Frequency and clinical impact of hyperkalaemia within a large, modern, real-world heart failure population
Abstract
Aims
This analysis qualitatively describes the impact of hyperkalaemia (HK) and renin-angiotensin-aldosterone system inhibitor (RAASi) use on clinical outcomes in patients with heart failure (HF).
Methods and results
Patients were included if they were ≥18 years old; had a serum potassium result between 1 January 2003 and 3 December 2018; had ≥2 separate, non-urgent care or emergency department encounters; and had an HF diagnosis. Criteria were met by 52 253 patients; 48 333 had sufficient follow-up for analysis. Patients were stratified by the presence/absence of HK (serum potassium >5.0 mmol/L) (n = 31 619 and n = 20 634, respectively) and by baseline left ventricular ejection fraction (LVEF) ≤40% or >40%. Compared with patients without HK (no-HK), those with HK had significantly higher rates of baseline cardiovascular risk factors, prior diagnoses, and greater RAASi use in both baseline and follow-up periods. Assessed outcomes included RAASi use, rate of 3 year major adverse cardiovascular events (MACE), and individual component rates. Between baseline and follow-up analyses, the proportion of patients on RAASi decreased by 5% in patients with HK but increased by 20% in no-HK patients. Overall, MACE and death were consistently highest in the presence of HK without RAASi treatment (63% and 62%, respectively) and lowest in no-HK but on RAASi (25% and 21%, respectively). After complete multivariable adjustment, these trends were consistent regardless of baseline LVEF.
Conclusions
In this large, real-world HF population, HK was common and linked to baseline clinical risk factors, declining use of RAASi treatment, and an increase in future MACE, regardless of baseline LVEF. Both HK and reduced RAASi use were independent predictors of future MACE.
Introduction
Hyperkalaemia (HK) can result from various acute and chronic conditions affecting potassium homoeostasis. HK commonly occurs with conditions such as chronic kidney disease (CKD) and can be complicated by co-morbidities such as heart failure (HF), diabetes mellitus (DM), and hypertension.1-3 Renin-angiotensin-aldosterone system inhibitors (RAASi's) are common treatments for these co-morbidities and may increase HK risk. HK may lead to potentially lethal arrhythmias or to discontinuation of RAASi's, potentially diminishing their clinical benefits.4
Heart failure patients are particularly susceptible to HK from HF-associated reductions in renal blood flow, as well as the aforementioned confounding co-morbidities and RAASi use. In 2013, an estimated 3.7 million US adults had HK, and prevalence has increased since 2010. In patients with CKD and/or HF, annual prevalence of HK was 6.4% in 2014, and about half of all patients with HK have CKD and/or HF.5
In the CHARM (Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) study, angiotensin-receptor blocker use either alone or in combination with an angiotensin-converting enzyme inhibitor was evaluated for symptomatic HF, including both preserved and decreased left ventricular ejection fraction (LVEF). Risk of HK increased with male gender, age ≥75 years, DM, prior use of angiotensin-converting enzyme inhibitors or spironolactone, baseline serum creatinine ≥176.8 μmol/L, and baseline serum potassium (sK) ≥5.0 mmol/L.6 Although these complications are known to occur, the true frequency and clinical impact of HK on patients with HF in real-world circumstances are not well characterized.
Methods
This was a retrospective, observational database study of Intermountain Healthcare patients. Intermountain Healthcare is a large, integrated health care network consisting of hospital, clinic, and physician networks in association with patients insured by a variety of commercial and governmental insurers, as well as Intermountain Healthcare's independent insurance companies. It provides service for approximately 60% of Utah, as well as parts of Idaho and Nevada.
Patients were included if they (i) were adult (≥18 years of age); (ii) had a nonspurious sK result between 1 January 2003 and 3 December 2018; (iii) had ≥2 non-urgent care or emergency department (ED) encounters at least 2 years apart; and (iv) had a documented HF diagnosis. Patients were categorized as never having any sK >5.0 mmol/L (no-HK group, n = 20 634) or as having at least one sK >5.0 mmol/L (HK group, n = 31 619). Likely spurious results (e.g. haemolysis) were excluded based on sK <2.5 or >10.0 mmol/L, OR sK >5.0 mmol/L AND text documentation of ‘haemolysis’ or ‘red cell lysis’, OR normokalaemia upon a repeat sK within 1 h of an sK >5.0 mmol/L. The index date was defined as the first sK after 1 January 2003 that was ≤5.0 mmol/L if in the no-HK group or the first sK >5.0 mmol/L for the HK group.
Patients were followed up for at least 3 years, and results were reported based on the presence or absence of HK and/or RAASi use and by baseline LVEF stratifications. Compared with the no-HK group, the HK group showed significantly higher rates of cardiovascular (CV) risk factors, prior diagnoses of interest, and RAASi use at baseline. Many of these risk factors were found to interact. These included hypertension with hyperlipidaemia, smoking, and renal failure; and smoking with hyperlipidaemia, diabetes, coronary artery disease, and renal failure. Supporting Information, Table S1 shows predictors of HK. Multivariable Cox regression was used to adjust for differences in baseline clinical variables. Outcomes assessed included RAASi use at baseline vs. follow-up period, rate of future 3 year major adverse CV events (MACE), and rates of individual MACE components, including death, myocardial infarction (MI), stroke, and HF hospitalization (HFH). Death was determined from hospital medical records, state of Utah death certificates, and the social security death master file. We obtained Intermountain international review board approval and a waiver of informed consent before study initiation. Our investigation conforms with the principles outlined in the Declaration of Helsinki.
Statistical analyses
The Student's t-test, analysis of variance, and the χ2 statistic were used to evaluate differences in baseline and clinical characteristics among the groups. Logistic regression was used to determine predictors of HK. The Kaplan–Meier survival estimate and the log-rank test of survival were used to evaluate initial associations of HK and RAASi categories to the endpoints. Multivariable Cox hazard regression analysis (SPSS, version 22.0) was used to evaluate study endpoints. Final models entered significant (P < 0.05) and confounding [10% change in hazard ratio (HR)] covariables. Two-tailed P-values of <0.05 were designated as nominally significant.
Results
A total of 52 253 patients met the HF subgroup criteria, of which 31 619 (61%) had HK and 20 634 (39%) did not have HK. A total of 48 333 patients (HK = 28 662; no-HK = 19 671) had 3 years of follow-up and were included for assessment. Baseline characteristics are presented in Table 1. The length of follow-up for HK patients was 4 ± 4 years vs. the no-HK group, which was 6 ± 5 years (P < 0.001).
| HK n = 31 619 (61%) | No-HK n = 20 634 (39%) | |
|---|---|---|
| Age (years)* | 73 ± 14 | 71 ± 16 |
| % ≥65 years old | 76% | 71% |
| Sex (male)* | 49% | 46% |
| Traditional CV risk factors | ||
| Hypertension* | 90% | 73% |
| Hyperlipidaemia* | 71% | 47% |
| Diabetes mellitus* | 50% | 29% |
| Smoking* | 35% | 24% |
| Renal insufficiency* | 35% | 9% |
| BMI, kg/m2, n = 41 136* | 31 ± 9 | 30 ± 8 |
| LVEF (%), n = 35 167† | 53 ± 19 | 52 ± 16 |
| LVEF ≤40% (n = 8071)* | 5466 (68%) | 2605 (32%) |
| LVEF >40% (n = 23 742)* | 16 217 (68%) | 7525 (32%) |
| Prior diagnoses | ||
| ASCVD* | 49% | 34% |
| CAD* | 20% | 13% |
| MI* | 10% | 7% |
| Stroke* | 4% | 2% |
| TIA* | 8% | 5% |
| PVD* | 2% | 1% |
| Atrial fibrillation* | 42% | 33% |
| Baseline medications | ||
| ACEi* | 57% | 27% |
| ARB* | 26% | 11% |
| Aldosterone inhibitor* | 17% | 4% |
| Any RAASi* | 71% | 35% |
| Follow-up medications | ||
| ACEi* | 47% | 40% |
| ARB* | 24% | 21% |
| Aldosterone inhibitor* | 21% | 12% |
| Any RAASi* | 66% | 55% |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ASCVD, atherosclerotic CV disease; BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; HK, hyperkalaemia; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PVD, peripheral vascular disease; RAASi, renin-angiotensin-aldosterone system inhibitor; TIA, transient ischaemic attack.
- † Ejection fraction taken closest to index (~70% of ejection fractions are taken with ±1 year of index).
- * P < 0.001.
The mean age in the HF subgroup was 73 ± 14 years in HK patients and 71 ± 16 years in the no-HK group (P < 0.001), with approximately 76% in the HK and 71% of patients in the no-HK groups being ≥65 years old. Traditional CV risk factors (e.g. hypertension, hyperlipidaemia, DM, smoking, and renal insufficiency) were significantly more common in HF patients with HK, as were prior CV conditions (e.g. atherosclerotic CV disease, coronary artery disease, MI, stroke, transient ischaemic attack, peripheral vascular disease, and atrial fibrillation). HK was common in the 8071 patients with LVEF ≤40% and in the 23 742 patients with LVEF >40% (in each group, HK: 68% vs. no-HK: 32%).
Rates of 3 year MACE outcomes are shown in Table 2, with frequencies higher in the HK vs. no-HK groups for MACE (47% vs. 35%), death (41% vs. 32%), and HFH (11% vs. 4%). MACE outcomes were also compared across the combined presence or absence of HK and RAASi's (Table 3). A total of 18 870 (39%) had HK and were treated with RAASi's, whereas 9792 (20%) patients had HK but were not on RAASi's. A total of 10 801 (22%) were in the no-HK group but treated with RAASi's; 8870 (18%) patients in the no-HK group were not on RAASi's (no-RAASi). Overall, event frequencies tended to be highest in the HK/no-RAASi group (MACE 63%, death 62%, MI <1%, stroke 1%, HFH 5%) and lowest in the no-HK/RAASi group (MACE 25%, death 21%, MI <1%, stroke 2%, HFH 6%). Compared with the no-HK/no-RAASi group, patients taking RAASi showed a reduced risk of MACE both with HK {adjusted HR = 0.67 [95% confidence interval (CI), 0.64, 0.70], P < 0.001} and without HK [adjusted HR = 0.51 (95% CI, 0.49, 0.54), P < 0.001] (Figure 1). However, patients with HK/no-RAASi had an increased MACE risk [HR = 1.37 (95% CI, 1.31, 1.42), P < 0.001] (Figure 1). There was no collinearity between the diagnosis of HK and any of the other independent predictors of 3 year MACE. The addition of renal insufficiency, as defined by creatinine (men: >123.8 μmol/L; women: >106.1 μmol/L), did not change the results.
| HK n = 28 662 | No-HK n = 19 671 | |
|---|---|---|
| Length of follow-up (years)* | 4 ± 4 (median: 3.2) | 6 ± 5 (median: 4.8) |
| MACE* | 47% | 35% |
| Death* | 41% | 32% |
| MI† | <1% | <1% |
| HFH* | 11% | 4% |
- HFH, heart failure hospitalization; HK, hyperkalaemia; MACE, major adverse cardiovascular events; MI, myocardial infarction.
- * P < 0.001.
- † P < 0.05.
| HK, RAASi | HK, no-RAASi | No-HK, RAASi | No-HK, no-RAASi | |
|---|---|---|---|---|
| HF patients | n = 18 870 | n = 9792 | n = 10 801 | n = 8870 |
| MACE* | 38% | 63% | 25% | 46% |
| Death* | 30% | 62% | 21% | 45% |
| MI* | <1% | <1% | <1% | <1% |
| Stroke* | 2% | 1% | 2% | 1% |
| HFH* | 13% | 5% | 6% | 2% |
| LVEF ≤40† | n = 4179 | n = 1287 | n = 1985 | n = 620 |
| MACE* | 48% | 71% | 32% | 57% |
| Death* | 32% | 69% | 21% | 54% |
| MI | <1% | 0% | <1% | <1% |
| Stroke‡ | 2% | <1% | 2% | 2% |
| HFH* | 25% | 11% | 15% | 8% |
| LVEF >40§ | n = 11 533 | n = 4684 | n = 5071 | n = 2454 |
| MACE* | 34% | 57% | 18% | 31% |
| Death* | 26% | 55% | 14% | 29% |
| MI‡ | <1% | <1% | <1% | <1% |
| Stroke‡ | 2% | 2% | 2% | 2% |
| HFH* | 12% | 6% | 6% | 3% |
- HF, heart failure; HFH, HF hospitalization; HK, hyperkalaemia; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MI, myocardial infarction; RAASi, renin-angiotensin-aldosterone system inhibitor.
- Results are shown overall and stratified by LVEF ≤40% and LVEF >40%.
- * P < 0.001.
- † Multivariable hazard ratios (HRs) for 3 year MACE: no-HK, RAASi vs. no-HK, no-RAASi: HR = 0.53 [95% confidence interval (CI), 0.46, 0.60], P < 0.001; HK, no-RAASi vs. no-HK, no-RAASi: HR = 1.29 (95% CI, 1.14, 1.46), P < 0.001; HK, RAASi vs. no-HK, no-RAASi: HR = 0.74 (95% CI, 0.66, 0.83), P < 0.001.
- ‡ P ≤ 0.05.
- § Multivariable HRs for 3 year MACE: no-HK, RAASi vs. no-HK, no-RAASi: HR = 0.56 (95% CI, 0.51, 0.61), P < 0.001; HK, no-RAASi vs. no-HK, no-RAASi: HR = 2.07 (95% CI, 1.91, 2.25), P < 0.001; HK, RAASi vs. no-HK, no-RAASi: HR = 1.03 (95% CI, 0.95, 1.11), P = not significant.
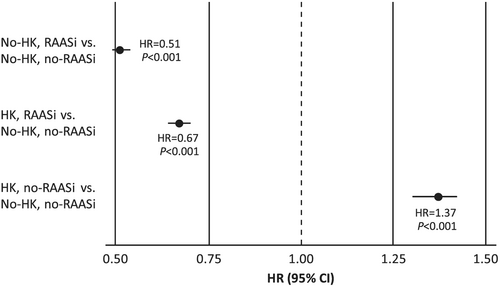
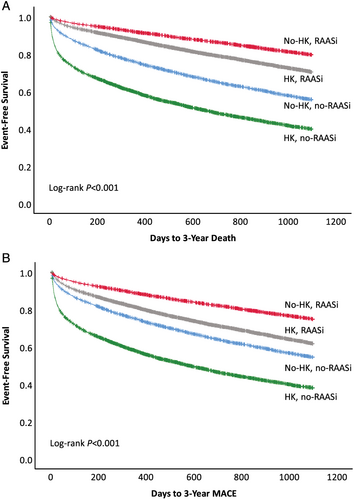
Major adverse CV event frequency by LVEF ≤40% or LVEF >40% is shown in Table 3. Frequency and risk trends in the LVEF ≤40% group were similar to the overall HF population. Among patients with LVEF >40% and compared with the no-HK/no-RAASi group, the no-HK/RAASi patients showed a decreased MACE risk [HR = 0.56 (0.51, 0.61), P < 0.001]; the HK/no-RAASi group showed an increased risk [HR = 2.07 (1.91, 2.25), P < 0.001]; and the HK/RAASi group showed similar risk [HR = 1.03 (0.95, 1.11), P = not significant]. Kaplan–Meier 3 year survival curves for death and MACE show a significant and favourable survival trend in patients on RAASi, regardless of presence or absence of HK, although no-HK is favourable to HK (Figure 2).
A total of 7430 (23.5%) patients had sK >5.5 mmol/L. Of those, 4380 (59.0%) received RAASi therapy. Patients with sK >5.5 mmol/L on RAASi therapy had lower frequencies of 3 year death (34.3% vs. 64.3%, P < 0.001) and MACE (43.0% vs. 65.4%). After adjustment by demographics, risk factors, and other medications, those on RAASi therapy had a decreased risk of 3 year MACE [HR = 0.53 (0.50, 0.57), P < 0.001].
Average total follow-up ED and inpatient annualized costs stratified by HK status are shown in Table 4, with both being significantly higher among the HK patients. The HK group had mean ED costs ($792 ± $4399) more than twice as high as the no-HK group ($312 ± $1555; P < 0.001). The median (interquartile range) costs were $133 (0, 656) and $ <1 (0, 281), respectively. Similarly, the HK group had mean inpatient costs ($22 490 ± $144 224) more than three-fold higher than the no-HK group ($7285 ± $55 595; P < 0.001). The median (interquartile range) costs were $3349 (0, 128 715) and $490 (0, 3765), respectively. A large majority of patients had only one HK-related ED visit and/or inpatient stay annually, as either a primary or any diagnosis (Table 4).
| HK | No-HK | |
|---|---|---|
| ED trends | ||
| Costs per year ($) | ||
| Mean ± SD* | 792 ± 4399 | 312 ± 1555 |
| Median (IQR) | 133 (0, 656) | <1 (0, 281) |
| Visits (any diagnosis), % (n) | ||
| Within Year 1 | 1.2% (373) | ND |
| Frequency ≥2 | 13% (138) | ND |
| Visits (primary diagnosis), % (n) | ||
| Within Year 1 | 0.3% (108) | ND |
| Frequency ≥2 | 10% (28) | ND |
| Inpatient trends | ||
| Costs per year ($) | ||
| Mean ± SD* | 22 490 ± 144 224 | 7285 ± 55 595 |
| Median (IQR) | 3349 (0, 128 715) | 490 (0, 3765) |
| Visits per year (any diagnosis), % (n) | ||
| Within Year 1 | 7.9% (2448) | ND |
| Frequency ≥2 | 30% (1735) | ND |
| Visits per year (primary diagnosis), % (n) | ||
| Within Year 1 | 0.4% (139) | ND |
| Frequency ≥2 | 11% (43) | ND |
- ED, emergency department; HK, hyperkalaemia; IQR, interquartile range; ND, no data; SD, standard deviation.
- * P < 0.001.
Table 4 shows the HK-related (either primary diagnosis or any diagnosis) ED and inpatient visits among HF patients with HK. Cost and utilization data were available for 1 year follow-up for 31 134 patients, illustrating greater than two-fold higher mean ED costs in the HK vs. no-HK groups ($792 and $312, respectively). Similarly, the impact on inpatient costs was three-fold higher ($22 490 vs. $7285, respectively). A majority of visits occurred within the first year after an HK event. When HK was a primary diagnosis, recurrent HK accounted for 10% of ED visits and 11% of inpatient visits. If we considered HK among any diagnosis, repeated visits contributed to 13% of ED visits and 30% of inpatient visits.
Discussion
Among this large, real-world, HF population, HK was common and associated with many baseline CV risk factors expected in this population. The average age was slightly higher in patients with HK compared with those without, but consistent with the average age of 73 years in an acute HF study conducted within the USA [OPTIMIZE (Organized Program to Initiate Life-Saving Treatment in Hospitalized Patients With Heart Failure)].7
In this study, HF patients with HK showed lower follow-up use of RAASi therapies relative to pre-HK baseline rates and higher rates of future MACE regardless of baseline LVEF. Patients in the no-HK/RAASi vs. those in the HK/no-RAASi groups showed the largest disparities in MACE and death risk, as well as survival, favouring RAASi treatment and HK avoidance. In this general HF population, both experiencing an episode of HK and having a reduction in the use of RAASi therapies were found to be independent predictors of future MACE.
Linde et al. investigated real-world associations of RAASi use, HK, and adverse clinical outcomes in a large cohort of RAASi-prescribed patients with new-onset CKD or HF. In both groups, patients with HK were at greater risk of RAASi modification; exposure to lower than guideline-recommended doses had a negative impact on CV outcomes and death. In the HF group, adjusted odds ratios showed increased risk of RAASi down-titration 1.33 (95% CI, 1.08, 1.62), MACE 1.85 (95% CI, 1.71, 1.99), and death 7.34 (95% CI, 6.35, 8.48).8 These results support current study findings and emphasize the need for strategies to maintain RAASi treatment in HF patients.
Further, despite infrequent ED and inpatient encounters, the HK group incurred annual costs two-fold and three-fold higher, respectively, than the no-HK group. According to the Health Care Cost and Utilization Project database in 2011, estimated Medicare total annual charges for HK-specific admissions were $697 million; mean length of stay was 2 to 3 days at an average of $24 085 per day; and one-third of patients were discharged to another short-term hospital or home health care.9, 10 Clinicians may consider such trends when participating in decision-making about RAASi treatment benefits in HF and optimally controlling HK risk.
To further clarify HK management in HF patients on RAASi, DIAMOND (NCT03888066) is an ongoing phase 3b study that will assess CV outcomes in HF patients at risk for HK on guideline-directed RAASi's who receive a novel potassium binder compared with those who have it withdrawn.
As with all observational studies, this study may reflect some residual confounding or bias, which we attempted to minimize with the statistical modelling described in the Methods section. Further, the use of potassium binders was too low to identify treatment-related associations, and we did not assess any non-drug HK management approaches. Because this was a single, geographically limited health-system study, we may not have captured all encounters outside the system. We had no way of ascertaining the chronicity of patients with renal insufficiency. Finally, our study population may not fully reflect patient characteristics from other regions.
Acknowledgements
Editorial support was provided by Lauren Burawski and Impact Communication Partners, Inc. and funded by Relypsa, Inc., a Vifor Pharma Group Company.
Conflict of interest
J.B.M., T.L.B., K.K.K., V.T.L., J.L.A., D.L.L., and H.T.M. report employment by Intermountain Healthcare, which conducted research funded by Relypsa, Inc., a Vifor Pharma Group Company. J.K. reports employment by Relypsa, Inc., a Vifor Pharma Group Company, and stock in Vifor Pharma.
Funding
This study was funded by a research grant from Relypsa, Inc., a Vifor Pharma Group Company, to the Intermountain Medical Center Heart Institute.




