Hospital readmissions of patients with heart failure from real world: timing and associated risk factors
Abstract
Aims
This study aims to investigate hospital readmissions and timing, as well as risk factors in a real world heart failure (HF) population.
Methods and results
All patients discharged alive in 2016 from Sahlgrenska University Hospital/Östra, Gothenburg, Sweden, with a primary diagnosis of HF were consecutively included. Patient characteristics, type of HF, treatment, and follow-up were registered. Time to first all-cause or HF readmission, as well as number of 1 year readmissions from discharge were recorded. In total, 448 patients were included: 273 patients (mean age 78 ± 11.8 years) were readmitted for any cause within 1 year (readmission rate of 60.9%), and 175 patients (mean age 76.6 ± 13.7) were never readmitted. Among readmissions, 60.1% occurred during the first quarter after index hospitalization, giving a 3 month all-cause readmission rate of 36.6%. HF-related 1 year readmission rate was 38.4%. Patients who were readmitted had significantly more renal dysfunction (52.4% vs. 36.6%, P = 0.001), pulmonary disease (25.6% vs. 15.4%, P = 0.010), and psychiatric illness (24.9% vs. 12.0%, P = 0.001). Number of co-morbidities and readmissions were significantly associated (P < 0.001 for all cause readmission rate and P = 0.012 for 1 year HF readmission rate). Worsening HF constituted 63% of all-cause readmissions. Psychiatric disease was an independent risk factor for 1 month and 1 year all-cause readmissions. Poor compliance to medication was an independent risk factor for 1 month and 1 year HF readmission.
Conclusions
In our real world cohort of HF patients, frequent hospital readmissions occurred in the early post-discharge period and were mainly driven by worsening HF. Co-morbidity was one of the most important factors for readmission.
Introduction
Prevalence of HF is estimated to be about 2% of the adult population in developed countries, including Sweden, rising to ≥10% with age over 70.1, 2 Readmission rates within the first 6 months was reported to be as high as 45%.3 Statistics Sweden (a government agency) predicts that in 10 years, the Swedish population over 80 years of age will have increased by 50%.4 Because demographics of the population is changing with a larger number of people reaching a higher age, and HF is a disease of the elderly, absolute numbers of HF patients are increasing.2 Health care costs for HF are high, where hospitalisations and costs for nursing facilities combined made up 75% of the cost.5, 6 The economic burden for HF is expected to increase unless the rate of hospital admissions decrease, because demographic changes will increase the total number of patients living with HF.7
Hospital readmissions in HF are multifactorial, and not only due to worsening HF itself (disease-centred factors), but also due to health care system factors, for example, suboptimal care (health care-centred factors), where certain types of follow-up such as multi-disciplinary team interventions have proven to reduce hospitalizations.8 Moreover, factors that affect the patient's clinical status and need for in-hospital care changes over time for many reasons. For instance, new treatments have been shown to reduce hospitalizations.9 Health care systems have also changed, such as introduction of home-based and/or palliative care and introduction of specific disease management teams that also provide home visits. Patients having the highest readmission rates, sometimes defined as three or more emergency admissions within a year, have been called high-impact users in a previous study.10 An important goal is to identify high-impact users to aim specific actions towards these patients in order to quell readmission rates. The identification of high-impact users have, however, been a challenge in previous studies.11 Previous risk models for prediction of readmissions have various limitations; for example, they were often developed on RCT populations and therefore not representative of the general HF population.12 The complexity and diversity of the mechanisms behind readmissions highlights the need for further studies of HF readmissions within a specific health care system. Sweden has a universal healthcare system that provides low-cost hospital care to all Swedish permanent residents. To date, there are limited data about timing of and risk factors for readmissions in a Swedish context. The aim of our study was to identify time window of hospital readmissions and related risk factors of HF patients in a real-world clinical setting.
Methods
Study cohort
Sahlgrenska University Hospital/Östra is a university hospital with a catchment area of 260 000 inhabitants. Patient records with a primary discharge diagnosis of HF from the department of internal medicine, including the cardiology ward, were identified from the local hospital discharge registry. The International Classification of Diseases (ICD10) is used when coding diagnosis in Swedish hospitals. In this study, the diagnosis code for HF, I50, was used. The code I42.0 was also screened, and patients were included in the study if, after review of the record, it was evident that the cause for hospitalization was deterioration or new onset of HF. The reason for this was that in our experience, code I42.0 is commonly used as an alternative to I50 among patients with dilated cardiomyopathy (DCM) as primary aetiology for their HF diagnosis. A Swedish study of validity of the HF diagnosis in medical records showed only 6% miscoded cases.13
A total of 516 unique patients were identified through the hospital discharge codes, who were admitted in 2016 with a primary diagnoses of HF. Because our aim was to study 1 year outcomes, all patients who died (n = 26) during the index hospitalization were excluded. For the same reason, patients who did not reside in the Västra Götaland region (n = 3) and therefore did not have a follow-up visit within our organization were excluded. Three cases were excluded due to wrong diagnosis. Patients with Grown Up Congenital Heart disease (GUCH) (n = 5) in addition to HF were excluded because they have a special follow-up programme in our department. Finally, patients who were registered in the intensive care unit (ICU) for administrative purposes but in reality treated in the intensive care clinic (n = 3) were excluded, leaving us with a total of 476 patients. In another 28 patients, an echocardiography had never been performed; thus, the HF diagnosis did not fulfil the criteria for HF according to European Society of Cardiology (ESC). Hence, these patients also were excluded from the analysis, leaving 448 patients.
Hospital readmissions
Time to first all-cause or HF readmission at different time periods (1 month, 3 months, 6 months, and 1 year) and number of readmissions during 1 year from day of discharge were recorded. Patients who were readmitted to hospital were compared with patients who had no readmissions.
Collection of data
Collection of data on patient characteristics such as co-morbidities, laboratory parameters, and physical parameters were registered in a pre-specified form (see Appendix A). The type of HF was specified according to ejection fraction (EF) and categorized according to the ESC guidelines as follows: HFrEF (EF < 40%), HFmrEF (EF 40–49%), and HFpEF (EF ≥ 50%). If an exact figure was not given, EF for each patient was recorded as the mean of the range given in the echocardiography report (e.g. 35–45 was recorded as 40%). HF was evaluated according to EF, wide versus narrow QRS-complex, and ischaemic versus non-ischaemic origin of HF. Patients were included in the group of HF with ischaemic origin if it was evident from the medical record that this was the judged reason for HF by the treating physician, or if it was the most likely cause of HF in light of the rest of the medical history. All other reasons for HF, such as hypertension, tachycardia, alcohol abuse, valve disease, and other reasons were included in the group of non-ischaemic HF. Laboratory parameters were recorded as the last value before discharge. Co-morbidities were recorded when occurring in the registered discharge diagnoses in the patients' medical record. Anaemia and renal failure were also recorded as a co-morbidity if the patient fulfilled the criteria according to laboratory reference values even though the diagnoses failed to be mentioned in the medical record. Psychiatric disease, where depression and anxiety were included, was recorded if the patient was treated with antidepressants in addition to ICD code. For thyroid disease, patients treated with thyroid hormone (Levothyroxine) were included even if the diagnosis was not included in the medical record. Poor compliance to medication was recorded if it was evident in the patients' medical record that they were not taking their medication as prescribed by the physician. Alcohol and drug abuse were recorded as a co-morbidity if it was included in the discharge diagnoses or if clearly stated in the discharge medical record. Specific co-morbidities included in the term systemic inflammatory disease were rheumatoid arthritis, systemic sclerosis, lupus erythromatosus, Sjögren syndrome, myositis, and mixed connective tissue disease. The review of the electronic medical records was carried out by two residents in cardiology. Collected data were directly transformed into a specially developed form and thereafter into an electronic file. On matters that were subject to interpretation, such as aetiology of HF, a senior cardiologist and professor was available for consultation.
Mortality
All patients who died during index hospitalization were excluded. Mortality was recorded as date of death according to our administrative database ELVIS, which is linked to the National Patient Register and Cause of Death Register, if the patient died within 1 year.
Statistical analysis
Baseline characteristics were compared between patients with and without all-cause readmissions within the 1 year follow-up. For continuous variables, Wilcoxon rank sum test was used. For categorical variables, χ2 test or Fisher exact test was used for non-ordered categorical variables; Wilcoxon rank sum test was used for ordered categorical variables. The all-cause and HF readmission rates from 1 month to 1 year after index discharge and distribution of the first readmission within 1 year follow-up were analysed. Univariable and multivariable Cox regression analyses were used to investigate predictors of short-term (1 month) and long-term (1 year) cause-specific readmissions. Baseline variables in Table 1, except number of co-morbidities and co-morbidities ≤3 vs. >3 (because they would cover up the role of one specific co-morbidity) were all entered into the univariable analysis model, and those with P-value <0.1 in the univariable analysis were further included into the multivariable model. Age and sex were mandatory into all the multivariable regression models. Variables entered into the multivariable analysis model for 1 month all-cause readmission included age, sex, systolic blood pressure (SBP), valvular disease, anaemia, renal dysfunction, psychiatric disease, poor compliance, serum sodium, N-terminal pro-brain type natriuretic peptide (NT-proBNP), and use of angiotensin converting enzyme inhibitors (ACEI)/angiotensin receptor blocker (ARB). For 1 year all-cause readmission, variables included age, sex, SBP, diabetes, atrial fibrillation, pulmonary disease, anaemia, renal dysfunction, systemic inflammatory disease, psychiatric disease, serum potassium, NT-proBNP, use of ACEIs/ARBs, diuretics, long-acting nitrates, and type of follow-up. For 1 month HF readmission, variables included age, sex, SBP, EF, alcohol or drug abuse, poor compliance, and use of β-blockers. For 1 year HF readmission, variables included age, sex, wide QRS-complex, diabetes, anaemia, renal dysfunction, systemic inflammatory disease, poor compliance, serum potassium, NT-proBNP, use of mineralocorticoid receptor antagonists (MRA), diuretics, and long-acting nitrates.
| Variables | Overall n = 448 | Not readmitted n = 175 | Readmitted n = 273 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Female, n (%) | 202 (45.1) | 79 (45.1) | 123 (45.1) | 0.985 |
| Age, mean (SD) | 77.5 (12.6) | 76.6 (13.7) | 78.0 (11.8) | 0.625 |
| SBP, mmHg, mean (SD) | 129 (21.6) n = 446 | 129 (21.8) | 128 (21.5) | 0.634 |
| DBP, mmHg, mean (SD) |
73.4 (11.6) n = 445 |
74.2 (12.2) | 72.9 (11.2) | 0.313 |
| Heart rate, b.p.m., mean (SD) |
74.3 (14.9) n = 445 |
74.4 (14.2) | 74.2 (15.3) | 0.940 |
| HF evaluation | ||||
| EF, mean (SD) | 42.3 (14.5) | 42.5 (14.0) | 42.2 (14.8) | 0.839 |
| Wide QRSa, n (%) | 159 (35.5) | 59 (33.7) | 100 (36.6) | 0.529 |
| HF type, n (%) | 0.831 | |||
| HFrEF | 184 (41.1) | 70 (40.0) | 114 (41.8) | |
| HFmrEF | 85 (19.0) | 39 (22.3) | 46 (16.8) | |
| HFpEF | 179 (39.9) | 66 (37.7) | 113 (41.4) | |
| Aetiology, n (%) | 0.575 | |||
| Ischaemic | 172 (38.4) | 70 (40.0) | 102 (37.4) | |
| Non-ischaemic | 276 (61.6) | 105 (60.0) | 171 (62.6) | |
| Co-morbidities, n (%) | ||||
| Hypertension | 266 (59.4) | 105 (60.0) | 161 (59.0) | 0.829 |
| Diabetes | 151 (33.7) | 51 (29.1) | 100 (36.6) | 0.102 |
| Ischaemic heart disease | 174 (38.8) | 68 (38.9) | 106 (38.8) | 0.995 |
| Atrial fibrillation | 265 (59.2) | 95 (54.3) | 170 (62.3) | 0.093 |
| Valvular disease | 51 (11.4) | 24 (13.7) | 27 (9.9) | 0.214 |
| Dilated cardiomyopathy | 25 (5.6) | 10 (5.7) | 15 (5.5) | 0.921 |
| Pulmonary disease | 97 (21.7) | 27 (15.4) | 70 (25.6) | 0.010 |
| Anaemia | 204 (45.5) | 68 (38.9) | 136 (49.8) | 0.023 |
| Renal dysfunction | 207 (46.2) | 64 (36.6) | 143 (52.4) | 0.001 |
| Sleep apnoea | 11 (2.5) | 4 (2.3) | 7 (2.6) | — |
| Dementia | 22 (4.9) | 9 (5.1) | 13 (4.8) | 0.856 |
| Cancer | 5 (1.1) | 3 (1.7) | 2 (0.7) | 0.383 |
| Alcohol or drug abuseb | 29 (6.5) | 11 (6.3) | 18 (6.6) | 0.897 |
| Systemic inflammatory disease | 19 (4.2) | 2 (1.1) | 17 (6.2) | — |
| Psychiatric disease | 89 (19.9) | 21 (12.0) | 68 (24.9) | 0.001 |
| Symptomatic hypotension | 9 (2.0) | 4 (2.3) | 5 (1.8) | — |
| Thyroid disease | 63 (14.1) | 27 (15.4) | 36 (13.2) | 0.505 |
| Poor compliance | 13 (2.9) | 3 (1.7) | 10 (3.7) | — |
| Co-morbidities number, mean (SD) | 4.0 (1.6) | 3.6 (1.7) | 4.2 (1.5) | — |
| Co-morbidities >3 | 264 (58.9) | 81 (46.3) | 183 (67.0) | 0.000 |
| Laboratory parameters | ||||
| Sodium, mmol/L, mean (SD) |
139.7 (3.4) n = 444 |
139.8 (3.2) | 139.6 (3.5) | 0.758 |
| Potassium, mmol/L, mean (SD) |
4.3 (0.4) n = 444 |
4.3 (0.4) | 4.2 (0.4) | 0.040 |
| Creatinine, μmol/L, mean (SD) |
113.1 (54.8) n = 443 |
104.6 (45.5) | 118.5 (59.4) | 0.002 |
| NT-proBNP, pg/mL, median (interquartile range) |
3,815 (1855, 7,400) n = 404 |
3,450 (1,515, 6,880) | 4,150 (2020, 7,960) | 0.065 |
| Medications, n (%) | ||||
| ACEIs/ARBs | 339 (75.7) | 140 (80.0) | 199 (72.9) | 0.087 |
| ACEIs/ARBs in max dose | 93 (20.8) | 42 (24.0) | 51 (18.7) | 0.176 |
| BBs | 395 (88.2) | 151 (86.3) | 244 (89.4) | 0.323 |
| BBs in max dose | 108 (24.1) | 39 (22.3) | 69 (25.3) | 0.471 |
| MRAs | 136 (30.4) | 52 (29.7) | 84 (30.8) | 0.813 |
| MRAs in max dose | 34 (7.6) | 14 (8.0) | 20 (7.3) | 0.793 |
| Digitalis | 52 (11.6) | 23 (13.1) | 29 (10.6) | 0.417 |
| Diuretics | 359 (80.1) | 132 (75.4) | 227 (83.2) | 0.046 |
| Nitrates | 56 (12.5) | 15 (8.6) | 41 (15.0) | 0.044 |
| CCBs | 77 (17.2) | 28 (16.0) | 49 (18.0) | 0.594 |
| Follow-up type, n (%) | 0.036 | |||
| HF clinic | 218 (48.7) | 96 (54.9) | 122 (44.7) | |
| Primary care | 230 (51.3) | 79 (45.1) | 151 (55.3) |
- HF with reduced ejection fraction (HFrEF): EF < 40%, HF with mid-range ejection fraction (HFmrEF): 40–49%, HF with preserved ejection fraction (HFpEF): EF ≥ 50%. Ischaemic HF was defined as HF with ischaemic origin, either if well-defined and described by the treating physician or if the previous medical history made it the most likely explanation. All non-ischaemic causes, such as tachycardia, valve disease, and more, were included in the non-ischaemic group.
- ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker; DBP, diastolic blood pressure; MRA, mineral corticoid antagonist; SBP, systolic blood pressure.
- a QRS-complex ≥120 ms.
- b If included in the patients discharge diagnoses or well described by the treating physician in the discharge medical record.
Because co-morbidities were demonstrated to have significant influence on readmission in the aforementioned analyses, we continued to explore the association between number of co-morbidities and readmission rate and association between number of co-morbidities and number of readmissions in 1 year follow-up. P for trend was tested for readmission rate and number of readmissions across the eight co-morbidities groups. Further, the cumulative incidence function curves were performed to compare the risk of 1 month and 1 year all-cause or HF readmission between patients with co-morbidities ≤3 vs. >3. The sub-distribution hazard ratio (sHR) and 95% confidence interval (CI) were calculated by the cumulative incidence function regression model (subdistribution hazard model) in which death was treated as a competing risk and age and sex were both adjusted. Survival data are shown using Kaplan–Meier curves and compared between the two groups with the log rank test. Statistical significance was set to P < 0.05 (two-tailed). All statistical analyses were performed by Stata version 16.0 (StataCorp LLC, College Station, Texas, USA).
Results
Baseline characteristics of the cohort
Baseline characteristics are shown in Table 1. There were no significant differences between patients with or without readmissions regarding demographic variables and data regarding EF. Patients readmitted within 1 year were more likely to have co-morbidities including pulmonary disease, anaemia, renal dysfunction, systemic inflammatory disease, and psychiatric disease. Both the number of co-morbidities and the proportion of patients with more than three co-morbidities were higher in patients readmitted within the 1 year follow-up. Serum potassium was lower while creatinine was higher in readmitted patients. In readmitted patients, NT-proBNP was higher than in never readmitted patients although it fell slightly short of statistical significance (P = 0.065). Patients readmitted were more frequently treated with diuretics and long-acting nitrates and were more often referred to follow-up in primary care than in an HF clinic.
Burden of readmission during subsequent 1 year follow-up after index hospitalization
The 1 month, 3 months, 6 months, and 1 year all-cause readmission rate was 20.3%, 36.6%, 47.1%, and 60.9%, respectively. HF readmissions were 11.4%, 21.0%, 29.2%, and 38.4% for the same time-periods, respectively. All-cause readmissions within the first 3 months after discharge accounted for 60.1% of all readmissions in the 1 year follow-up. The proportion of all-cause readmissions that occurred in the second, third, and fourth quarters was 17.2%, 15.4%, and 7.3%, respectively. HF readmissions in the 1 year follow-up was 54.7% in the first quarter, 21.5% in the second quarter, 14.5% in the third quarter, and 9.3% in the fourth quarter (Figure 1).
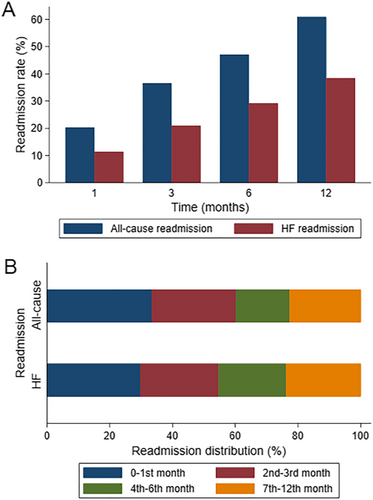
Cause-specific readmissions
Psychiatric disease was an independent risk factor for 1 month all-cause readmission, while serum sodium a protective factor (Table 2). Regarding 1 year all-cause readmissions, the independent predictors included pulmonary disease, systemic inflammatory disease, psychiatric disease, NT-proBNP, treatment with long-acting nitrates, and follow-up in primary care. For HF readmissions, the 1 month readmission predictors were poor compliance and use of β-blockers, and the 1 year readmission predictors were systemic inflammatory disease, poor compliance, use of MRA, and nitrates (Table 2).
| Hazard ratio (95% CI) | P value | |
|---|---|---|
| All-cause readmission | ||
| 1 month readmission | ||
| Psychiatric disease, yes vs. no | 1.78 (1.09–2.91) | 0.022 |
| Sodium, by 10 mmol/L increase | 0.51 (0.27–0.96) | 0.037 |
| 1 year readmission | ||
| Pulmonary disease, yes versus no | 1.34 (1.00–1.81) | 0.050 |
| Systemic inflammatory disease, yes versus no | 1.96 (1.12–3.40) | 0.018 |
| Psychiatric disease, yes versus no | 1.60 (1.18–2.16) | 0.002 |
| NT-proBNP, by 1 log-unit increase | 1.18 (1.04–1.34) | 0.011 |
| Nitrates, yes versus no | 1.55 (1.09–2.23) | 0.016 |
| Follow up type, HF clinic versus primary care | 0.70 (0.53–0.94) | 0.017 |
| HF readmission | ||
| 1 month readmission | ||
| Poor compliancea, yes versus no | 3.62 (1.17–11.14) | 0.025 |
| Beta-blockers, yes versus no | 0.35 (0.17–0.69) | 0.003 |
| 1 year readmission | ||
| Systemic inflammatory disease, yes versus no | 1.97 (1.02–3.82) | 0.045 |
| Poor compliance, yes versus no | 3.82 (1.66–8.81) | 0.002 |
| MRA, yes versus no | 1.42 (1.01–1.99) | 0.044 |
| Nitrates, yes versus no | 1.75 (1.15–2.66) | 0.009 |
- MRA, mineralcorticoid receptor antagonists.
- a If well described by the treating physician in the discharge medical record that the patient was not taking medication according to prescription.
Association of co-morbidities and readmission
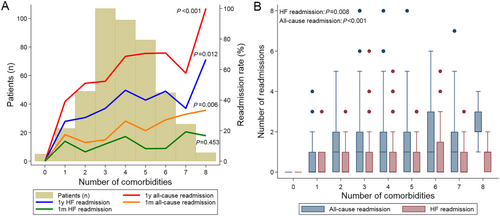
Most HF patients had three to five co-morbidities. The 1 month (P for trend 0.006) and 1 year (P for trend <0.001) all-cause readmission rate and 1 year HF readmission rate (P for trend 0.012) increased significantly with increasing number of co-morbidities (Figure 2). The number of all-cause and HF readmissions also grew with increasing numbers of co-morbidities (Figure 2). Compared with patients with ≤3 co-morbidities, HF patients with >3 co-morbidities had significantly higher risk of cumulative incidence of 1 year all-cause readmission (HR 1.75, 95% CI 1.36–2.27, P < 0.001), 1 month all-cause readmission (HR 2.15, 95% CI 1.33–3.48, P = 0.002), and 1 year HF readmission (HR 1.53, 95% CI 1.10–2.11, P = 0.011) (Figure 3). The 1 year mortality risk was also higher in HF patients with >3 co-morbidities than those with ≤3 co-morbidities.
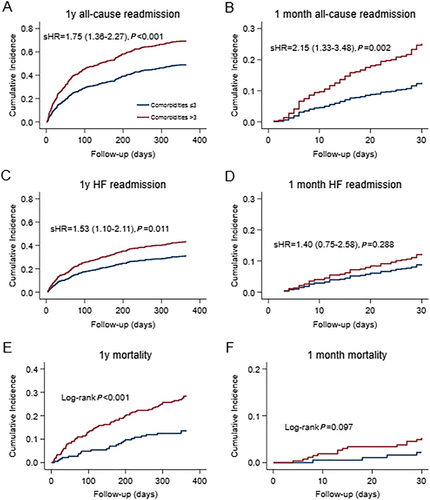
Mortality
The total 1 month mortality rate was 4.2%. For patients who were never readmitted, the figure was 4.1% and for readmitted patients 4.3%; hence, there was no significant difference between groups. Mortality rate within 1 year was in total 22.8%. There was a significant difference in 1 year mortality rate between patients who were readmitted, where the rate was 29.0%, and patients who were never readmitted, where the mortality rate was 12.8% (P = 0.000).
Discussion
In this real-world cohort of HF patients, hospital readmissions frequently occurred early during the post-discharge period, mainly driven by worsening HF. Moreover, risk factors varied among hospital readmissions of different causes and at different time periods.
Our study population was old with a mean age of 77.5 compared with other larger scale observational registry studies such as the EURObservational programme, where mean age was 69 years in the acute HF-group.14 Our study included 45% female patients compared with 37.4% in the EURObservational programme. Renal failure was more common in our cohort with 46.2% of patients suffering from this condition compared with 26% in the EURObservational programme. We had a lower percentage of patients with HF of ischaemic origin, 38.4% compared with 50% in the EURObservational programme.14 One probable explanation for the lower presence of HF with ischaemic origin in our cohort could be that our population was older and with a larger proportion of women, a subset of patients where HF with preserved ejection fraction (HFpEF) is more common, a type of HF that is more often related to other risk factors than ischaemic heart disease.15
Thirty day readmission rate in our study was 20.3% for all-causes, the same order of magnitude as in another study.16 The majority of all readmissions occurred in the first 3 months with 60.1% of readmissions. A readmission rate of 20% in the first month with a total readmission rate of 60.9% within a year makes early readmission account for a relatively large part of readmissions, similar to a previous Australian study.17 In our study, however, HF-related readmissions constituted slightly more than half of the readmissions, with 11.4% HF-readmissions within 30 days. This is inconsistent with other studies that have reported two thirds of early readmissions being primarily for non-HF-related causes.17, 18 The fact that our population was older and with more females and therefore had a significant subset of patients with HF with preserved ejection fraction (HFpEF) without proven effective therapy could be one part of the explanation.
Previous investigations have tried to identify patients who utilize a high proportion of health care by having many hospital readmissions. A large British registry study from 2018 by Rao et al. investigated hospital admissions of HF patients and divided them into high-impact versus low-impact users, that is, patients with few hospital admissions compared with patients with numerous admissions.19 No exact cut-off in number of readmissions was reported; rather, patients were grouped according to similar readmission patterns. In contrast to our results, with no difference in age distribution between readmitted and never readmitted patient groups in our study, Rao et al. reported a higher percentage of people >75 years of age in the high-impact group compared with the low-impact group.19 Both short-term and chronic high-impact users in the Rao study were much more likely to have been diagnosed as an inpatient (84%) compared with low impact users where only a small part (1.4%) were diagnosed as inpatient, making our entire study population more similar to the high-impact users.
Contrary to other studies, we did not see any correlation between type of HF and readmission rates.20 For example, the distribution of HFrEF in readmitted and not readmitted patients was similar.
We observed a clear correlation between burden of co-morbidities and readmissions, as observed earlier.19 One year all-cause and HF readmission rates as well as number of readmissions rouse significantly with increasing number of co-morbidities in our cohort. This relationship suggests that it might be appropriate to put an effort into targeting individuals with >3 co-morbidities with more intense follow-up with a disease management team, as this type of follow-up has previously proven to be efficient in preventing hospitalizations.21, 22 There was no significant correlation between co-morbidities and HF readmission rates within the first month. A possible explanation for this is that early readmissions due to worsening HF is often due to HF itself, for instance residual congestion, and moreover predominantly attributable to cardiac causes during this post-discharge vulnerable period.
Specific co-morbidities such as diabetes mellitus, hypertension, and ischaemic heart disease and their association to readmission have been analysed in a review article by Ross et al., where none of those specific co-morbidities were consistently associated with readmission.23 Other co-morbidities such as psychiatric disorder, systemic inflammatory disease, and pulmonary disease were significantly associated with all-cause readmissions in our study. Psychiatric disease has been included in later studies of prediction models and seem to be a significant risk factor for readmission in these studies like in our material.16, 24 Pulmonary disease was analysed in several of the studies included in the review by Ross et al. and were not consistently associated with readmission.23
Poor compliance to medication was an independent risk factor for HF readmission in our study. Adherence to HF treatment is crucial because specific medication reduce both mortality and morbidity.25, 26 Targeting specific individuals at risk for readmission with a disease management programme can improve compliance to medication and hereby lessen the burden of mortality as well as morbidity.27, 28
For all cause 1 year readmissions, higher levels of NT-proBNP were an independent risk factor for readmissions, concordant with other studies, and a consistent risk factor in studies included in a review of predictive models.23 This could be a marker of remaining congestion and an important sign to observe and act on before hospital discharge.29
Our findings are clinically relevant as frequent hospital readmissions occurred during the first quartile after index hospitalization and was mainly driven by worsening HF rather than high age and burden of co-morbidities. This implies that both post-discharge management and timely optimization of HF care are crucial for preventing hospital readmission. Moreover, co-morbidities are associated with frequent hospital readmission, which underscores the importance of involving other specialities when managing HF patients.
Strengths and limitations
Major strengths are that we have real-life data extrapolated from medical records and not registries. We have consecutively included all patients hospitalized during 1 year, which avoids seasonal variations, for HF in our institution who were appropriate to study regarding follow up, minimizing selection bias.
Review of records was performed by two residents in cardiology making some of the parameters subject to interpretation bias. Access to a senior cardiologist and professor of cardiology for support and final decision making in unclear cases we believe minimized this risk.
Another limitation is the relatively small size of the study. On the other hand, the strength is our access to complete clinical data through original medical records. This was a retrospective study, and limitations connected to this type of study must be considered.
Conclusion
In this real-world cohort of HF patients, hospital readmissions frequently occurred early during the post-discharge period and were mainly driven by worsening HF. Moreover, risk factors varied among hospital readmissions of different causes and at different time periods. By targeting high-risk HF population for hospital readmission and directing appropriate interventions towards these patients, many hospital readmissions should be preventable.
Acknowledgement
We thank Dr Carl Amilon for comments on the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by the Swedish agreement between the government and the county councils concerning economic support for providing an infrastructure for research and education of doctors, the Swedish Research Council (ALF 73400), and the Regional Development Fund, Västra Götaland County, Sweden (FOU-VGR).
Appendix A: Form for review of medical records
Personal identification number:Patient study number:
Release date index hospitalization:
Ward number:
Date of first readmission due to heart failure:
Date of first readmission due to all causes:
Number of readmissions due to heart failure:
Number of readmissions due to all-cause:
Diseased within follow-up period of 1 year: yes/no
Date of death:
Patient information
Gender: Male/Female
Type of follow up
Primary care Heart clinic
Type of heart failure
HFrEF HFpEF HFmrEF Biventricular heart failure Undefined (no ultrasound available)
ECG
Heart rhythm: sinus rhythm/atrial fibrillation or flutter/ventricular pacing
QRS-width >120 ms: yes/no
Cardiac ultrasound
EF: Severe aortic stenosis: yes/no
Mitral regurgitation/aortic regurgitation > grade 2: yes/no
Underlying cause of heart failure
Ischaemic heart disease Dilated cardiomyopathy Prescription drugs Tachyarrhythmia Hypertension Valvular disease Alcohol or drug abuse Other
Co-morbidities
Renal failure Ischaemic heart disease Diabetes Hypertension Atrial fibrillation/flutter Thyroid disease Asthma/COPD Systemic inflammatory disease Metastasized cancer Dementia Lack of compliance to medication Drug/alcohol abuse Anaemia Psychiatric disorder Valvular heart disease Syncope/symptomatic hypotension
Laboratory parameters, pulse, blood pressure
Potassium: ______ mmol/L Creatinine: _____ μmol/L Sodium: ______ mmol/L NTproBNP: _____ ng/L Systolic blood pressure: _____mmHg Diastolic blood pressure:____ mmHg Pulse:_____ b.p.m.
Medication at date of release from hospital:
ACEI: yes/no maximum tolerated dose: yes/no
ARB: yes/no maximum tolerated dose: yes/no
BB: yes/no maximum tolerated dose: yes/no
MRA: yes/no maximum tolerated dose: yes/no
ARNI: yes/no maximum tolerated dose: yes/no
Hydralazine: yes/no
Loop-diuretics: yes/no >80 mg of furosemide: yes/no
Thiazide diuretics: yes/no
Digoxin: yes/no
CCB: yes/no
Long acting nitrates: yes/no




