 90.3% were on an ACEI/ARB, 87.9% were on a BB, 70.6% were on an MRA, and 30.8% were on ivabradine. However, few patients reached ‘target’ doses of recommended therapies at any point during the 18 month follow up period, with little evidence of uptitration from baseline through 18 months: the initial dose of drug at baseline remaining unchanged for the majority of patients.
At baseline, patients treated with an ACEI were more likely to be on target dose compared with an ARB, and there was only a modest increase in the proportion of patients on target dose at follow-up for either class. Uptitration was more likely in younger patients, those with a higher blood pressure, and in those without the co-morbidities of T2DM or CKD—suggesting either clinical caution or clinical inertia.
Under-dosing at all time points was particularly apparent with BBs although uptitration was again more common in younger patients and also in those with a higher BMI or resting heart rate or lower LVEF. Perhaps surprisingly, those with underlying CAD (as classified by the study doctor) were less likely to be uptitrated, possibly due to their older age and higher LVEF (data not shown).
For MRAs, more patients were on target dose at enrolment than for other drug groups, but the proportion did not change during follow-up, again suggesting little attention to drug uptitration.
Similarly, few patients were on the target dose of ivabradine at baseline, with little change during follow-up. A higher resting heart rate was associated with a higher probability of uptitration, which is clinically unsurprising as the drug's initiation and uptitration should be modified by the resting heart rate.16
By providing baseline and 18 month follow-up data on uptitration of recommended HFrEF disease-modifying medications from a global population, these data extend our understanding of dosing patterns reported in previous studies, which tended to be regional rather than global.
In the BIOSTAT-CHF registry of patients with HFrEF suboptimally treated at baseline, recruited in Europe from December 2010–December 2012, only 22% achieved target dose of a renin-angiotensin system (RAS) inhibitor at a median 21 months' follow-up, and 12% target BB dose—lower proportions than in our study.6 Similarly to our data, the registry not only showed an association between lack of RAS inhibitor uptitration and lower BMI and eGFR but also reported less uptitration in women. For BB, older age, lower heart rate, and lower blood pressure were associated with less uptitration, similarly to our data.
The ESC Heart Failure Long-Term Registry of patients, recruited with acute and chronic HF from May 2011–April 2013, showed similar levels of drug usage to QUALIFY for RAS blockers, BBs, and MRAs, with somewhat lower proportions at target dose. However, many physicians were still uptitrating their patients.9 No clear reason for failing to reach target dose was recorded for 29% of patients prescribed ACEIs, ARBs, or BBs and 47% of those prescribed MRAs.
A comparison of QUALIFY dosing data with those from two other recently published registries, CHECK-HF17, 18 and CHAMP,8, 19 is presented in Figure 3.
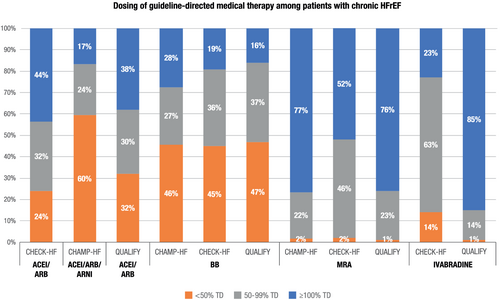
Baseline data from the Dutch CHECK-HF registry (2013–2106) showed relatively high use of evidence-based treatment in HFrEF, especially in younger patients. However, only half of eligible patients achieved ≥100% of target doses for ACEI/ARB and MRAs and 19% for BBs,17 similar to our data. Older age, lower BMI, and lower blood pressure were associated with lower dosage across drug classes, similar to our data.18
Baseline prescribing levels of ACEI/ARB, BB, and MRA in the CHAMP-HF (Change the Management of Patients with Heart Failure) registry in the USA (December 2015–March 2017) were lower than in QUALIFY.8, 19 During 12 months' follow-up, initiation and uptitration only occurred in a small minority of patients, with associated factors similar to those we report: for ACEI/ARBs—higher systolic blood pressure; for BB—younger age, higher systolic blood pressure, or higher heart rate. The presence of CAD was associated with a higher likelihood of BB dose decrease or medication discontinuation.
Evidence of sub-optimal dosing is not limited to European and US studies. In the ASIAN-HF registry (October 2012–December 2015), guideline-recommended target doses were achieved in only 17% of patients treated with ACEI/ARB, 13% for BB, and 29% for MRAs.7 This was lower than European or US data, although there were marked differences between countries. Similar factors were associated with target dosage as in our study: younger age and no CKD for ACEI/ARBs; younger age, higher BMI and hypertension history for BBs; and older age, higher systolic blood pressure and hypertension history for MRAs. Similar to BIOSTAT-CHF,6 ASIAN-HF reported a clear association between dosage of ACEI/ARB or BB achieved and subsequent risk of all-cause mortality or HF hospitalization,7 as has also been reported in the large multiyear national registry of HF in the UK.20
The results of our study, and those mentioned earlier, contrast with those from randomized controlled trials in which at least 50–60% of patients achieved target doses.21-25 Patients in clinical practice are generally older and with more co-morbidities than those in clinical trials, and this is likely to affect prescribing decisions and the perception of risk and benefit of these therapies. Additionally, some trials have a run-in period prior to randomization, which is likely to lead to an overestimation of drug tolerability in real-world practice.26
The QUALIFY data show that clinical factors, including age, lower blood pressure, and lower BMI, along with co-morbidities such as CAD, DM, and CKD are associated with lower likelihood of appropriate uptitration of drug therapy, even when under the supervision of a specialist. This translates into an inability to achieve target doses of medication, a target that has consistently been shown to be associated with better outcomes.6, 8, 12, 19 Such patients are at higher absolute risk and are therefore missing out on the potential to gain important clinical benefits.
The lack of appropriate drug optimisation for patients with HFrEF has been reported before,27 and many initiatives have attempted to improve the quality of care, including the Get With The Guidelines programme.28 Disease management programmes have been introduced, often including early review after hospital discharge, HF nurse specialists, or pharmacists to support patient education and medication optimization. Financial incentives may encourage hospitals or general practitioners to achieve better process and outcome measures (e.g. 30 day readmission penalties and best practice tariffs).29, 30 Some clinical features associated with inability to achieve target doses may be physiological and clinically appropriate, but the wide variation in achievement within studies6-8, 20 suggests that there are potentially many patients who could tolerate more appropriate drug doses, given the opportunity.
A recent review highlighted the practical considerations that might extend maximal medical therapy for patients with HFrEF26 including better education, more clinical time for patients (either face-to-face or remotely), adjustment of concomitant medication to allow better use of HF drugs, management of side-effects, and optimized timing of drug dosages.
Collection of data from routine practice, and benchmarking against standards, will allow poor performance to be identified, but the responsibility to improve performance is likely to lie locally, and local champions are required to drive improvement. Improved clinical decision support, coupled with healthcare professional and patient/family education, and benchmarking of key process and outcome measures, are likely to be front and centre of any solution.
Strengths and limitations
Strengths of this study include the large number of patients recruited and followed up for 18 months from many centres in 36 countries.
The relatively young age of the QUALIFY population may not be typical of that seen in clinical practice in Western countries but reflects the international nature of the registry. QUALIFY centres were selected by national coordinators, on a voluntary basis, and selection bias cannot be excluded.
A large majority of QUALIFY patients (90%) were treated by cardiologists, which does not reflect routine practice in some countries. The failure to uptitrate HF medicines and reach target doses may be even more apparent in patients treated by a non-cardiologist workforce.
At the time the QUALIFY registry was initiated, international HF guidelines did not include a recommendation for neprilysin inhibitor therapy, and this drug was not available.10 As a result, data on its use were not collected.
We did not have complete follow-up data on all patients enrolled in the QUALIFY registry and had to limit our analyses to those who did. There were no major differences in clinical and demographic characteristics between those for whom we did, or did not, have such data, but we cannot exclude unmeasured confounding.
Conclusions
In this international HFrEF patient cohort, despite good adherence to the use of guideline-directed therapy, few patients attain target doses of drug classes, with little evidence of drug uptitration over time for any individual patient. Optimization of drug therapy in HFrEF remains a global challenge. This problem is not likely to reduce unless more specifically targeted and incentivized by healthcare systems and quality improvement initiatives.
Acknowledgements
Editorial support for this manuscript was provided by Jenny Bryan BSc and was funded by Servier.
Conflict of interest
M. C. reports consultancy and speaker fees from AstraZeneca, Boehringer-Ingelheim, Lilly, RestMed, Servier, Novartis, Pfizer, Bayer, Medtronic, Boston Scientific, Abbott, Amgen, and MSD. J. S. reports no conflicts of interest. M. B. and S. W. are supported by the Deutsche Forschungsgemeinschaft (SFB TTR 219, S-01). M. B. reports personal fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Servier, Medtronic, Vifor, Novartis, RECor, and Abbott outside the submitted work. S. W. reports grants from Servier Affaires Medicales during the conduct of the study. L. T. reports trial committee member and member of speakers bureau for Servier (within the submitted work) and trial committee member for CVIE Therapeutics (outside the submitted work). P. P. reports grants, personal fees, and other from Servier during the conduct of the study; personal fees and other from Amgen, Novartis, Berlin Chemie, Bayer, Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Cibiem, Respicardia, Abbott Vascular, and Renal Guard Solutions outside the submitted work. S. D. A. reports personal fees from Servier during the conduct of the study; personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, Cardiac Dimension, Impulse Dynamics, and Novartis outside the submitted work, and grant support from Abbott Vascular and Vifor Pharm outside the submitted work. G. S. F. reports that he was a committee member of trials and registries sponsored by Boehringer Ingelheim, Medtronic, Bayer, Novartis, Servier, and Vifor. M. K. reports consulting/invited speaker fees from Servier, Novartis, AstraZeneca, Torrent, Sanofi, and Bayer.
Funding
This study was funded by Servier. Servier sponsored QUALIFY. The steering committee was responsible for the trial design and supervised patient recruitment and clinical management of the trial. The sponsor curated the data and made it available to the authors to facilitate the statistical modelling at the Institute for Medical Biometry, Epidemiology and Medical Informatics, Saarland University, Homburg, Germany. The steering committee oversaw analysis and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. The sponsor could comment on the manuscript before submission, but all final decisions were made by the authors.




