The prevalence, predictors, and outcomes of spontaneous echocardiographic contrast or left ventricular thrombus in patients with HFrEF
Abstract
Aims
This study aimed to determine prevalence, predictors, and association with ischaemic stroke risk of spontaneous echocardiographic contrast (SEC) or left ventricular thrombus (LVT) in patients with heart failure with reduced ejection fraction (HFrEF).
Methods and results
Clinical, echocardiographic, and follow-up data from January 2009 through February 2019 were retrospectively extracted from electronic medical records of patients with heart failure with left ventricular ejection fraction < 40% by echocardiography on admission, with follow-up to February 2020. Of 9485 consecutive patients with HFrEF, 123 (1.3%) presented LVT and 331 (3.5%) presented SEC. Patients with vs. those without SEC/LVT had larger left ventricular end-diastolic volume (199.5 ± 77.7 vs. 165.8 ± 61.3 mL, P < 0.001), lower left ventricular ejection fractions (29.5 ± 7.0% vs. 33.7 ± 5.5%, P < 0.001), and more often ischaemic cardiomyopathy, apical aneurysm, chronic kidney diseases, and smoking habit. In Cox regression analysis, SEC and LVT were independent predictors for ischaemic stroke occurrence [hazard ratio (HR) = 2.40, 95% confidence interval (CI): 1.74–3.31; HR = 4.52, 95% CI: 2.77–7.40, both P < 0.001]. In patients with those without SEC or LVT, stroke risk was higher among those not on anticoagulants (HR = 2.55, 95% CI: 1.85–3.53; HR = 4.71, 95% CI: 2.84–7.81, both P < 0.001), but similar among those on anticoagulants (P > 0.05). In patients with sinus rhythm, the associations between SEC/LVT and ischaemic stroke persist with HRs of 2.57 (95% CI: 1.69–3.92) and 5.74 (95% CI: 3.38–9.75).
Conclusions
In patients with HFrEF, SEC was not uncommon and increased risk of ischaemic stroke as well as LVT. Anticoagulants could play a role in the reduction of stroke risk, suggesting that patients with SEC/LVT, even those in sinus rhythm, would benefit from systemic anticoagulation treatment.
Introduction
Left ventricular thrombus (LVT) frequently complicates acute anterior myocardial infarction, dilated cardiomyopathy, and severe left ventricular dysfunction, increasing the risk for embolic events by four-fold and long-term mortality by two-fold.1, 2 Among patients with severe systolic dysfunction, LVT prevalence ranges from 2.1% to 7.0%, being higher in patients with worse left ventricular ejection fraction (LVEF) and ischaemic aetiology.1-3 In patients with LVEF < 45% at first echocardiography during the first week after acute myocardial infarction, LVT was diagnosed by either echocardiography or cardiac magnetic resonance in 7% of patients at first week and 22% at first month.4 LVT is also associated with systemic embolism in nearly 11% of patients.5 In current guidelines, anticoagulant therapy use is considered reasonable for patients with ST-segment elevated myocardial infarction and asymptomatic LVT (Class IIa; Level of Evidence C)6 and is recommended for over 3 months in patients with ischaemic stroke or transient ischaemic attacks in sinus rhythm following LVT.7 In patients with LVT, anticoagulation should be administered for up to 6 months guided by repeated imaging (IIa, C).8 However, routine use of anticoagulation for patients with severe left ventricular dysfunction remains a matter of debate.2, 9
Spontaneous echocardiographic contrast (SEC) also predisposes to intracardiac thrombus.5, 10 The prevalence of SEC detection is reported to be between 4.2% and 9.6% in patients with recent myocardial infarction.11 As per limited data, SEC in left ventricle may increase the risk of stroke in patients with left ventricular dysfunction, warranting close echocardiographic follow-up for possible LVT development. Close follow-up, however, might not preclude risk for potential ischaemic stroke particularly without anticoagulant therapy.
The present study of SEC/LVT in patients with heart failure and reduced ejection fraction (HFrEF) aimed to determine its prevalence, related factors, stroke risk, and whether anticoagulant therapy use is effective for stroke prevention.
Methods
Study design
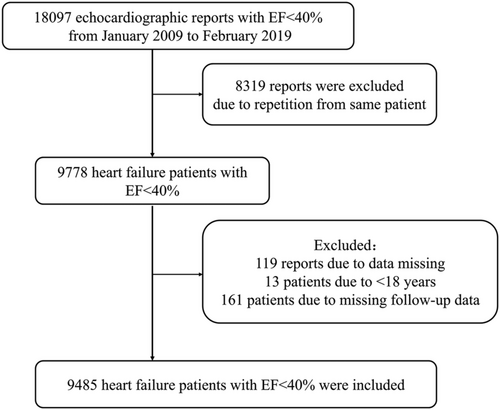
Analysable patient records from January 2009 through February 2019 were extracted from the electronic medical records database at the First Affiliated Hospital of Wenzhou Medical University. Eligible patients with heart failure were those ≥18 years of age and with a first LVEF of <40% by quantitative echocardiography assessment. Each patient's first echocardiography was performed at the time of presentation to the hospital. Exclusion criteria were presence of thrombus in left atrium (LA) or right cardiac cavity, presence of prosthetic valves and infective endocarditis or cardiac tumours, incomplete echocardiography examination, or missing relevant clinical data including follow-up data. Age, gender, previous medical history, laboratory markers, diagnosis, treatment, and follow-up data from analysable patient records were also extracted from the electronic medical records database. From the 18 097 echocardiographic records with standard image quality diagnosed with ejection fraction < 40% that were extracted and reviewed according to definitive echocardiography diagnosis, 8319 reports were excluded due to repetition from same patients. From the remaining echocardiography reports, 193 patients were excluded due to data errors, age < 18 years, or missing follow-up data. Finally, 9485 patients with heart failure with ejection fraction < 40% were included in the analysis. A study flow diagram is presented in Figure 1. The study conforms to the principles outlined in the Declaration of Helsinki. The study was approved by the ethics committee of the First Affiliated Hospital of Wenzhou Medical University Ethics Committee. Due to the retrospective design of the study and because data were acquired in the context of clinical practice, written informed consent was not obtained from patients.
Definitions
This analysis focused on the prevalence of ischaemic stroke diagnosed as clinically relevant focal neurological symptoms detected by computed tomography or magnetic resonance imaging and confirmed by a neurologist during follow-up until February 2020. Ischaemic cardiomyopathy (ICM) was defined as congestive heart failure in the presence of cardiomyopathy associated with a documented history of myocardial infarction, coronary revascularization, or obstructive coronary artery disease (>50% stenosis).12 Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate less than 60 mL/min/1.73 m2.13 Each echocardiographic evaluation was reviewed independently by two experienced readers using a commercially available imaging system in accordance with the American Society of Echocardiography guidelines. A consensus was made on the diagnosis of SEC/LVT. Echocardiographic measurements included left ventricular end-diastolic dimension (LVEDD), left atrial diameter, left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), LVEF, and the presence of SEC and LVT. LVEF was determined using the biplane Simpson's method in the apical two-chamber and four-chamber views. HFrEF was defined as heart failure with LVEF < 40%.14 SEC was defined by dynamic smoke-like echoes with characteristic swirling motion distinct from white noise artefact.15 In this study, SEC was considered as SEC only in left ventricle rather than LA or right cavity. LVT was diagnosed as an echogenic mass adjacent to but distinguishable from left ventricular endocardium in an area of wall-motion abnormality.15
Anticoagulants were used according to patients' indications and contraindications from the up-to-date guideline at that time and in combination with the patients' intentions.6, 7 For high-risk patients (such as those with previous bleeding issues, renal failure, or anaemia), the use of anticoagulants was individualized according to specialist physician's guidance. Some patients with atrial fibrillation (AF) or LVT were not eligible for regular anticoagulant use due to the presence of high-risk bleeding factors, contraindications, or poor compliance. Anticoagulants included warfarin and novel oral anticoagulants such as dabigatran and rivaroxaban, which were available in Chinese markets.
Statistical analysis
Data analyses were performed with SPSS software (SPSS Version 23.0 for Windows, IBM Corporation). Clinical data are presented as mean ± standard deviation for continuous variables and as frequency (%) for categorical variables and were compared using independent Student's t-test and χ2 test, respectively. Non-normally distributed variables are presented as median (interquartile range) and were compared using the Mann–Whitney U test. In patients with HFrEF, factors associated with SEC/LVT were evaluated using univariate and multivariate logistic regression analyses, and ischaemic stroke risk was assessed using Cox regression survival analysis. To further explore the effectiveness of anticoagulation, subjects were divided by use or not of anticoagulation. Because the presence of AF may interfere with the association between SEC/LVT and stroke, patients in sinus rhythm at baseline were extracted for subgroup analysis to reduce confounding parameters. Kaplan–Meier survival analyses were conducted for non-SEC/LVT, SEC, and LVT groups. A two-sided P < 0.05 was considered as statistically significant.
Results
Subject characteristics
In the overall population of 9485 patients studied, mean age was 66.4 ± 14.5 years, mean LVEF was 33.5 ± 5.7%, 70.9% were men, 21.1% had AF, 11.7% had ICM, and 7.5% had apical aneurysm. The prevalence of SEC was 3.5% (331 patients), and that of LVT was 1.3% (123 patients). Twenty-four patients of the latter also presented SEC and were considered as part of the LVT group. Demographics and clinical characteristics of the study cohort, overall and stratified by SEC and LVT categories, are shown in Table 1. Compared with the non-SEC/LVT group, patients with SEC/LVT were younger, more often men, with hypertension, ICM, apical aneurysm, a history of previous stroke, and smoking or drinking habit; higher levels of haemostatic markers (D-dimer, fibrinogen, and platelet); larger LVEDD, LVESV, and LVEDV; and lower LVEF. The detailed characteristics of the SEC and LVT groups, separately, are presented in Table 2. Relative to patients with LVT, those with SEC were older and had larger left atrial diameter, LVEDD, LVESV, and LVEDV; lower D-dimer level; and more often ICM, AF, apical aneurysm, or previous stroke.
|
Overall N = 9485 |
Non-SEC/LVT N = 9031 |
SEC/LVT N = 454 |
P-value | |
|---|---|---|---|---|
| Age, years | 66.4 ± 14.5 | 66.7 ± 14.4 | 62.0 ± 14.5 | <0.001 |
| Male | 6726 (70.9%) | 6341 (70.2%) | 385 (84.8%) | <0.001 |
| Hypertension | 4447 (46.9%) | 4291 (47.5%) | 156 (34.4%) | <0.001 |
| Diabetes | 2059 (21.7%) | 1969 (21.8%) | 90 (19.8%) | 0.351 |
| Dyslipidaemia | 679 (7.2%) | 649 (7.2%) | 30 (6.6%) | 0.709 |
| ICM | 2621 (29.0%) | 2791 (29.4%) | 170 (37.4%) | <0.001 |
| AF | 2000 (21.1%) | 1888 (20.9%) | 112 (24.7%) | 0.059 |
| Apical aneurysm | 713 (7.5%) | 641 (7.1%) | 72 (15.9%) | <0.001 |
| DVT | 237 (2.5%) | 221 (2.4%) | 16 (3.5%) | 0.163 |
| Previous stroke | 411 (4.3%) | 381 (4.2%) | 30 (6.6%) | 0.024 |
| CKD | 1961 (20.7%) | 1831 (20.3%) | 98 (28.6%) | <0.001 |
| Smoking | 2831 (29.8%) | 2636 (29.2%) | 195 (43.0%) | <0.001 |
| Drinking | 1823 (19.2%) | 1698 (18.8%) | 125 (27.7%) | <0.001 |
| Haemoglobin, g/L | 120.8 ± 30.9 | 120.5 ± 31.1 | 126.8 ± 26.5 | <0.001 |
| Platelet, 109/L | 199.5 ± 85.6 | 199.1 ± 85.7 | 207.0 ± 83.2 | 0.064 |
| BNP, ng/mL | 989.0 (385.0, 2378.0) | 965.0 (374.0, 2329.0) | 1438.0 (624.5, 2819.0) | <0.001 |
| D-dimer, mg/L | 1.3 (0.8, 2.5) | 1.2 (0.7, 2.5) | 1.5 (0.9, 2.5) | <0.001 |
| Fibrinogen, g/L | 4.3 ± 1.5 | 4.3 ± 1.5 | 4.6 ± 1.7 | <0.001 |
| Left atrium diameter, mm | 46.2 ± 7.4 | 46.1 ± 7.3 | 47.3 ± 8.1 | <0.001 |
| LVEDD, mm | 59.7 ± 8.9 | 59.5 ± 8.8 | 63.6 ± 10.3 | <0.001 |
| LVESV, mL | 112.7 ± 47.1 | 111.3 ± 45.5 | 141.7 ± 64.5 | <0.001 |
| LVEDV, mL | 167.4 ± 62.6 | 165.8 ± 61.3 | 199.5 ± 77.7 | <0.001 |
| LVEF, % | 33.5 ± 5.7 | 33.7 ± 5.5 | 29.5 ± 7.0 | <0.001 |
| Anticoagulant use | 1090 (11.5%) | 960 (10.6%) | 130 (28.6%) | <0.001 |
| Warfarin | 587 (6.2%) | 518 (5.7%) | 69 (15.2%) | <0.001 |
| NOAC | 503 (5.3%) | 442 (4.9%) | 61 (13.4%) | <0.001 |
| Antiplatelet use | 3924 (41.4%) | 3725 (41.2%) | 199 (43.8%) | 0.283 |
- AF, atrial fibrillation; BNP, Type B natriuretic peptide; CKD, chronic kidney disease; DVT, deep vein thrombosis; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVT, left ventricular thrombus; NOAC, novel oral anticoagulants; SEC, spontaneous echocardiographic contrast.
- Data are presented as mean ± standard deviation, median (interquartile range), or n (%).
|
SEC N = 331 |
LVT N = 123 |
P | |
|---|---|---|---|
| Age, years | 63.5 ± 14.1 | 58.0 ± 14.8 | <0.001 |
| Male | 283 (85.5%) | 102 (82.9%) | 0.556 |
| Hypertension | 118 (35.6%) | 38 (30.9%) | 0.375 |
| Diabetes | 62 (18.7%) | 28 (22.8%) | 0.355 |
| Dyslipidaemia | 22 (6.6%) | 8 (6.5%) | 1.000 |
| ICM | 108 (32.6%) | 62 (50.4%) | <0.001 |
| AF | 100 (32.5%) | 12 (9.8%) | <0.001 |
| Apical aneurysm | 45 (13.6%) | 27 (22.0%) | 0.042 |
| DVT | 9 (2.7%) | 7 (5.7%) | 0.152 |
| Previous stroke | 20 (6.0%) | 10 (8.1%) | 0.404 |
| CKD | 98 (29.6%) | 32 (26.0%) | 0.485 |
| Smoking | 141 (42.6%) | 54 (43.9%) | 0.831 |
| Drinking | 94 (28.4%) | 31 (25.2%) | 0.555 |
| Haemoglobin, g/L | 126.4 ± 23.5 | 127.9 ± 33.4 | 0.661 |
| Platelet, 109/L | 201.9 ± 81.1 | 221.0 ± 87.6 | 0.036 |
| BNP, ng/mL | 1359.0 (606.3, 2722.0) | 1584.0 (771.0, 3075.0) | 0.502 |
| D-dimer, mg/L | 1.3 (0.8, 3.1) | 1.8 (1.0, 5.2) | 0.012 |
| Fibrinogen, g/L | 4.7 ± 1.7 | 4.2 ± 1.7 | 0.011 |
| Left atrium, mm | 48.0 ± 8.2 | 45.7 ± 7.4 | 0.008 |
| LVEDD, mm | 64.3 ± 10.7 | 61.6 ± 8.6 | 0.007 |
| LVESV, mL | 146.5 ± 67.9 | 130.8 ± 54.7 | 0.016 |
| LVEDV, mL | 206.5 ± 82.0 | 184.4 ± 74.7 | <0.001 |
| LVEF, % | 29.5 ± 7.0 | 30.1 ± 7.0 | 0.373 |
| Anticoagulant use | 80 (24.2%) | 50 (40.7%) | 0.001 |
| Warfarin | 38 (11.5%) | 31 (25.2%) | 0.001 |
| NOAC | 42 (12.7%) | 19 (15.4%) | 0.442 |
| Antiplatelet use | 140 (42.3%) | 59 (48.0%) | 0.289 |
- AF, atrial fibrillation; BNP, Type B natriuretic peptide; CKD, chronic kidney disease; DVT, deep vein thrombosis; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVT, left ventricular thrombus; NOAC, novel oral anticoagulants; SEC, spontaneous echocardiographic contrast.
- Data are presented as mean ± standard deviation, median (interquartile range), or n (%).
Factors associated with SEC/LVT
Univariate logistic regression analysis was performed to define potential clinical risk factors associated with SEC/LVT. Parameters associated with SEC/LVT included male gender, age, smoking, drinking, ICM, apical aneurysm, CKD, left atrial diameter, LVEF, LVEDD, LVEDV, and LVESV (Table 3). The relevant variables were added to a multivariate model to analyse independent risk factors of SEC/LVT in patients with HFrEF. As shown in Table 3, male gender [odds ratio (OR) = 1.73, 95% confidence interval (CI): 1.25–2.37, P = 0.001], age (OR = 0.98, 95% CI: 0.97–0.99, P < 0.001), smoking (OR = 1.45, 95% CI: 1.15–1.83, P = 0.002), ICM (OR = 1.74, 95% CI: 1.31–2.31, P < 0.001), apical aneurysm (OR = 2.35, 95% CI: 1.64–3.38, P < 0.001), CKD (OR = 1.93, 95% CI: 1.53–2.44, P < 0.001), LVEF (OR = 0.91, 95% CI: 0.90–0.93, P < 0.001), and LVEDV per 1 mL (OR = 1.00, 95% CI: 1.00–1.01, P < 0.001) were independently associated with SEC/LVT.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR | P | OR | P | |
| Male | 2.367 (1.825, 3.070) | <0.001 | 1.725 (1.254, 2.374) | 0.001 |
| Age | 0.980 (0.974, 0.986) | <0.001 | 0.980 (0.973, 0.987) | <0.001 |
| Smoking | 1.827 (1.509, 2.212) | <0.001 | 1.451 (1.152, 1.828) | 0.002 |
| Drinking | 1.656 (1.338, 2.049) | <0.001 | ||
| AF | 1.239 (0.995, 1.543) | 0.055 | ||
| ICM | 1.464 (1.204, 1.780) | <0.001 | 1.740 (1.311, 2.309) | <0.001 |
| Apical aneurysm | 2.467 (1.894, 3.213) | <0.001 | 2.351 (1.636, 3.377) | <0.001 |
| CKD | 1.578 (1.279, 1.946) | <0.001 | 1.930 (1.528, 2.436) | <0.001 |
| D-dimer | 1.464 (1.204, 1.780) | <0.001 | ||
| Fibrinogen | 1.146 (1.079, 1.218) | <0.001 | ||
| Haemoglobin | 1.008 (1.004, 1.011) | <0.001 | ||
| Left atrium | 1.023 (1.010, 1.035) | <0.001 | ||
| LVEF | 0.901 (0.888, 0.914) | <0.001 | 0.912 (0.897, 0.928) | <0.001 |
| LVEDD | 1.050 (1.039, 1.060) | <0.001 | ||
| LVEDV | 1.007 (1.006, 1.008) | <0.001 | 1.004 (1.003, 1.006) | <0.001 |
| LVESV | 1.001 (1.009, 1.012) | <0.001 | ||
- AF, atrial fibrillation; CKD, chronic kidney disease; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic dimension; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; OR, odds ratio.
Presence of SEC was associated with increased risk of stroke embolism in patients with HFrEF
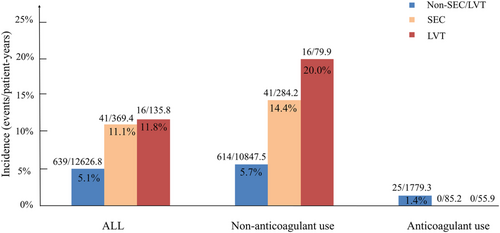
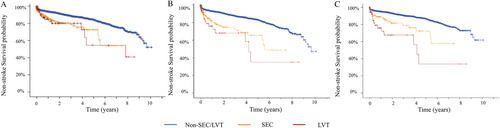
During total 13 132.0 patient-years follow-up with median 1.38 year follow-up, 697 of the 9485 patients suffered ischaemic stroke events, with 5.3% events/patient-years. Detailed characteristics of the latter patients are presented in Table 4. As shown in Figure 2, the non-SEC/LVT, SEC, and LVT groups had a stroke embolism rate of 5.1% (639/12 626.8), 11.1% (41/369.4), and 11.8% events/patient-years (16/135.8), respectively (P < 0.001). As shown in Table 5, in unadjusted model compared with the non-SEC/LVT group, the hazard ratios (HRs) for SEC and LVT were 2.060 (95% CI: 1.501–2.827) and 2.230 (95% CI: 1.377–3.611), respectively. The adjusted HRs for ischaemic stroke were computed to assess the predictive value of SEC/LVT presence for stroke embolism in patients with HFrEF. After adjusting for potential confounders (age, male gender, hypertension, diabetes, AF, deep vein thrombosis, previous stroke, anticoagulant use, smoking, drinking, LVEDD, and LVEF), the HR for SEC and LVT was 2.40 (95% CI: 1.74–3.31) and 4.52 (95% CI: 2.77–7.40), respectively (all P < 0.001). In Figure 3, Kaplan–Meier analysis was used to estimate the stroke-free rates for patients with HFrEF and SEC/LVT. The probability of ischaemic stroke for patients with SEC or LVT was higher than that for the non-SEC/LVT group (P < 0.001). In patients with HFrEF, the SEC and LVT groups had similar stroke-free rates (P = 0.767).
|
Stroke N = 697 |
No stroke events N = 8788 |
P-value | |
|---|---|---|---|
| Age, years | 69.3 ± 12.0 | 66.2 ± 14.6 | <0.001 |
| Male | 515 (73.9%) | 6211 (70.7%) | 0.076 |
| Hypertension | 393 (56.4%) | 4054 (46.1%) | <0.001 |
| Diabetes | 182 (26.1%) | 1877 (21.4%) | 0.004 |
| Dyslipidaemia | 53 (7.6%) | 644 (7.3%) | 0.647 |
| ICM | 187 (26.8%) | 2604 (29.6%) | 0.063 |
| AF | 261 (37.4%) | 1739 (19.8%) | <0.001 |
| Apical aneurysm | 51 (7.3%) | 662 (7.5%) | 0.882 |
| DVT | 42 (6.0%) | 195 (2.2%) | <0.001 |
| Previous stroke | 18 (2.6%) | 393 (4.5%) | 0.016 |
| CKD | 126 (18.1%) | 1835 (20.9%) | 0.080 |
| Smoking | 256 (36.7%) | 2575 (29.3%) | <0.001 |
| Drinking | 192 (27.5%) | 1631 (18.6%) | <0.001 |
| Haemoglobin, g/L | 124.6 ± 28.5 | 120.5 ± 31.1 | <0.001 |
| Platelet, 109/L | 197.1 ± 79.1 | 199.7 ± 86.1 | 0.441 |
| BNP, ng/mL | 924.0 (390.3, 2003.0) | 993.0 (384.0, 2396.0) | 0.245 |
| D-dimers | 1.2 (0.7, 2.5) | 1.4 (0.8, 3.3) | 0.002 |
| Fibrinogen | 4.3 ± 1.5 | 4.3 ± 1.5 | 0.957 |
| Left atrium, mm | 46.6 ± 7.1 | 46.1 ± 7.4 | <0.001 |
| LVEDD, mm | 58.3 ± 8.2 | 59.8 ± 9.0 | <0.001 |
| LVESV, mL | 105.8 ± 42.4 | 113.3 ± 47.3 | <0.001 |
| LVEDV, mL | 157.8 ± 55.6 | 168.2 ± 63.1 | <0.001 |
| LVEF, % | 33.8 ± 5.5 | 33.5 ± 5.7 | 0.156 |
- AF, atrial fibrillation; BNP, Type B natriuretic peptide; CKD, chronic kidney disease; DVT, deep vein thrombosis; ICM, ischaemic cardiomyopathy; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume.
- Data are presented as mean ± standard deviation, median (interquartile range), or n (%).
| Unadjusted HR | P-value | Adjusted HR | P-value | |
|---|---|---|---|---|
| All | ||||
| Non-SEC/LVT | Ref | Ref | ||
| SEC | 2.060 (1.501, 2.827) | <0.001 | 2.400 (1.737, 3.314) | <0.001 |
| LVT | 2.230 (1.377, 3.611) | <0.001 | 4.524 (2.767, 7.395) | <0.001 |
| No anticoagulant use | ||||
| Non-SEC/LVT | Ref | Ref | ||
| SEC | 2.448 (1.783, 3.360) | <0.001 | 2.551 (1.846, 3.525) | <0.001 |
| LVT | 3.126 (1.902, 5.138) | <0.001 | 4.709 (2.839, 7.812) | <0.001 |
| Anticoagulant use | ||||
| Non-SEC/LVT | Ref | Ref | ||
| SEC | — | — | — | — |
| LVT | 1.341 (0.181, 9.912) | 0.774 | 2.194 (0.249, 19.332) | 0.479 |
- HR, hazard ratio; LVT, left ventricular thrombus; SEC, spontaneous echocardiographic contrast.
- Adjusted model is adjusted for age, gender, hypertension, diabetes, atrial fibrillation, deep vein thrombosis, previous stroke, anticoagulant use, smoking, drinking, left atrium diameter, left ventricular end-diastolic diameter, and left ventricular ejection fraction.
Anticoagulant use may reduce risk of stroke in patients with SEC and HFrEF
As shown in Table 5, in Cox regression analysis of study patients on or not on anticoagulants, the adjusted HRs of patients with SEC and LVT for stroke embolism were 2.55 (95% CI: 1.85–3.53) and 4.71 (95% CI: 2.84–7.81) in patients not on anticoagulants, while it did not differ significantly in patients with LVT on anticoagulation compared with the control group. Figure 3 and 3 shows the long-term stroke-free interval estimates for patients with SEC or LVT or non-SEC/LVT, stratified by the use or not of anticoagulation. Without anticoagulation, the stroke-free time of patients with SEC/LVT was shorter than that of patients without SEC/LVT (P < 0.001). However, among the 1115 patients on anticoagulation pre-stroke, incidence rates of stroke embolism were similar in patients with vs. without SEC/LVT (P = 0.352).
Patients with HFrEF with SEC/LVT on sinus rhythm have higher risk than those without SEC/LVT and may benefit from anticoagulation treatment
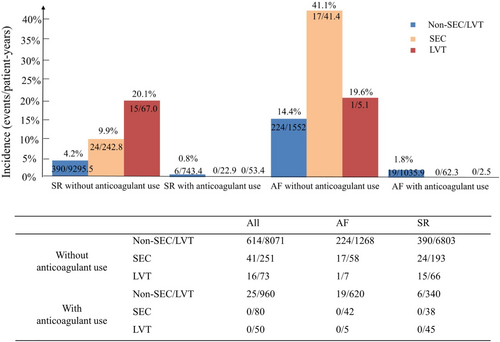
To circumvent the impact of AF as a cofounding factor because of its strong association with ischaemic stroke, the relationship between SEC/LVT and stroke was analysed in patients on sinus rhythm at baseline as a separate subgroup. As shown in Figure 4, patients with SEC or LVT on sinus rhythm who did not receive anticoagulants demonstrated a higher stroke embolism rate of 9.9% events/patient-years and 20.1% events/patient-years compared with patients without SEC and LVT (4.2% events/patient-years, P < 0.001, respectively) (Figure 4). Of 231 patients with HFrEF and SEC, 38 patients received anticoagulants, and 193 patients did not. Twenty-four (9.9% events/patient-years) ischaemic stroke events occurred in patients with sinus rhythm and SEC without anticoagulant use at 242.8 patient-years follow-up, while no ischaemic stroke events occurred in patients with sinus rhythm and SEC who received anticoagulants at 22.9 patient-years follow-up (P < 0.001). Of 340 patients with sinus rhythm and non-SEC/LVT who received anticoagulant therapy, six (0.8% events/patient-years) ischaemic stroke events occurred at 743.4 patient-years follow-up. Of 38 patients with sinus rhythm and SEC who received anticoagulants, no ischaemic stroke events occurred at 22.9 patient-years follow-up. Under anticoagulant use, the risk of ischaemic stroke did not differ significantly between the two subgroups (P > 0.05).
As shown in Table 6, Cox regression analysis revealed that unadjusted HR for ischaemic stroke of patients with SEC and LVT was 2.06 (95% CI: 1.50–2.83) and 2.230 (95% CI: 1.38–3.61), respectively (P < 0.001). After adjustment for correlative confounders, the presence of SEC and LVT was associated with even greater risk for ischaemic stroke, with HRs of 2.57 (95% CI: 1.69–3.92) and 5.74 (95% CI: 3.38–9.75) (P < 0.001, respectively).
| SR without anticoagulant use | Unadjusted HR | P-value | Adjusted HR | P-value |
|---|---|---|---|---|
| Control | Ref | Ref | ||
| SEC | 2.060 (1.501, 2.827) | <0.001 | 2.573 (1.688, 3.923) | <0.001 |
| LVT | 2.230 (1.377, 3.611) | <0.001 | 5.738 (3.379, 9.745) | <0.001 |
- HR, hazard ratio; LVT, left ventricular thrombus; SEC, spontaneous echocardiographic contrast; SR, sinus rhythm.
- Adjusted model is adjusted for age, gender, hypertension, diabetes, deep vein thrombosis, previous stroke, anticoagulant use, smoking, drinking, left atrium diameter, left ventricular end-diastolic diameter, and left ventricular ejection fraction.
Discussion
Main findings
The present retrospective single-centre study yielded the following main findings: (i) in patients with HFrEF, the prevalence of SEC and LVT was 3.5% and 1.3%, respectively; (ii) in multivariate analysis, the presence of SEC/LVT was associated with male gender, age, smoking, ICM, apical aneurysm, CKD, LVEF, and LVEDV; (iii) relative to controls, stroke risk doubles in those with SEC and quadruples in those with LVT; (iv) without anticoagulant use, the presence of SEC increased by 2.5-fold the risk of stroke embolism in patients with HFrEF, whereas patients with SEC/LVT on anticoagulation showed similar low risk of ischaemic stroke events to patients without SEC/LVT; and (v) patients in sinus rhythm with SEC/LVT and no anticoagulant treatment had a two-fold to five-fold risk of ischaemic stroke.
The prevalence and potential mechanisms of SEC/LVT formation
In previous studies, the prevalence of LVT in selected patient populations (acute myocardial infarction, ICM, dilated cardiomyopathy, and reduced LVEF) ranged from 2.1% to 7.0%.1-3 In the present study of patients with HFrEF, the prevalence of SEC and LVT was 3.5% and 1.3%, respectively. The lower prevalence might be explained as follows: firstly, differences in population selection may translate into a lower proportion of acute myocardial infarction cases; in the present study, ICM, AF, and apical aneurysm were present in only 29.0%, 21.1%, and 7.5% of patients, respectively. Secondly, the presence of SEC/LVT was determined by transthoracic echocardiography, which albeit non-invasive and commonly used may yield lower detection efficacy than transesophageal echocardiography and magnetic resonance. Thirdly, true prevalence may be underestimated because of the retrospective study design.
Although the mechanisms underlying LVT formation have not been well established, SEC/LVT occurs under various clinical and pathological conditions, including dilated cardiac chambers, ventricular aneurysm, and hypercoagulable state.7, 15 Left ventricular dysfunction has been associated with a pro-thrombotic state and a dysfunctional coagulation system.6, 16 Left ventricular regional wall akinesia and dyskinesia result in blood stasis due to low output in dilated cardiac chambers.17, 18 Prolonged ischaemia aggravates subendocardial tissue injury and thus contributes to the inflammatory process. Altered neurohormonal mechanisms and endothelial dysfunction contribute to platelet aggregation, increased thrombin activation, and a hypercoagulable state.17-19
SEC is associated with increased risk of ischaemic stroke as well as LVT
Spontaneous echocardiographic contrast is considered to trigger a hypercoagulable state, and its presence has been associated with coagulation markers.20 Heppell et al. demonstrated that altered haemostatic markers such as thrombin–antithrombin complexes, D-dimers, β-thromboglobulin, and von Willebrand factor were independently associated with SEC and thrombus formation.21 A systematic review and meta-analysis confirmed that D-dimer level was a predictor for SEC with a pooled sensitivity and specificity of 75% and 81%, respectively.22 Several studies have shown that SEC in the LA and left atrial appendage increased incidence of systemic embolization.11, 23, 24 However, anticoagulants have been used in certain patients with HFrEF and LVT, but not studied in patients following SEC from left ventricle. The present study documented that patients with SEC exhibited an increased risk of stroke embolism as did patients with HFrEF and LVT.
Need for anticoagulation in patients with HFrEF and SEC/LVT
Based on CHADS2/CHA2DS2-VASc score, anticoagulant use is reasonable for patients with AF, especially in the presence of SEC/LVT.25 The presence or absence of SEC/LVT may be of great importance in the management of HFrEF, even in patients with sinus rhythm.25 Because no major clinical trials had been specifically conducted, for the early guideline, effectiveness of anticoagulation aiming for stroke prevention was not well established in patients with HFrEF without AF or a previous thromboembolic event (Class IIb; Level of Evidence B).25-27 As the largest randomized trial of oral anticoagulation in sinus rhythm and reduced LVEF, the Warfarin vs. Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial was designed to compare the primary combined endpoint of ischaemic stroke, ICM, or death.28 The WARCEF trial included 2305 patients with 6 year follow-up and demonstrated that warfarin and aspirin had the same effect on the primary composite outcome with HR = 0.93 (95% CI: 0.79–1.10)28; however, statistical comparisons were stratified by first ever stroke or transient ischaemic attack (0.72 vs. 1.36 events per 100 patient-years; HR = 0.52, 95% CI: 0.33–0.82).28 The current guideline is based on the WARCEF trial and represents an update of the recommendation that anticoagulants are reasonable for patients with heart failure who do not have AF or a previous thromboembolic event (Class IIa; Level of Evidence B).25, 28 These guidelines do not specifically refer to patients with SEC/LVT. Our results substantiate the notion that patients with SEC/LVT have a higher risk of ischaemic stroke. Furthermore, patients with AF require more careful consideration regarding anticoagulation treatment based on CHADS2/CHA2DS2-VASc score. In contrast to patients with AF, the stroke risk of patients with HFrEF on sinus rhythm may be underestimated, and careful evaluation of the presence of SEC/LVT and consequent embolic risk is crucial. Based on our data, patients with HFrEF on sinus rhythm with LVT would benefit from anticoagulant use, in accordance with previous guidelines.8 Unfortunately, during most of the follow-up period, the majority of high-risk patients with SEC and HFrEF did not receive oral anticoagulants, with as few as 24.2% receiving no oral anticoagulants pre-stroke. We posit that close routine detection of SEC and more aggressive use of anticoagulation are important to reduce ischaemic stroke risk due to SEC.
Limitations
Generalizability of findings in this study is limited by several factors. Firstly, this is a retrospective observational study with potential selection bias because SEC and LVT were detected by routinely performed transthoracic echocardiography in patients on admission and at follow-up. Although some modalities such as left ventricular contrast, CTA, or magnetic resonance imaging may improve diagnosis of SEC or LVT, these modalities were not routinely performed during the study, which might have led to underestimation of the number of patients with LVT. Secondly, because of the retrospective design, and duration and dose of anticoagulant therapy, grade of SEC and visualization of SEC/LVT, which may affect the association between the presence of SEC/LVT and stroke risk, were not included in the analysis. Detailed echocardiographic variables such as cardiac output and quantified diastolic function were not routinely measured in this study. Further investigation is required to identify therapy duration and the efficacy and safety of oral anticoagulants using more echocardiographic variables in this clinical setting. Thirdly, although the sample size of our study was large, the number of patients in the SEC/LVT subgroup was relatively small, resulting in wide 95% CIs for C-statistics. Fourthly, AF was diagnosed at the first exam in our study. Clinical data including history of paroxysmal or persistent AF were retrospectively extracted from electronic medical records to the best of our ability. Although the majority of hospitalized patients were monitored with a cardiac telemetry system and some outpatients received Holter monitoring, some patients with history of paroxysmal AF or who developed AF during follow-up may have been missed due to the retrospective study design.
Conclusions
In the present retrospective single-centre study of patients with HFrEF, SEC in left ventricle was not uncommon, and the presence of LVT indicated a poor prognosis with two-fold and four-fold increased ischaemic stroke risk, respectively. Anticoagulant use may improve the outcomes of patients with SEC/LVT and HFrEF, especially those in sinus rhythm, which warrants further study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81600341), the Natural Science Foundation of Zhejiang Province (LQ15H020005), Wenzhou Science Technology Bureau Foundation (Y20190616), and Medical Health Science and Technology Projects of Zhejiang Province (2021RC091).
Conflict of interest
None declared.




