A novel echocardiographic method for estimation of pulmonary artery wedge pressure and pulmonary vascular resistance
Abstract
Aims
This study aimed to evaluate a novel echocardiographic algorithm for quantitative estimation of pulmonary artery wedge pressure (PAWP) and pulmonary vascular resistance (PVR) in patients with heart failure and pulmonary hypertension (PH) scheduled to right heart catheterization (RHC).
Methods and results
In this monocentric study, 795 consecutive patients (427 men; age 68.4 ± 12.1 years) undergoing echocardiography and RHC were evaluated. Multiple regression analysis was performed to identify echocardiographic predictors of PAWP and PVR measured by RHC in the derivation group (the first 200 patients). The diagnostic accuracy of the model was then tested in the validation group (the remaining 595 patients). PH was confirmed by RHC in 507 (63.8%) patients, with 192 (24.2%) cases of precapillary PH, 248 (31.2%) of postcapillary PH, and 67 (8.4%) of combined PH. At regression analysis, tricuspid regurgitation maximal velocity, mitral E/e′ ratio, left ventricular ejection fraction, right ventricular fractional area change, inferior vena cava diameter, and left atrial volume index were included in the model (R = 0.8, P < 0.001). The model showed a high diagnostic accuracy in estimating elevated PAWP (area under the receiver operating characteristic curve = 0.97, 92% sensitivity, and 93% specificity, P < 0.001) and PVR (area under the receiver operating characteristic curve = 0.96, 89% sensitivity, and 92% specificity, P < 0.001), outperforming 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations (P < 0.001) and Abbas' equation (P < 0.001). Bland–Altman analysis showed satisfactory limits of agreement between echocardiography and RHC for PAWP (bias 0.7, 95% confidence interval −7.3 to 8.7) and PVR (bias −0.1, 95% confidence interval −2.2 to 1.9 Wood units), without indeterminate cases.
Conclusions
A novel quantitative echocardiographic approach for the estimation of PAWP and PVR has high diagnostic accuracy in patients with heart failure and PH.
Introduction
Right heart catheterization (RHC) is the gold standard for the evaluation of cardiopulmonary haemodynamics, because it allows to (i) diagnose pulmonary hypertension (PH) by using mean pulmonary artery pressure (PAPm ≥ 25 mmHg at rest according to the European Society of Cardiology/European Respiratory Society guidelines1 or >20 mmHg according to the 6th World Symposium on Pulmonary Hypertension recommendations2) and (ii) distinguish by using pulmonary artery wedge pressure (PAWP) and pulmonary vascular resistance (PVR) between precapillary PH [PAWP ≤ 15 mmHg and PVR ≥ 3 Wood units (WU)], isolated postcapillary PH (PAWP > 15 mmHg and PVR < 3 WU), and combined PH (PAWP > 15 mmHg and PVR ≥ 3 WU).1 Precapillary PH is usually associated with pulmonary arterial hypertension, post-embolic PH, or PH due to lung disease or hypoxia, while isolated postcapillary and combined PH are mainly due to heart failure (HF) or valvular diseases. Therefore, misclassification of the PH subtype may cause incorrect therapy and adverse prognosis.3
Echocardiography has emerged as a non-invasive tool to evaluate cardiopulmonary haemodynamics.4-7 Following the complex 2009 American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) recommendations for diastolic function assessment,8 in 2016, an ASE/EACVI update proposed a simplified approach to estimate PAWP,9 providing however a semiquantitative and not quantitative measurement of PAWP. As a result, PVR cannot be calculated, and the distinction between precapillary and postcapillary PH remains elusive. Abbas et al. developed an algorithm to calculate PVR in a population of patients with precapillary PH, but this method may lead to false-positive results in patients with HF and elevated PAWP.5
Considering that PAPm is composed of PAWP plus the transpulmonary pressure gradient (TPG), we developed a mathematical model to predict the ratio between PAWP and PAPm by using standard echocardiographic variables in a derivation cohort. From this model, we obtained a quantitative echocardiographic estimate of both PAWP and PVR (PAWPecho and PVRecho), and we then tested the accuracy of the model against RHC in a large validation cohort.
Methods
In this monocentric study, patients referred for RHC from the Department of Cardiology and the Department of Pneumology of Fondazione Toscana G. Monasterio, Pisa, Italy, between 2013 and 2019 were consecutively evaluated. Exclusion criteria were the presence of uncorrected intra-cardiac or extra-cardiac shunts and poor echocardiographic image quality, including inadequate Doppler signals of tricuspid regurgitation (TR) and pulmonary regurgitation (PR).
All patients underwent a complete echocardiographic Doppler examination within 24 h from but always before RHC. Diuretics, inotropes, and vasodilators were not administered between echocardiography and RHC. Beyond echocardiography and RHC, all patients also underwent a thorough clinical evaluation and laboratory characterization including plasma N-terminal fragment of pro-B-type natriuretic peptide (NT-proBNP, ECLIA monoclonal method, Roche Diagnostics®, Basel, Switzerland) and high-sensitivity cardiac troponin T (hs-cTnT, Roche Diagnostics).
Right heart catheterization
Right heart catheterization with a flow-directed pulmonary artery catheter was used to measure at end-expiration right atrial pressure (RAPcath), right ventricular (RV) pressure, systolic pulmonary artery pressure (PAPscath), diastolic pulmonary artery pressure (PAPdcath), PAPmcath, and PAWPcath. The right position of the Swan–Ganz catheter when measuring PAWP was verified during the procedure by fluoroscopy, with deflation, retraction, repositioning, and reinflation in a different branch of the pulmonary artery in case of suboptimal PAWP measurement.
Cardiac output (CO) was calculated by thermodilution as a mean of three or five consecutive measurements (varying <10%) in patients with sinus rhythm and atrial fibrillation (AF), respectively. TPG was calculated as PAPmcath − PAWPcath, while PVRcath as the TPG/CO ratio. We followed the 2015 European Society of Cardiology/European Respiratory Society guidelines,1 in which PAPm ≥ 25 mmHg constitutes the threshold for the definition of PH, instead of the more recent 6th World Symposium on Pulmonary Hypertension recommendations,2 because the latter does not address the patient subgroup with PAWP ≤ 15 mmHg and PVR < 3 WU.
Transthoracic Doppler echocardiography
Echocardiography (Philips iE33 xMATRIX echocardiography system, Andover, MA) was performed according to international recommendations.10-13 Sonographers (C. T. and E. P.) were blinded to RHC results. Every recorded image consisted of at least three or five cardiac cycles in patients in sinus rhythm and AF, respectively.
Stroke volume was calculated by multiplying the left ventricular (LV) outflow tract area by the LV outflow tract velocity–time integral measured by pulsed-wave Doppler. In patients with significant aortic regurgitation, RV outflow tract was used. CO was calculated by multiplying stroke volume by heart rate. RAPecho was assessed by measuring inferior vena cava (IVC) diameter and its variations during the respiratory cycle, including a brief sniff to elicit the inspiratory response.6-11 PAPsecho was calculated by adding RAPecho to the maximal systolic pressure gradient from tricuspid regurgitation velocity (TRV). PAPdecho was calculated by adding RAPecho to the PR end-diastolic gradient (PREDG). PAPmecho was calculated as (PAPsecho + 2 · PAPdecho)∕3.
Left atrial volume index (LAVi) was estimated with the disc summation algorithm (Simpson's technique) in a biplane approach from the apical four-chamber and two-chamber view.11 The grading of mitral regurgitation (MR) was assessed by measuring the vena contracta, with proximal isovelocity surface area method when assessable, dominant E wave in the mitral inflow (>1.5 m/s), systolic flow reversal in the pulmonary veins, and jet swirling used as supportive indices of severe MR.12 LV filling pressure was evaluated according to current recommendations using a comprehensive approach, which included the estimation of TRV, LAVi, isovolumic relaxation time, and different E/e′ ratio thresholds in special conditions, such as severe MR and AF.9, 14
To maximize image quality and decrease the likelihood of discarding patients for poor acoustic windows, we also employed off-axis approaches, such as the RV inflow tract for TR, subcostal view for PR, and left lateral approach for IVC dimension and variations.
Statistical methods
Data were analysed with SPSS version 23.0 (IBM Corp., Armonk, NY). Continuous measures were expressed as mean ± standard deviation or median and inter-quartile range and compared by using Student's t-test or Mann–Whitney U test, as appropriate. Categorical variables were presented as numbers and percentages and compared by using the χ2 test. The correlation coefficient R (or Spearman's rho) was assessed when necessary. The ANOVA or Kruskal–Wallis test was used to analyse the differential distribution of data among >2 groups, with subsequent post hoc corrections for multiple comparisons (i.e. Tukey–Kramer or Conover test, respectively).
Determination of the echocardiographic model of pulmonary artery wedge pressure and pulmonary vascular resistance prediction
Considering that PAWP should always be inferior to (being a component of) PAPm, we used the PAWP/PAPm ratio as the dependent variable of the model.
Univariate linear regression analysis was first performed to search for echocardiographic and laboratory predictors of the ratio. Variables with a significance probability value (maximized partial likelihood ratio) ≤0.05 were successively entered into a stepwise multivariable linear regression analysis showing unstandardized and standardized regression coefficients to compare the strength of the effect of each independent variable with that of the dependent variable, with variance inflation factor (VIF) used to exclude multicollinearity. The result of the multivariable linear regression analysis was used to obtain the model of prediction of PAWP/PAPm (as shown in Equation 1), which was thus completely data driven. From this model, we then calculated the echocardiographic estimate of PAWP and PVR (PAWPecho and PVRecho, as shown in Equations 2 and 3).
To test the intra-observer and inter-observer variability, three independent observers (V. C., N. R. P., and V. S.) reanalysed the parameters of the model (including CO), from 50 randomly selected patients in the ‘derivation cohort’, and reproducibility was measured with the intraclass correlation coefficient.
Validation of the echocardiographic model of pulmonary artery wedge pressure and pulmonary vascular resistance prediction
Pulmonary artery wedge pressure and PVR estimates from the echocardiographic model were then validated against RHC data in the validation cohort, through areas under the receiver operating characteristic (ROC) curve (AUCs) and their associated 95% confidence intervals (CIs). McNemar test was used to compare the accuracy of the model with previous algorithms. Bland–Altman plot analysis was also used to assess bias and limits of agreement (defined as 95% CI around the mean) between echocardiographic and RHC data in the whole cohort and in specific patient subgroups.
Results
Patient population characteristics
Among 968 patients screened, 15 (1.6%) were excluded because of the presence of uncorrected intra-cardiac or extra-cardiac shunt and 158 (16.3%) due to inadequate TR and/or PR Doppler signals. Finally, 795 patients were included in the analysis, as shown in Table 1.
| Variables | Overall (n = 795) | Non-PH (n = 288) | PH (n = 507) | P value |
|---|---|---|---|---|
| Age (years) | 68.4 ± 12.1 | 67.0 ± 11.8 | 69.4 ± 11.6 | ns |
| Men, n (%) | 427 (53.7) | 161 (55.9) | 266 (52.5) | ns |
| BMI (kg/m2) | 27.2 ± 9.4 | 27.4 ± 14.2 | 27.1 ± 5.1 | ns |
| BSA (m2) | 1.9 ± 0.2 | 1.9 ± 0.2 | 1.9 ± 0.2 | ns |
| Systolic blood pressure (mmHg) | 134.2 ± 24.9 | 135.0 ± 26.2 | 133.7 ± 24.2 | ns |
| Diastolic blood pressure (mmHg) | 72.7 ± 12.0 | 71.2 ± 11.2 | 73.6 ± 12.3 | <0.05 |
| Heart rate (b.p.m.) | 73.8 ± 15.2 | 69.6 ± 13.2 | 76.0 ± 15.7 | <0.001 |
| Sinus rhythm, n (%) | 534 (67.2) | 222 (77.1) | 312 (61.5) | <0.001 |
| Atrial fibrillation, n (%) | 205 (25.8) | 54 (18.7) | 151 (29.8) | <0.001 |
| Paced rhythm, n (%) | 56 (7.0) | 12 (4.2) | 44 (8.7) | <0.05 |
| eGFR (mL/min/1.73 m2) | 72.5 ± 27.2 | 76.7 ± 26.3 | 70.2 ± 27.5 | ns |
| NT-proBNP (ng/L) | 1227 (373.5–3128) | 557 (191–1634) | 1715.5 (612.3–4364.3) | <0.001 |
| hs-cTnT (ng/L) | 18.5 (10.5–34.3) | 14.0 (8.7–24.6) | 21.8 (12.6–39.0) | <0.001 |
| Echocardiographic data | ||||
| LVEF (%) | 49.7 ± 17.7 | 49.6 ± 16.0 | 49.6 ± 18.6 | ns |
| LVEDV index (mL/m2) | 76.3 ± 32.2 | 79.1 ± 29.9 | 74.7 ± 33.3 | ns |
| LVESV index (mL/m2) | 42.9 ± 31.6 | 43.3 ± 28.4 | 42.6 ± 33.3 | ns |
| LVM index (g/m2) | 112.1 ± 38.4 | 112.8 ± 37.2 | 111.7 ± 39.2 | ns |
| LVEDD (mm) | 53.2 ± 10.2 | 54.1 ± 9.4 | 52.7 ± 10.6 | ns |
| LVESD (mm) | 39.9 ± 13.0 | 40.5 ± 11.9 | 39.6 ± 13.6 | ns |
| MR Grade 1, 2, 3, n (%) | 314 (39.5)/314 (39.5)/78 (9.8) | 136 (47.2)/96 (33.3)/16 (5.6) | 178 (35.1)/218 (43.0)/62 (12.2) | <0.001 |
| Mitral E/A ratio | 1.3 ± 0.9 | 1.1 ± 0.6 | 1.5 ± 1.0 | <0.001 |
| Mitral E/e′ ratio | 13.9 ± 8.3 | 11.3 ± 5.2 | 15.3 ± 9.3 | <0.001 |
| LAVi (mL/m2) | 41.4 ± 15.1 | 37.9 ± 13.3 | 43.4 ± 15.6 | <0.001 |
| PREDG (mmHg) | 9.7 ± 5.0 | 6.4 ± 2.9 | 11.5 ± 5.0 | <0.001 |
| RVFAC (%) | 35.4 ± 9.7 | 40.3 ± 8.6 | 33.1 ± 9.4 | <0.001 |
| TAPSE (mm) | 18.9 ± 5.3 | 20.4 ± 5.1 | 18.0 ± 5.2 | <0.001 |
| TR Grade 3, n (%) | 67 (8.4) | 10 (3.8) | 57 (11.2) | <0.001 |
| TRV (cm/s) | 324.1 ± 65.2 | 279.6 ± 38.5 | 351.2 ± 58.1 | <0.001 |
| RAP (mmHg) | 7.8 ± 3.6 | 6.1 ± 2.8 | 8.8 ± 3.6 | <0.001 |
| IVC diameter (mm) | 19.6 ± 5.5 | 17.0 ± 4.8 | 21.1 ± 5.2 | <0.001 |
| Right heart catheterization | ||||
| Cardiac output (L/min) | 5.0 ± 1.4 | 5.4 ± 1.5 | 4.9 ± 1.4 | <0.001 |
| PAWP (mmHg) | 15.9 ± 7.9 | 11.3 ± 4.5 | 18.4 ± 8.2 | <0.001 |
| PVR (WU) | 2.8 ± 2.6 | 1.4 ± 0.8 | 3.6 ± 2.9 | <0.001 |
| Systolic PAP (mmHg) | 46.8 ± 16.2 | 31.3 ± 6.5 | 55.6 ± 14.1 | <0.001 |
| Mean PAP (mmHg) | 28.7 ± 10.1 | 18.5 ± 3.6 | 34.5 ± 7.8 | <0.001 |
| Diastolic PAP (mmHg) | 19.4 ± 7.7 | 12.3 ± 3.4 | 23.4 ± 6.4 | <0.001 |
| RAP (mmHg) | 7.8 ± 4.8 | 5.5 ± 3.4 | 9.1 ± 5.0 | <0.001 |
- BMI, body mass index; BSA, body surface area; eGFR, estimated glomerular filtration rate; hs-cTnT, high-sensitivity cardiac troponin T; LAVi, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LVM, left ventricular mass; MR, mitral regurgitation; NT-proBNP, N-terminal fragment of pro-B-type natriuretic peptide; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PREDG, pulmonary regurgitation end-diastolic gradient; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVFAC, right ventricular fractional area change; TR, tricuspid regurgitation; TRV, tricuspid regurgitation velocity; WU, Wood units.
- Data are presented as mean and standard deviation if normally distributed or median and inter-quartile range if not normally distributed or as number and %.
At RHC, PH was diagnosed in 507 patients (64%, Table 1). Beyond showing higher pulmonary artery pressures (PAPs, PAPd, and PAPm), patients with PH also showed a worse CO, diastolic profile, mitral regurgitation severity, and RV function compared with patients without PH (all P < 0.001). They also showed higher hs-cTnT and NT-proBNP plasma levels (all P < 0.001). Overall, there were 77 (10%) cases of pulmonary arterial hypertension, 345 (43%) of left heart disease, 49 (6%) of lung disease, and 36 (5%) of chronic thromboembolic PH (Figure 1 and Supporting Information, Table S1). Among patients with left heart disease, 335 (97%) had HF, due to HF with reduced ejection fraction in 166 (48%), HF with mid-range ejection fraction in 31 (9%), and HF with preserved ejection fraction (HFpEF) in 138 (40%).
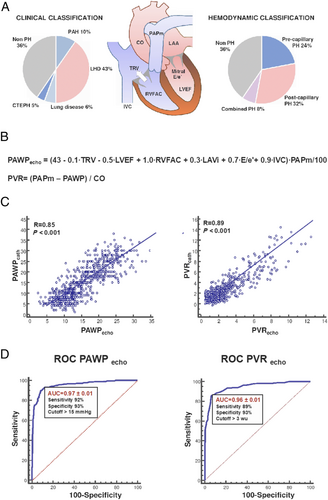
At RHC, precapillary, isolated postcapillary, and combined PH were found in 192 (24.2%), 248 (31.2%), and 67 (8.4%) patients, respectively (Table 2 and Figure 1). Patients with postcapillary and combined PH had worse LV systolic function, as well as higher LV filling pressures, compared with patients with precapillary PH (all P < 0.05, Table 2). Patients with precapillary PH had the highest TRV values, while patients with combined PH showed the worst profile in terms of RV pressure overload and RV systolic function (all P < 0.05, Table 2). Likewise, patients with combined PH had the highest values of NT-proBNP and hs-cTnT and the lowest estimated glomerular filtration rate (all P < 0.05, Table 2).
| Variables | Precapillary PH (n = 192) | Postcapillary PH (n = 248) | Combined PH (n = 67) |
|---|---|---|---|
| Age (years) | 67.7 ± 13.3 | 69.7 ± 10.6 | 73.5 ± 8.9*,† |
| Men, n (%) | 86 (46.7) | 148 (57.8) | 32 (47.8%) |
| BMI (kg/m2) | 26.5 ± 4.6 | 27.7 ± 5.4* | 26.5 ± 4.8 |
| BSA (m2) | 1.8 ± 0.2 | 1.9 ± 0.2* | 1.8 ± 0.2† |
| Systolic blood pressure (mmHg) | 132.6 ± 21.0 | 132.2 ± 26.9 | 135.2 ± 24.0 |
| Diastolic blood pressure (mmHg) | 74.9 ± 11.7 | 72.4 ± 12.8* | 72.2 ± 12.5* |
| Heart rate (b.p.m.) | 74.4 ± 13.4 | 76.3 ± 16.3 | 77.8 ± 16.6 |
| Sinus rhythm, n (%) | 154 (83.7) | 129 (50.4) | 27 (40.3%) |
| Atrial fibrillation, n (%) | 30 (16.3) | 95 (37.1) | 27 (40.3%) |
| Paced rhythm, n (%) | 0 (0) | 32 (12.5) | 13 (19.4%) |
| eGFR (mL/min/1.73 m2) | 73.0 ± 28.1 | 70.4 ± 27.8 | 61.5 ± 21.6* |
| NT-proBNP (ng/L) | 717 (210–2951.5) | 1964 (989–4671)* | 3884 (1384–8090)*,† |
| hs-cTnT (ng/L) | 16.1 (8.8–30.3) | 23.8 (15.1–44.3)* | 29.3 (17.9–50.8)*,† |
| Echocardiographic data | |||
| LVEF (%) | 63.7 ± 10.0 | 40.8 ± 17.2* | 44.3 ± 18.6* |
| LVEDV index (mL/m2) | 52.9 ± 14.9 | 89.5 ± 34.8* | 77.7 ± 32.8* |
| LVESV index (mL/m2) | 19.9 ± 10.6 | 57.4 ± 35.0* | 48.5 ± 33.2* |
| LVM index (g/m2) | 85.4 ± 24.7 | 129.1 ± 38.2* | 118.0 ± 36.3* |
| LVEDD (mm) | 45.4 ± 5.8 | 57.8 ± 10.2* | 53.1 ± 10.8* |
| LVESD (mm) | 29.4 ± 6.2 | 46.3 ± 13.0* | 41.6 ± 13.8* |
| MR grade (mild/moderate/severe) | 114 (62.0)/28 (15.2)/1 (0.5) | 47 (18.4)/153 (59.8)/49 (19.1) | 17 (25.4)/37 (55.2)/12 (17.9) |
| Mitral E/A ratio | 0.9 ± 0.5 | 2.0 ± 1.1* | 2.1 ± 1.1* |
| Mitral E/e′ ratio | 10.2 ± 5.1 | 18.6 ± 10.4* | 20.1 ± 10.8* |
| LAVi (mL/m2) | 31.1 ± 8.9 | 50.0 ± 14.9* | 50.0 ± 14.8* |
| PREDG (mmHg) | 13.6 ± 5.6 | 9.6 ± 3.6* | 13.1 ± 5.1† |
| RVFAC (%) | 30.1 ± 7.6 | 37.0 ± 9.0* | 29.7 ± 10.1* |
| TAPSE (mm) | 19.1 ± 5.6 | 18.0 ± 4.9* | 15.4 ± 4.3*,† |
| TR grade, n (%) | 17 (9.2) | 25 (9.6) | 15 (22.4)*,† |
| TRV (cm/m2) | 388.5 ± 58.2 | 320.4 ± 38.1* | 363.9 ± 69.6† |
| RAP (mmHg) | 6.1 ± 2.9 | 10.1 ± 3.0* | 11.3 ± 2.9* |
| IVC diameter (mm) | 18.6 ± 4.7 | 22.3 ± 5.1* | 23.2 ± 4.2* |
| Right heart catheterization | |||
| Cardiac output (L/min) | 5.0 ± 1.3 | 5.0 ± 1.4 | 4.0 ± 1.0*,† |
| Systolic PAP (mmHg) | 59.7 ± 16.2 | 50.2 ± 9.3* | 65.2 ± 13.4† |
| Mean PAP (mmHg) | 35.8 ± 9.0 | 31.8 ± 5.0* | 41.3 ± 8.2† |
| Diastolic PAP (mmHg) | 23.4 ± 7.1 | 22.2 ± 4.6* | 28.9 ± 7.9† |
| PAWP (mmHg) | 9.4 ± 2.9 | 23.7 ± 4.8* | 23.4 ± 4.7* |
| RAP (mmHg) | 5.9 ± 3.8 | 10.6 ± 4.6* | 12.1 ± 5.2* |
| PVR (WU) | 5.9 ± 3.3 | 1.7 ± 0.7* | 4.6 ± 1.5*,† |
- BMI, body mass index; BSA, body surface area; eGFR, estimated glomerular filtration rate; hs-cTnT, high-sensitivity cardiac troponin T; IVC, inferior vena cava; LAVi, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVESV, left ventricular end-systolic volume; LVM, left ventricular mass; MR, mitral regurgitation; NT-proBNP, N-terminal fragment of pro-B-type natriuretic peptide; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PREDG, pulmonary regurgitation end-diastolic gradient; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVFAC, right ventricular fractional area change; TR, tricuspid regurgitation; TRV, tricuspid regurgitation velocity; WU, Wood units.
- Data are presented as number and %, mean and standard deviation if normally distributed, or median and inter-quartile range if not normally distributed.
- * P < 0.05 in comparison with the group of precapillary PH.
- † P < 0.05 in comparison with the group of postcapillary PH.
Non-invasive echo-Doppler algorithm for simultaneous pulmonary artery wedge pressure and pulmonary vascular resistance estimation in the derivation group
The echocardiographic, haemodynamic, and laboratory characteristics of the derivation cohort (n = 200) are reported in Supporting Information, Table S2.
| Variables | Univariable analysis | Stepwise multivariable analysis | ||||
|---|---|---|---|---|---|---|
| B | P | B unstandardized coefficient | B standardized coefficient | P | VIF | |
| LVEF (%) | −0.71 ± 0.09 | <0.001 | −0.44 ± 0.04 | −0.34 | <0.001 | 1.35 |
| LVEDV index (mL/m2) | 0.18 ± 0.02 | <0.001 | — | — | — | — |
| LVESV index (mL/m2) | 0.19 ± 0.03 | <0.001 | — | — | — | — |
| LVM index (g/m2) | 0.30 ± 0.04 | <0.001 | — | — | — | — |
| MR grade (mild/moderate/severe) | 13.58 ± 2.20 | <0.001 | — | — | — | — |
| Mitral E/A ratio | 12.95 ± 2.37 | <0.001 | — | — | — | — |
| Mitral E/e′ ratio | 0.86 ± 0.18 | <0.001 | 0.54 ± 0.08 | 0.18 | <0.001 | 1.15 |
| LAVi (mL/m2) | 0.83 ± 0.11 | <0.001 | 0.30 ± 0.05 | 0.19 | <0.001 | 1.42 |
| PREDG (mmHg) | −1.99 ± 0.31 | <0.001 | — | — | — | — |
| RVFAC (%) | 0.97 ± 0.17 | <0.001 | 1.01 ± 0.07 | 0.41 | <0.001 | 1.27 |
| TAPSE (mm) | −0.03 ± 0.38 | ns | — | — | — | — |
| TRV (cm/m2) | −0.18 ± 0.02 | <0.001 | −0.09 ± 0.01 | −0.27 | <0.001 | 1.33 |
| IVC diameter (mm) | 1.12 ± 0.23 | <0.001 | 0.78 ± 0.12 | 0.18 | <0.001 | 1.26 |
| Inspiratory collapse of IVC > 50% | −9.94 ± 3.59 | <0.01 | — | — | — | — |
| eGFR (mL/min/1.73 m2) | −0.01 ± 0.07 | ns | — | — | — | — |
| NT-proBNP (ng/L) | 0.01 ± 0.01 | ns | — | — | — | — |
| hs-cTnT (ng/L) | 0.23 ± 0.03 | ns | — | — | — | — |
- eGFR, estimated glomerular filtration rate; IVC, inferior vena cava; LAVi, left atrial volume index; LVEDV, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume index; LVM, left ventricular mass; MR, mitral regurgitation; NT-proBNP, N-terminal fragment of pro-B-type natriuretic peptide; PAPm, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PREDG, pulmonary regurgitation end-diastolic gradient; RHC, right heart catheterization; RVFAC, right ventricular fractional area change; TRV, tricuspid regurgitation velocity; VIF, variance inflation factor.
| Model | R | R2 | Adjusted R2 | SD | Predictors |
|---|---|---|---|---|---|
| 1 | 0.53 | 0.27 | 0.27 | 18.8 | 94 − 0.8 · EF |
| 2 | 0.63 | 0.39 | 0.40 | 17.1 | 133 − 0.6 · EF − 0.2 · TRV |
| 3 | 0.70 | 0.48 | 0.47 | 16.0 | 120 − 0.5 · EF − 0.2 · TRV + 0.9 · E/e′ |
| 4 | 0.74 | 0.55 | 0.54 | 14.9 | 83 − 0.6 · EF − 0.1 · TRV + 0.9 · E/e′ + 0.75 · RVFAC |
| 5 | 0.76 | 0.58 | 0.57 | 14.4 | 52 − 0.5 · EF − 0.1 · TRV + 0.8 · E/e′ + 1.0 · RVFAC + 1.1 · IVC |
| 6 | 0.78 | 0.60 | 0.59 | 14.0 | 43 − 0.5 · EF − 0.1 · TRV + 0.7 E/e′ + 1.0 · RVFAC + 0.9 · IVC + 0.3 · LAVi |
- EF, ejection fraction; IVC, inferior vena cava; LAVi, left atrial volume index; PAPm, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; RVFAC, right ventricular fractional area change; SD, standard deviation; TRV, tricuspid regurgitation velocity.
Considering the number of variables in the equation, a list of simplified regression equations for clinical use, according to the stepwise method, is also provided in Table 4.
The intra-observer and inter-observer agreements for all the parameters included in the equations were satisfactory (all ICCs > 0.9 and >0.8, respectively).
Diagnostic accuracy of the model in the validation group
The echocardiographic model was then tested in the validation cohort (n = 595). The echocardiographic, haemodynamic, and laboratory characteristics of the validation cohort are reported in Supporting Information, Table S3.
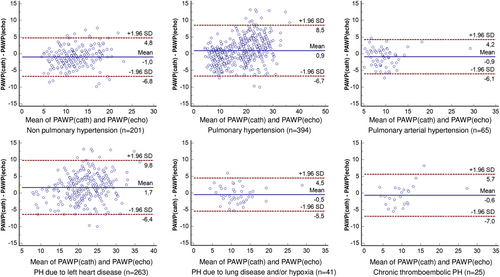
A strong correlation was found between PAWPecho and PAWPcath (R = 0.85, P < 0.001; Table 5 and Figure 1), which was confirmed both in patients with PH (R = 0.84, P < 0.001) and without PH (R = 0.72, P < 0.001). At ROC analysis, PAWPecho accurately predicted PAWPcath > 15 mmHg (AUC = 0.97, optimal cut-off value = 15 mmHg, 92% sensitivity, and 93% specificity, P < 0.001; Figure 1), with the lowest accuracy being observed in patients with chronic thromboembolic PH (AUC 0.90, 100% Sensitivity, 82% Specificity; Supporting Information, Figure S1). The current model showed a higher diagnostic accuracy in identifying LV filling pressure as compared with the 2016 recommendations,9 both including patients with AF (92% sensitivity and 93% specificity vs. 54.5% sensitivity and 87.6% specificity, respectively; P < 0.001) and excluding patients with AF (94% sensitivity and 91% specificity vs. 61.6% sensitivity and 86.9% specificity, respectively; P < 0.001).
| Variable | Echo | RHC | R | P |
|---|---|---|---|---|
| PAPm (mmHg) | ||||
| Overall (n = 595) | 30.2 ± 9.1 | 29.6 ± 9.8 | 0.90 | <0.001 |
| Non-PH (n = 201) | 21.5 ± 4.2 | 19.0 ± 3.4 | 0.67 | <0.001 |
| Pulmonary arterial hypertension (n = 65) | 37.0 ± 11.0 | 36.4 ± 9.1 | 0.89 | <0.001 |
| PH due to left heart disease (n = 263) | 32.9 ± 6.4 | 33.4 ± 6.7 | 0.79 | <0.001 |
| PH due to lung disease and/or hypoxia (n = 41) | 39.7 ± 8.0 | 38.8 ± 7.9 | 0.80 | <0.001 |
| Chronic thromboembolic PH (n = 25) | 40.0 ± 8.0 | 39.5 ± 9.2 | 0.81 | <0.001 |
| PAWP (mmHg) | ||||
| Overall (n = 595) | 15.6 ± 5.9 | 16.4 ± 7.9 | 0.85 | <0.001 |
| Non-PH (n = 201) | 12.5 ± 3.8 | 11.6 ± 4.5 | 0.71 | <0.001 |
| Pulmonary arterial hypertension (n = 65) | 10.7 ± 3.6 | 9.5 ± 3.5 | 0.58 | <0.001 |
| PH due to left heart disease (n = 263) | 19.3 ± 5.2 | 21.8 ± 6.7 | 0.74 | <0.001 |
| PH due to lung disease and/or hypoxia (n = 41) | 12.0 ± 5.4 | 11.2 ± 6.8 | 0.88 | <0.001 |
| Chronic thromboembolic PH (n = 25) | 11.1 ± 4.0 | 10.5 ± 6.1 | 0.71 | <0.001 |
| PVR (WU) | ||||
| Overall (n = 595) | 3.0 ± 2.0 | 2.9 ± 2.4 | 0.89 | <0.001 |
| Non-PH (n = 201) | 1.8 ± 0.8 | 1.6 ± 0.9 | 0.64 | <0.001 |
| Pulmonary arterial hypertension (n = 65) | 5.3 ± 2.7 | 5.5 ± 2.6 | 0.91 | <0.001 |
| PH due to left heart disease (n = 263) | 2.9 ± 1.5 | 2.6 ± 1.8 | 0.80 | <0.001 |
| PH due to lung disease and/or hypoxia (n = 41) | 6.8 ± 2.0 | 6.7 ± 2.6 | 0.81 | 0.002 |
| Chronic thromboembolic PH (n = 25) | 5.9 ± 2.2 | 6.2 ± 3.1 | 0.88 | <0.001 |
- PAPm, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; WU, Wood units.
- Variables are presented as mean and standard deviation if normally distributed or median and inter-quartile range if not normally distributed.
Using Bland–Altman analysis, reasonable limits of agreement were observed between PAWPecho and PAWPcath (Figure 2) in the overall group (bias 0.7, 95% CI −7.3 to 8.7), in patients with PH (bias 0.9, 95% CI −6.7 to 8.5), and in patients without PH (bias −1.0, 95% CI −6.8 to 4.8 mmHg). Satisfactory limits of agreement were also achieved in the different clinical PH groups, with the widest limits being observed in PH due to left heart disease (Figure 2).
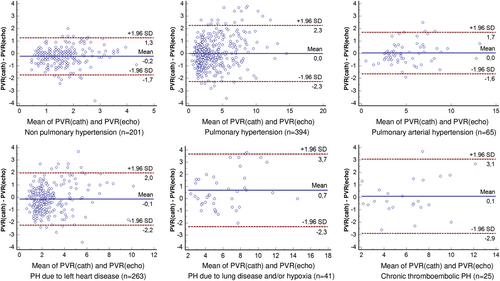
A strong correlation was also found between PVRecho and PVRcath (R = 0.89, P < 0.001; Table 5 and Figure 1), which was also confirmed in patients with PH (R = 0.88, P < 0.001) and without PH (R = 0.64, P < 0.001). At ROC analysis, PVRecho accurately predicted PVRcath > 3 WU (AUC = 0.96, cut-off value = 3 WU, 89% sensitivity, and 92% specificity, P < 0.001; Figure 1). We observed 80% sensitivity and 95% specificity (AUC = 0.95) in the group without PH and 89% sensitivity and 91% specificity (AUC = 0.95) in the group with PH, with lowest accuracy being observed in patients with pulmonary arterial hypertension (AUC = 0.91, 84% sensitivity, and 89% specificity) (Supporting Information, Figure S2). The obtained model showed a higher diagnostic accuracy, when compared with the echocardiographic algorithm developed by Abbas' group for PVR prediction,5 which in our population showed 61% sensitivity and 72% specificity (P < 0.001).
Bland–Altman analysis confirmed satisfactory limits of agreement between PVRecho and PVRcath in the overall population (bias −0.1, 95% CI −2.2 to 1.9), in patients without PH (bias −0.2, 95% CI −1.7 to 1.3), and in patients with PH (bias 0.0, 95% CI −2.3 to 2.3 WU), as well as in different clinical classes of PH (Figure 3).
Subgroup analysis was also performed in the challenging subgroup of patients with AF (n = 142, 23.9% of the validation group). Again, a good correlation was found between PAWPecho and PAWPcath (R = 0.72, P < 0.001), as well as between PVRecho and PVRcath (R = 0.80, P < 0.001). PAWPecho accurately predicted PAWPcath (AUC 0.94, 93% sensitivity, and 88% specificity), and PVRecho accurately predicted PVRcath (AUC 0.90, 91% sensitivity, and 84% specificity) also in patients with AF (Supporting Information, Figure S3). Likewise, a good diagnostic accuracy of PAWPecho and PVRecho was shown in patients with moderate-to-severe MR and paced rhythm (Supporting Information, Figures S4 and S5).
By using the PAWPecho and PVRecho obtained by the current algorithm, patients were significantly better allocated to precapillary PH, isolated postcapillary PH, and combined PH subgroups, compared with a combination of the algorithm proposed by the guidelines and the Abbas equation (all P < 0.001, Table 6).
| RHC | Guidelines + Abbas | Novel algorithm | P value | |
|---|---|---|---|---|
| Indeterminate | 0 | 99 (16.6%) | 0 | <0.001 |
| Precapillary PH | 148 | 48 (32.4%) | 115 (77.7%) | <0.001 |
| Postcapillary PH | 192 | 111 (57.8%) | 167 (87.0%) | <0.001 |
| Combined PH | 54 | 20 (37.0%) | 40 (74.1%) | <0.001 |
- PH, pulmonary hypertension; RHC, right heart catheterization.
The diagnostic accuracies relative to PAWPecho vs. PAWPcath and PVRecho vs. PVRcath according to the simplified equations are reported in Supporting Information, Figure S6.
Discussion
In our study, a novel echocardiographic method for the quantitative estimation of both PAWP and PVR was validated in a large population of patients with and without HF. The robustness of the model, which was completely data driven, was demonstrated against RHC across a wide range of cardiac and pulmonary diseases and regardless of the underlying cardiac rhythm. ROC analysis showed a higher diagnostic accuracy than current international recommendations.5, 9 Despite a satisfactory agreement at Bland–Altman analysis, there was still some imprecision, especially for extreme values. Nonetheless, patients' allocation to precapillary, postcapillary, and combined postcapillary was still correct in most cases and in a larger proportion (improvement in allocation ranging from 30% to 45%) as compared with current recommendations, without indeterminate cases (0% vs. 16%).5, 9 Notably, all variables included in the model should be routinely acquired during a standard echocardiographic examination11 and may be easily incorporated in a reporting system immediately providing PAWP/PVR values for clinical use. Nonetheless, a list of simplified equation with a reasonable diagnostic performance is also provided to balance precision with quickness of execution, depending on the clinical need.
At present, echocardiography is the only non-invasive technique that allows estimation of pulmonary and LV filling pressures in HF/PH. According to PH guidelines, assessment of PVR is necessary to distinguish precapillary from isolated and combined postcapillary PH.1, 2 Nevertheless, PAWP and PVR are not routinely assessed in most echocardiographic laboratories but only in cardiac catheterization laboratories. Drawbacks of echocardiography include inadequate accuracy15-19 and insufficient precision.20 Most of the previous studies have been conducted using a qualitative approach,21-29 while there are only few data on the non-invasive quantitative evaluation of PAWP mainly conducted in small, highly selected populations (e.g. HF with reduced EF, or valve disease, or post-acute myocardial infarction) and usually excluding patients with AF and moderate-to-severe valvular regurgitation.23, 24, 30-39
The 2016 ASE/EACVI recommendations have improved the reliability and clinical utility of LV filling pressure estimation, as compared with the previous 2009 recommendations.14 However, the number of patients classified as indeterminate is not negligible (>15% in the multicentre Euro-Filling study, similar to 16% testing the same algorithm in our study).14 Andersen et al. proposed in patients with cardiac disease a novel echocardiographic algorithm, which showed a high diagnostic accuracy for prediction of LV filling pressure (87% sensitivity and 88% specificity), reducing the number of patients in whom the evaluation was inconclusive (7%). However, a different algorithm was used for patients with severe MR and paced rhythms,40 two challenging conditions for echocardiography, similar to HFpEF or extremely reduced LVEF.41, 42 We analysed a large heterogeneous population: among patients with PH (64%), the large majority had left heart disease (43%) mainly due to HF (including patients with HF with mid-range ejection fraction and HFpEF), but also patients with respiratory diseases were included (21%). Notably, the same algorithm remains highly valuable also in patients with AF and severe MR, without inconclusive cases (0%).
Some of the proposed variables in Equation 2 are already included in the latest ASE/EACVI algorithm (i.e. TRV, average E/e′, and LAVi). IVC size and dynamics are already used for RAP and consequently pulmonary artery pressure estimation.11 LVEF is pivotal when choosing the algorithm for grading of LV diastolic dysfunction from current recommendations,11 and, being load dependent, it may also add precision to the current model.43 RVFAC may reflect the effect of the increased LV filling pressure and/or PVR on the right ventricle, which is even more susceptible to pressure overload.44 Finally, PAPm mathematically encompasses PAWP and TPG, reflecting the steady component of the pulmonary circulation, differently from the pulsatile component of PAPs, which has greater respiratory and individual variability.45 The echocardiographic estimation of PAPm, although not routinely performed in the current practice, is actually advised by the guidelines6 and, in our population, does show a good correlation with PAPm measured at RHC (R = 0.85, P < 0.001). In line with our findings, Barbier et al. showed that an algorithm based on PREDG could accurately predict increased PAWP, with higher accuracy than the ASE/EACVI algorithm, in a cohort of patients with cardiac disease (183 subjects; 117 with an EF < 50%).46 Noteworthy, the model is mathematically independent of mitral regurgitation grade, which influences PAWP,47 but it is challenging to evaluate, particularly in intermediate grade.11 Biomarkers were also excluded by the stepwise regression analysis, similar to previous studies.34, 35
With regard to PVR estimation, Abbas et al. developed an algorithm (a ratio between TRV or TRV2 and velocity–time integral in the pulmonary artery) in a population characterized by precapillary PH (mean PAWP = 13 mmHg) and preserved LVEF (mean LVEF = 58%).5 However, this method may lead to false-positive results in patients with HF with reduced ejection fraction and postcapillary PH, using TRV as a surrogate of TPG. In our population, a modest performance of Abbas' algorithm was indeed found (61% sensitivity and 72% specificity), with a ‘virtual gap’ in PVR values between 3 and 4.5 WU, due to the use of two different formulas.
Study limitations
The feasibility of the proposed method highly depends on image quality and the presence of TR and PR signals. In particular, obtaining complete PR jets can be challenging.12 Using different approaches (such as the RV inflow tract for TR, subcostal view for PR, and left lateral approach for IVC), only 16.3% of patients were excluded because of poor Doppler signal.
The presence of multiple variables in the model might appear time consuming, but the algorithm incorporation in the electronic reporting system or the use of simplified equations may balance accuracy with clinical straightforwardness.
We observed satisfactory limits of agreement between echocardiography and RHC parameters, but considering the confidence limits, a certain imprecision is still present, especially in some subgroups. However, the reclassification capability of the model in identifying PH subtypes is still striking as compared with current recommendations.
We did not use speckle tracking and strain analysis, which showed a potential role in predicting PAWP in preliminary studies.48-50 In particular, left atrial deformation during atrial systole strongly correlates with PAWP at RHC and may be used to calculate both PAWP and PVR with good diagnostic accuracy.50 Nevertheless, the strength of the study is the use of currently established and widely available echocardiographic techniques.
Finally, the results of this monocentric study, albeit promising, are based on internal validation only and should be replicated in larger studies with multicentric design and external validation.
Conclusions
The novel echocardiographic method for quantitative assessment of PAWP and PVR proposed in this study is feasible and provides a reliable estimation of cardiopulmonary haemodynamics in patients with HF and PH, even in the presence of AF. The model accurately identified patients with precapillary, isolated postcapillary, and combined PH, with no cases of undetermination and outperforming current echocardiographic algorithms, by using variables routinely acquired in echocardiographic laboratories. The use of an electronic reporting system with the equation embedded (simplified equation also provided) may warrant in real time, with no delay two relevant parameters as PAWP and PVR for daily clinical use, as follow-up and treatment optimization of patients with either HF or PH.
Conflict of interest
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.




