Time-sensitive approach in the management of acute heart failure
Abstract
Acute heart failure (AHF) has become a global public health burden largely because of the associated high morbidity, mortality, and cost. The treatment options for AHF have remained relatively unchanged over the past decades. Historically, clinical congestion alone has been considered the main target for treatment of acute decompensation in patients with AHF; however, this is an oversimplification of the complex pathophysiology. Within the similar clinical presentation of congestion, significant differences in pathophysiological mechanisms exist between the fluid accumulation and redistribution. Tissue hypoperfusion is another vital characteristic of AHF and should be promptly treated with appropriate interventions. In addition, recent clinical trials of novel therapeutic strategies have shown that heart failure management is ‘time sensitive’ and suggested that treatment selection based on individual aetiologies, triggers, and risk factor profiles could lead to better outcomes. In this review, we aim to describe the specifics of the ‘time-sensitive’ approach by the clinical phenotypes, for example, pulmonary/systemic congestion and tissue hypoperfusion, wherein patients are classified based on pathophysiological conditions. This mechanistic classification, in parallel with the comprehensive risk assessment, has become a cornerstone in the management of patients with AHF and thus supports effective decision making by clinicians. We will also highlight how therapeutic modalities should be individualized according to each clinical phenotype.
Introduction
Heart failure (HF) is becoming a global pandemic because of the high incidence rates with significant socio-economic implications.1, 2 In addition, the HF patient population continues to increase in the developed countries—where the population is ageing owing to the increased longevity ensured by improved healthcare systems.3, 4 Primarily, the hospitalization and acute-phase management of HF, especially of acute HF (AHF), presents challenges to healthcare systems while necessitating substantial efforts in achieving favourable outcomes.5, 6
Mainstay of the AHF treatment is medications (e.g. diuretics, vasodilators, and inotropes) and/or devices, such as non-invasive ventilation (NIV) or temporary mechanical circulatory support devices. Various large-scale randomized controlled trials were conducted to test novel treatments; however, none yielded solid evidence in AHF management, while only a few being clinically useful. Given the dearth of quality evidence on acute treatment approaches, there remains a lack of a universally recognized treatment protocol for AHF management. Overall consensus among the experts is that, excluding the variation in regional and societal clinical guidelines, not many changes were added in the recommended AHF management guidelines during the past few decades.7-9
More recently, some of the clinical trials and observational studies demonstrated that HF treatment is time sensitive.10-12 In particular, the early treatment has been effective in improving the clinical outcomes of patients with respiratory failure and/or cardiogenic shock. Thus, time-sensitive treatments based on individual pathophysiology and risk factors are crucial for more efficient and effective clinical management of AHF patients.
Clinical significance of early intervention for acute heart failure
In the management of cardiovascular diseases including acute coronary syndrome (ACS), time and prognosis are directly related. For example, door-to-balloon or door-to-reperfusion time is regarded as an important quality indicator.13, 14 Previous reports have also emphasized the benefits of early intervention in AHF. During the acute phase of haemodynamic deterioration (i.e. within a few hours of hospital arrival), acute kidney injury and/or multiple organ dysfunction syndrome (MODS) may occur, which could lead to poor outcomes. At this point, providing initial treatment may improve pulmonary congestion and restore tissue perfusion.10, 15-19 In addition, the significance of early intervention was confirmed by randomized controlled trials and observational studies to be similar to that in other acute circulatory system diseases (Table 1). Consequently, the clinical practice guidelines embedded recommendations on a time-sensitive approach for the treatment of AHF.7-9
| Author, year (ref. #) | Exposure/intervention | Comparison | Design | Number of patients | Primary outcome | Main findings |
|---|---|---|---|---|---|---|
| Takahashi et al., 201120 | EMS transportation | Observational | 1218 | In-hospital death from all causes | Longer transportation time by emergency medical service (EMS) was associated with increased mortality rates | |
| Plaisance et al., 200710 | Non-invasive ventilation | Early CPAP from scene vs. late CPAP from ambulance | RCT | 124 | Dyspnoea score and ABG | Early CPAP improved respiratory status, and subsequent rates of tracheal intubation and mortality as secondary outcomes |
| Peacock et al., 200911 | Vasoactives (vasodilators and/or inotropes) | Observational | 46 811 | In-hospital death from all causes | Early initiation of vasoactives (as well as vasodilators or inotropes separately) was associated with lower mortality rates and the adjusted odds of death increased 6.8% for every 6 h of treatment delay | |
| Packer et al., 201721 | Ularitide | Placebo | RCT | 2157 | Cardiovascular death during a median follow-up 15 months and a hierarchical composite endpoint during the initial 48 h | No between-group differences were observed with respect to both short-term and long-term outcomes, although the ularitide group showed a greater reduction in NT-proBNP levels than the placebo group |
| Metra et al., 201922 | Serelaxin | Placebo | RCT | 6545 | Cardiovascular death at 180 days and worsening heart failure at 5 days | No between-group differences were observed with respect to both short-term and midterm outcomes as well as renal failure at 180 days and length of hospital stay |
| Maisel et al., 200823 | Diuretics | Observational | 58 465 | In-hospital death from all causes | Delays in diuretics administration was associated with increased mortality | |
| Matsue et al., 201712 | Furosemide | Observational | 1291 | In-hospital death from all causes | Early intravenous administration of furosemide was associated with a lower mortality rate | |
| Park et al., 201824 | Furosemide | Observational | 2761 | In-hospital and post-discharge death from all causes | No differences in both in-hospital and post-discharge mortality rates were observed between the early and late treatment groups | |
| Gul and Bellumkonda, 201925 | IABP | Observational | 193 | 30 day mortality from all causes | Early use of IABP in cardiogenic shock patients was associated with improvement in mortality regardless of aetiology (i.e. ACS or not) | |
| Dangers et al., 201726 | VA-ECMO | Observational | 105 | 1 year mortality from all causes | Early ECMO use prior to the development of multiple organ failure was associated with a better survival rate |
- ABG, arterial blood gas; ACS, acute coronary syndrome; AHF, acute heart failure; CPAP, continuous positive airway pressure; IABP, intra-aortic balloon pump; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RCT, randomized controlled trial; VA-ECMO, venous–arterial extracorporeal membrane oxygenation.
However, applying a ‘one-size-fits-all’ treatment strategy [e.g. shorter door to balloon in ST-segment elevation myocardial infarction (STEMI) patients] for extremely heterogeneous AHF cases is not practically viable. Clinical congestion, considered as the hallmark of AHF, was characterized by the (i) intravascular fluid retention for days to weeks and the (ii) sudden fluid redistribution of effective circulating volume from systemic to pulmonary blood vessels. Previously, most trials and observational studies on AHF included patients with acute pulmonary congestion and/or subacute worsening of peripheral congestion with no dyspnoea while sitting upright at rest but with orthopnoea and dyspnoea on minor exertion.27-30 Conversely, tissue hypoperfusion is a dominant predictor of mortality in AHF, and a target for drug development to improve survival outcomes as well. While numerous studies agree on prognostic impacts of congestion and tissue hypoperfusion, the current therapeutic approaches for them using monotherapies (i.e. medications and device therapies) and each single modality (i.e. chest X-ray, electrocardiogram, and echocardiogram) have been unsatisfactory. Even in ACS patients, outcomes were improved by classifying patients into the STEMI and non-STEMI groups. Thus, we believe that this may be true for AHF patients as well regarding not only their presentation but also their underlying HF phenotype and management.
When considering a ‘time-sensitive’ intervention in AHF, the pathophysiology and potential reversibility of the disease need to be considered. Regardless of left ventricular ejection fraction (EF), AHF does not occur abruptly but rather progresses gradually. A previous study on ambulatory patients with AHF showed that intracardiac pressure/pulmonary artery pressure, measured by implantable haemodynamic monitoring devices, increased gradually a few weeks prior to AHF decompensation, requiring immediate hospitalization.31 However, the patients had various clinical courses and manifestations despite the similarity in the increased filling pressures. Thus, classifying patients appropriately according to the underlying pathophysiology and clinical phenotypes is crucial.
Clinical manifestations and phenotypes of acute heart failure that require early intervention
Clinical practice guidelines and expert opinions proposed to evaluate several phenotypes of AHF to identify patients with a high risk who require tailored treatment approaches. Early patient classification according to individual clinical presentation is also key for determining the appropriate initial treatment. For example, the clinical scenario classification is useful for classifying patients with AHF according to pathophysiological conditions, with reference to the systolic blood pressure (Table 2).32 Such classification facilitates rapid risk stratification of AHF patients, which supports effective clinical decision making. We describe the pathophysiological mechanisms and time-sensitive management of three major patterns of AHF manifestations (pulmonary and/or systemic congestion, and tissue hypoperfusion) in the following sections.
| Clinical characteristics | |
|---|---|
| CS1 |
• Systolic blood pressure >140 mmHg • Symptoms develop abruptly • Predominantly diffuse pulmonary oedema • Minimal systemic oedema (patient may be euvolemic or hypovolemic) • Acute elevation of filling pressure often with preserved ejection fraction • Vascular pathophysiology |
| CS2 |
• Systolic blood pressure 100–140 mmHg • Symptoms develop gradually, together with a gradual increase in body weight • Predominantly systemic oedema • Minimal pulmonary oedema • Chronic elevation of filling pressure, including increased venous pressure and elevated pulmonary arterial pressure • Manifestations of organ dysfunction (renal impairment, liver dysfunction, anaemia, and hypoalbuminemia) |
| CS3 |
• Systolic blood pressure <100 mmHg • Rapid or gradual onset of symptoms • Predominantly signs of hypoperfusion • Minimal systemic and pulmonary oedema • Elevation of filling pressure Two subsets: ⁃ Early stage: no signs/symptoms of hypoperfusion/cardiogenic shock ⁃ Late stage: clear hypoperfusion or cardiogenic shock |
| CS4 |
• Symptoms and signs of acute heart failure • Evidence of acute coronary syndrome • Isolated elevation of cardiac troponin is inadequate for CS4 classification |
| CS5 |
• Rapid or gradual onset • No pulmonary oedema • Right ventricular dysfunction • Sings of systemic venous congestion |
Pulmonary congestion
Pathophysiology and epidemiology
Approximately 80–90% of patients with AHF present with dyspnoea and symptoms of pulmonary congestion on physical and radiographic examinations.33, 34 The severity of pulmonary congestion varies across the clinical profile spectrum of AHF29, 35: acute cardiogenic pulmonary oedema (ACPE; or flash pulmonary oedema), which results from the breakdown of cardiac function and cardiovascular interaction, is the worst phenotype with a prevalence of 10–20% in unselected patients with AHF (Table 3).36-38 A rapid increase in pulmonary capillary hydrostatic pressure (>25 mmHg), leading to interstitial and intraalveolar fluid infiltration in the lungs, results in severe respiratory failure, which worsens with hypercapnia and acidosis.39 Thus, ACPE cases require early and immediate intervention upon presentation. Moreover, a previous study showed a higher mortality rate in ACPE patients than those with ACS-related or hypertensive HF, although signs of pulmonary congestion and elevated blood pressure are common findings at presentation among patients with both ACPE and hypertensive HF,37, 38 which leads to misclassification of AHF patients.
| Clinical criteria (all of them) |
| • Acute respiratory distressa |
| • Physical examinationsb |
| • Orthopnoea |
| • Respiratory failurec |
| Diagnostic confirmation (at least two of the following) |
| • Clear signs of pulmonary congestion on chest radiograph or CT scan |
| • Multiple B-lines on lung ultrasoundd |
| • Elevated pulmonary capillary pressure on catheterization |
| • Increased total lung water on pulse contour and thermodilution analysis system |
| • Signs of elevated filling pressures on echocardiographye |
| • Significant elevation of natriuretic peptidesf |
- CT, computed tomography.
- a Respiratory distress: acute increase in the work of breathing (assessed by single inspection), significant tachypnoea (respiratory rate >25 breaths/min), may be with the use of accessory muscles or abdominal paradox.
- b Crackles ± wheezes over the lungs, third heart sound.
- c Oxygen saturation on room air by pulse oximetry (SpO2) < 90%. Arterial blood gases may be also show PaO2 < 60 mmHg, PaCO2 > 45 mmHg, or PaO2/FiO2 < 300 mmHg.
- d ≥3 B-lines in two chest zones on each hemithorax.
- e E/e′ > 15. Other parameters of elevated left atrial pressure may also be considered.
- f N-terminal pro-B-type natriuretic peptide >900 pg/mL (or >1800 pg/mL in older than 75 years).
Two distinct pathophysiological pathways of the haemodynamic deterioration, including fluid accumulation and redistribution, exist in cases of pulmonary congestion.40 Fluid accumulation advances gradually via renal and dietary mechanisms (slow pathway), while fluid redistribution develops relatively rapidly through direct stimulation of the sympathetic nervous system, inflammation, drugs, or hormones (fast pathway), which leads to systemic vasoconstriction and/or mobilization of venous reservoir where mainly splanchnic vessels that contain anywhere from 20% to 50% of the total blood volume are transiently recruited.41, 42 In patients who have fluid redistribution, haemodynamic deterioration could also be triggered easily by various activities of daily living associated with sympathetic nervous system activation (e.g. exercise) and primarily present with pulmonary congestion.43 A previous hypothesis-generating study highlighted these pathophysiological mechanisms, showing that splanchnic nerve block leads to a significant reduction in intracardiac filling pressure and systemic vascular resistance.44
Time-sensitive management of acute cardiogenic pulmonary oedema
While managing AHF patients with predominant pulmonary congestion, clinicians prioritize to relieve the pulmonary oedema to avoid respiratory complications (Figure 1). The degree of increased left atrial pressure (indicating pulmonary congestion) has been reported to be significantly associated with the time to death in animal model-based studies45; clinical studies also showed that early intervention improves outcomes for patients with ACPE, including the risk of mortality.10, 15, 16, 46-48 Such patients should not be administered a large amount of diuretics in all cases; the key approach to sufficiently reduce the afterload and venous return includes the use of vasodilators and NIV. NIV can dramatically ameliorate respiratory failure without major adverse events, particularly in patients presenting with pulmonary congestion and is used by paramedics in the pre-hospital setting in several countries.49
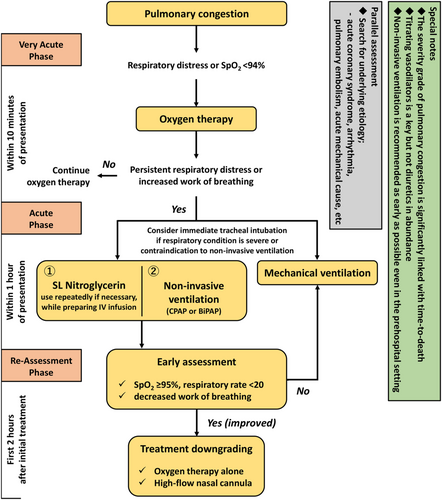
Non-invasive ventilation
Early treatment for pulmonary congestion, such as NIV, is practically relevant to improve oxygenation and haemodynamic instability. Compared with conventional oxygen therapy, NIV improves oxygenation and rapidly reduces the breathing effort,50 thereby leading to a prompt restoration of respiratory function, consequently reducing the risk of tracheal intubation and mortality.51, 52 The Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial was the largest (n = 1069) randomized controlled study comparing the effects of conventional oxygen therapy with NIV in patients with apparent pulmonary congestion. It showed that NIV was associated with a greater improvement in patient-reported dyspnoea, acidosis, and hypercapnia; however, it did not show a positive effect on mortality (9.8% vs. 9.5%; P = 0.87) or intubation rates (2.8% vs. 2.9%; P = 0.90).53 Nonetheless, the shift of the patients of the conventional oxygen therapy group to the NIV therapy group was noteworthy and possibly influenced the results. In addition, the 3CPO trial excluded critically ill patients; thus, both intubation and mortality rates were lower than those in prior studies.54 In addition, the latest meta-analysis including the 3CPO trial, showed that NIV could reduce the intubation rate and the mortality rate in patients with pulmonary congestion and high risk, including those with ACS, respectively.55 Furthermore, in the pre-hospital setting, early NIV initiation reduced the intubation rate and subsequently the mortality rate in patients with dominant pulmonary congestion.10, 15, 16 Specifically, early NIV initiation has been recommended in patients with pulmonary congestion with complications such as acidosis and/or hypercapnia.56 In recent years, high-flow nasal cannula has also been considered to be a potential therapeutic option to restore acute respiratory failure, including AHF, especially in patients at a mild-to-moderate risk without hypercapnia or who downgrade the respiratory support (i.e. from mechanical ventilation/NIV to high-flow nasal cannula).57
Vasodilators
Patients with pulmonary congestion predominantly showing fluid redistribution could benefit from vasodilating agents, such as nitrates or natriuretic peptides, as vasodilators restore ventricular function by reducing afterload and alleviate symptoms by reducing the cardiac filling pressure.46, 58 Previous studies reported that early administration of vasodilators, even in the pre-hospital setting, is associated with a lower morality risk in patients with pulmonary congestion.11, 49, 59 Hence, recent clinical practice guidelines for HF have pragmatically recommended vasodilators as the first-line medical therapy for patients presenting with pulmonary congestion,7-9 despite no confirming evidence on their long-term benefits associated with reduced mortality other than improved haemodynamics and dyspnoea.21, 22, 60, 61 However, hypotension may occur as an adverse effect of vasodilating agents and has been previously associated with unfavourable effects on morbidity and mortality, thereby limiting the beneficial vasodilatory effects.62-64 Therefore, vasodilating agents should be used cautiously in normotensive patients with the risk of hypotension.61, 62, 65 Furthermore, natriuretic peptide agents reduce the blood pressure more than nitrates, making them unsuitable as first-line vasodilators for normotensive patients with AHF.32, 62, 65
Systemic congestion
Pathophysiology and epidemiology
Generally, AHF patients have a gradual onset of HF symptoms, typically over the course of days to weeks. The aforementioned mechanism that leads to systemic congestion through renal and dietary factors possibly contributes to the development of AHF. In fact, increases in filling pressure and sometimes in body weight occur at least 1 week before the onset of acute decompensation.31, 66 A previous report also showed that patients with a higher ejection fraction (EF), renal dysfunction, and higher intracardiac filling pressures at baseline (e.g. central venous pressure and diastolic pulmonary artery pressure) experience acute decompensation earlier.67
Often described as ‘acute-on-chronic heart failure’ (i.e. acute decompensated HF), systemic congestion is the most frequently presented phenotype, accounting for >60% of patients with AHF in observational studies, and is highly heterogeneous and overlaps with pulmonary congestion.37, 38, 68, 69 The prognosis of patients with dominant systemic congestion is comparable with that of patients with other AHF phenotypes, except cardiogenic shock.37, 38 Nevertheless, the prognosis varies considerably depending on several conditions, such as de novo/recently diagnosed HF vs. worsening HF and reduced vs. preserved EF.70-72
Time-sensitive management of systemic congestion
Systemic congestion causes chronically elevated venous pressure, resulting in end-organ dysfunction, such as liver and/or kidney failure. Treating patients with systemic congestion is based on aggressive diuresis, which optimizes the preload condition and afterload adjustment to achieve adequate cardiac output, thereby promoting diuresis (Figure 2). These patients seem to have less time-sensitive features, thereby warranting prompt recognition and standardized goal-directed care, compared with those with ACPE and/or tissue hypoperfusion (i.e. cardiogenic shock).
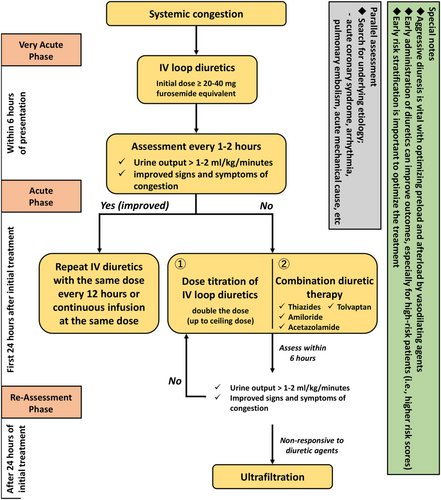
The latest large-scale AHF trial with vasodilators, including an AHF population who had overlapping symptoms with pulmonary and systemic congestion, failed to show favourable effects of the experimental drugs (such as ularitide and serelaxin) with early initiation and dose titration on patient outcomes.21, 22, 61 Thus, in this heterogeneous phenotype, clinicians should identify unique populations to guide the administration of individual therapies with the early treatment strategy. However, based on previous studies, stratification according to systolic blood pressure and natriuretic peptide levels is not enough to identify the population who could benefit from early intervention. Nevertheless, the comprehensive risk prediction tools that incorporate clinical and biomarker parameters could potentially serve as a cornerstone to improve the outcomes of AHF patients by analysing the accurate timing of medical, invasive, or palliative therapies. Other observational studies supported such approaches in identifying patients who could benefit from early intervention.12, 23, 73 In the Acute Decompensated Heart Failure National Registry, early administration of diuretics was associated with a reduced risk of mortality in AHF patients with a high level of B-type natriuretic peptide (BNP) at admission.23 Also, the Registry Focused on Very Early Presentation and Treatment in Emergency Department of Acute Heart Failure Syndrome (REALITY-AHF) showed a positive relationship between early intravenous administration of furosemide and in-hospital mortality in patients hospitalized with AHF after presenting at the emergency department and reported further numerical trends in favour of early treatment in patients with a higher GWTG-HF (Get With The Guideline-Heart Failure) risk score,12 which predicts in-hospital mortality in patients with AHF.74, 75 Natriuretic peptides, such as BNP and N-terminal pro-B-type natriuretic peptide (NT-proBNP), are ideal biomarkers for evaluating AHF that aid in easily identifying the target (high-risk) population in addition to contributing in AHF diagnosis, risk stratification, and treatment response evaluation.
Moreover, the evaluation of AHF patients admitted in the emergency department necessitates comprehensive risk-scoring tools available within the first 1 to 2 h. These should be objective indicators not prone to inter-observer variability/errors. However, to our knowledge, such profiling systems have not been prospectively assessed in randomized studies specifically with therapies that could alter the clinical course of AHF. Some evidence suggests inaccuracy in bedside physical examinations to estimate haemodynamic status, which is used in medical decision making.76, 77 After identifying useful clinical profiles for the heterogeneous AHF population with dominant systemic congestion and establishing appropriate treatment strategies for patient subgroups based on the underlying pathology, new, successful therapies for AHF could be developed (such as the early initiation of sodium–glucose cotransport protein 2 inhibitors to prevent acute kidney injury).
Tissue hypoperfusion
Pathophysiology and epidemiology
Cardiogenic shock is the most critical manifestation of AHF; it is defined as the state involving an ineffective cardiac output due to a primary cardiac disorder, which results in both clinical and biochemical manifestations of inadequate tissue perfusion (Table 4).8, 78, 79 Inadequate circulatory compensation accelerates the development of cardiogenic shock. Nitric oxide-dependent maintenance of vascular tone is regulated through activated inflammatory mediators followed by reciprocal peripheral vasoconstriction.80 The inflammatory response triggered by inadequate tissue perfusion or cell death (as in myocardial infarction), which releases cytokines, leads to inappropriate vasodilation resulting in capillary leakage and microcirculatory dysfunction. This systemic inflammatory response syndrome (SIRS) further affects the tissue perfusion and results in MODS, which could implicitly result in further deterioration without restoring the adequate cardiac output.81 Bleeding and transfusion or haemolysis triggered by mechanical circulatory support could also influence the onset of SIRS.17 In addition to haemodynamic collapse (i.e. haemodynamic cardiogenic shock), other detrimental mechanisms of SIRS and MODS (i.e. hemometabolic cardiogenic shock) are strongly associated with a considerably high mortality rate in patients with cardiogenic shock.17, 78, 82, 83
| Clinical criteria (one of them) |
| • SBP < 90 mmHg with adequate volume |
| • Requiring catecholamines to maintain SBP > 90 mmHg |
| Diagnostic confirmation (at least one of clinical or laboratory findings) |
| • Clinical findings: cold extremities, oliguria (urine output <30 mL/h), mental confusion, narrow pulse pressure |
| • Laboratory findings: metabolic acidosis, elevated serum lactate (>2.0 mmol/L), elevated serum creatinine |
- SBP, systolic blood pressure.
- Haemodynamic information has been regarded as ancillary findings: cardiac index of ≤2.2 L/min/m2 and pulmonary capillary wedge pressure of ≥15 mmHg.
Cardiogenic shock is primarily linked to ACS, with frequencies of approximately 5% in AHF.79, 84, 85 The in-hospital mortality rates of patients with cardiogenic shock range from 27% to 51%, which may be influenced by differences in the disease severity across its profile spectrum.82 Although only a few treatment strategies are based on evidence from randomized controlled trials, there is evidence to show that early revascularization for STEMI improves clinical outcomes, irrespective of the presence of cardiogenic shock78, 86, 87; discussions and detailed reviews of early revascularization for ACS-related cardiogenic shock are presented elsewhere and are beyond the scope of this study. Non-ACS-related cardiogenic shock (e.g. cardiomyopathy, myocarditis, and valvular disease) is reported to account for up to 30% of cardiogenic shock cases.88 Although the prognosis of non-ACS-related cardiogenic shock varies, depending on the severity and/or the underlying aetiology, it remains substantially worse than that of other AHF phenotypes.88, 89
Time-sensitive management of cardiogenic shock
The principal therapeutic goal for cardiogenic shock is restoring tissue perfusion followed by relieving both systemic and pulmonary congestion. A number of studies emphasized on early intervention in patients with tissue hypoperfusion, while time-sensitive management is widely accepted not only for cardiovascular disease management but also in other clinical practice guidelines for emergency medicine.90, 91 Patients with cardiogenic shock have a high mortality rate in the early course of hospitalization (>50% died within 24 h), which suggests the importance of early intervention to restore haemodynamic stability and improve tissue perfusion.18, 19 As non-ACS-related cardiogenic shock is less common relative to ACS-related cardiogenic shock, few evidence-based therapeutic interventions for the former exist. Some patients are treated with disease-specific therapies, including those for arrhythmia, acute mechanical causes such as mitral regurgitation, and pulmonary embolism, while conventional supportive therapies (e.g. inotropes and vasoactive agents) are administered in numerous cases.8 Currently, the standardized and effective treatment strategy for patients with cardiogenic shock, regardless of the aetiology (i.e. ischaemic or non-ischaemic) includes a timely assessment and tailored interventions prior to the development of the aforementioned detrimental cascade constituting shock status (i.e. haemodynamic to hemometabolic derangement) (Figure 3).92 In addition, patients with cardiogenic shock are often complicated by pulmonary congestion, which also requires an early treatment strategy.
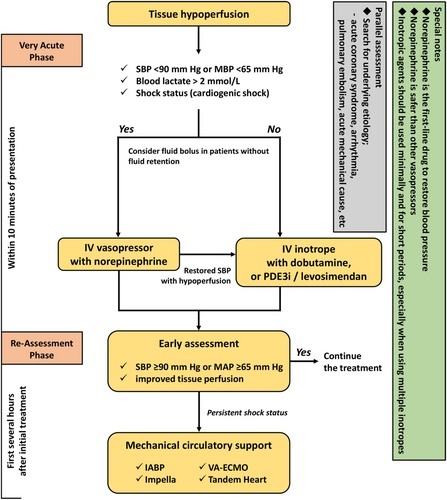
Inotropes and vasopressors
Inotropes and vasopressors are frequently used to restore and maintain sufficient tissue perfusion in patients with cardiogenic shock. Delaying the use of these drugs may increase mortality, per a recent report.11 Thus, they are highly recommended for use during the early phase as necessary. Although the pharmacological suitability for patients with tissue hypoperfusion is not well evidenced, norepinephrine may be recommended as the first-line medication to instantaneously restore the blood pressure. Norepinephrine was reported to be safer than other vasopressors (i.e. dopamine, vasopressin, or epinephrine) and is associated with a lower risk of arrhythmias.93-96 In the Sepsis Occurrence in Acutely Ill Patients II (SOAP II) study, treatment with dopamine was linked to an increased mortality rate and arrhythmia event compared with norepinephrine in patients with AHF.93 However, the trial included patients with various haemodynamic phenotypes of shock (e.g. obstructive, valvular or post-cardiotomy). Further, norepinephrine, at time, negatively affects cardiac function by augmenting systemic afterload and could potentially increase myocardial oxygen consumption.97
For treating patients with a low cardiac output, inotropes (e.g. dobutamine) may improve the stroke volume after achieving adequate perfusion pressure with vasopressors.83 However, some inotropic agents with vasodilation properties, including phosphodiesterase 3 inhibitors and levosimendan, could provide more comparable effects than dobutamine in patients who received beta-blockers or those with a non-ischaemic aetiology of HF; nonetheless, clinicians should pay particular attention to using excessive vasodilation that can cause hypotension and arrhythmias.64, 98, 99 In addition, dopamine is associated with an increased risk of short-term and long-term mortality compared with dobutamine or levosimendan in AHF patients.100 Hence, inotropic agents, in particular when using multiple inotropes,101 should be used minimally and for shorter periods, considering the risk vs. haemodynamic benefit balance.
Mechanical circulatory support
In clinical practice, the intra-aortic balloon pump (IABP) is used most widely as a mechanical circulatory support device for cardiogenic shock cases.102 However, the Intra-aortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial, which enrolled patients with myocardial infarction-related shock, failed to show the effect of the routine use of IABP on the 30 day mortality (primary endpoint) and the 1 year outcomes (secondary endpoint).79 Several years after the IABP-SHOCK II trial, the rate of IABP use has subsequently declined,103, 104 and IABP use was downgraded to a class IIIB recommendation for ACS-related cardiogenic shock in recent clinical practice guidelines in Europe.105, 106 However, the subgroup analysis of the IABP-SHOCK II trial reported benefits of using IABP for younger patient populations with a history of myocardial infarction.79 Further, early initiation of IABP in patients with cardiogenic shock is associated with a significant improvement in 30 day mortality, regardless of the aetiology.25
Venous–arterial extracorporeal membrane oxygenation (VA-ECMO) is used to support cardiovascular and respiratory systems, which establishes a large amount of right-to-left shunt by draining venous blood from the right atrium and returning it after oxygenation to the ascending aorta (central cannulation) or the iliac artery (peripheral cannulation), and strongly improves end-organ perfusion. Although no randomized controlled trials evaluated the effectiveness of VA-ECMO in cardiogenic shock, a meta-analysis of observational studies showed an association of VA-ECMO in cardiogenic shock with reduced 30 day mortality,107 and early initiation (before the onset of MODS) was also related to favourable outcomes in patients with cardiogenic shock.26, 108 Currently, ongoing clinical trials are investigating the use of VA-ECMO in patients with cardiogenic shock (i.e. ECLS-SHOCK, NCT036372205, ANCHOR, NCT03813134, ECMO-CS, and NCT02301819).
Furthermore, currently available mechanical circulatory support devices include the TandemHeart and Impella (i.e. 2.5, CP, and 5.0 systems). In a meta-analysis comparing mechanical circulatory support devices (TandemHeart and Impella) with IABP, the devices were not associated with an improvement in mortality compared with IABP despite their initial beneficial effects on mean arterial pressure and arterial lactate in patients with cardiogenic shock.109 However, several observational studies showed that active Impella placement should be combined with early initiation before the percutaneous coronary intervention, which is related to a reduced mortality rate in patients with ACS-related cardiogenic shock.101, 110, 111 Although sufficiently powered trials of mechanical circulatory support devices are scarce, small explorative randomized controlled trials comparing Impella (2.5 and CP) with IABP for patients with ACS-related cardiogenic shock showed no significant difference in 30 day mortality between the devices, despite some benefit in haemodynamic variables.112, 113 Moreover, a recent large-scale registry data from the USA (n = 28 304) showed that compared with IABP, Impella was associated with a significantly higher risk of in-hospital mortality and bleeding in cardiogenic shock cases related to acute myocardial infarction.114 Because these results were derived from small-scale trials and observational studies with limitations by selection bias and confounding, a sophisticated designed clinical trial is warranted to investigate the effective strategy with the use of Impella in cardiogenic shock cases (i.e. DanGer and NCT01633502). Also, combinations of mechanical circulatory support devices (i.e. VA-ECMO and Impella or VA-ECMO and IABP) could be more effective than when used individually.115, 116 Nonetheless, as several unsolved problems regarding the timing, device, subject, and approach (e.g. central or peripheral cannulation; transfemoral, brachial, or subclavian approach; and choice of left ventricular venting) exist,17, 117 further research and evidence are needed to appropriately select a targeted population to optimize outcomes, efficiently allocate resources, and avoid medical futility.
Pulmonary artery catheter
Prompt interventions to improve outcomes of patients with cardiogenic shock would require many clinical and laboratory measures for haemodynamic monitoring and guiding treatments appropriately.82 A pulmonary artery catheter (PAC), such as Swan–Ganz catheter, is used for haemodynamic assessments among critically ill patients admitted into intensive and/or cardiovascular care units; however, prior large-scale randomized clinical trials and meta-analyses failed to demonstrate the efficacy of PAC-guided treatment on patient outcomes.118-121 In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial, PAC-guided treatment did not affect the overall mortality and hospitalization but increased the in-hospital adverse events (e.g. PAC-related infection and bleeding) in severe AHF cases but not in cardiogenic shock.122 Thus, the current clinical practice guidelines for HF do not recommend the routine use of PAC to monitor haemodynamics and guide treatment.7, 8 However, severe cardiogenic shock cases often complicated by infection/sepsis and right ventricular dysfunction or when patients are unresponsive to initial therapies, PAC can be useful to identify therapy targets by integrating other serial markers reflecting the haemodynamic status.85, 123, 124 Although no clear targeted value for AHF treatment was established, maintaining sufficient tissue perfusion through a comprehensive monitoring system, including PAC, remains clinically significant.
Implications for the design of clinical trials
Clinical phenotyping and absolute risk assessment play important roles in improving the quality of care and subsequently clinical outcomes across a wide spectrum of patients with AHF. The specific phenotypes associated with high mortality rates and severe symptoms, such as ACPE and cardiogenic shock, could be identified at the initial presentation and treated early, resulting in improved outcomes. However, in most patients with a gradual onset of symptoms, except those with ACS-related AHF, the effect of the time-sensitive approach remains to be clarified. Hence, early interventions may influence the outcomes considerably for high-risk AHF patients without an ischaemic episode based on a comprehensive risk assessment; however, to our knowledge, a risk-based approach involving early intervention has not been assessed prospectively and in a randomized manner in the context of designing therapies that alter the natural history of AHF. Further investigations are needed that prospectively confirm the improvement of outcomes for patients with AHF using a risk-based approach.
To timely intervene in patients with AHF, an accurate diagnosis and understanding of the pathology are clinically significant; nonetheless, recognizing haemodynamic derangement in the early phase of decompensation of HF is challenging. After intracardiac devices, such as implantable pulmonary artery pressure monitoring devices, were introduced, we could directly detect an increased intracardiac filling pressure, which enables the treatment of HF decompensation with diuretics and vasodilators at an early phase. Such approaches can improve clinical outcomes of patients with HF, especially HF readmissions.125, 126 Taken together, the use of biomarkers is attractive to indirectly evaluate the pathophysiology and haemodynamics and might aid in identifying patients who may or may not benefit from early intervention and specific drug therapies. A previous study showed that the prognosis is worse in patients with AHF who had an elevated natriuretic peptide level after in-hospital treatment than those with clinically and biologically stabilized status.127 Thus, such high-risk patients should be treated with a tailored management strategy, including aggressive diuretic therapies. Although a recent randomized controlled trial failed to show the benefit from the natriuretic peptide-guided therapy in AHF patients, this study's result should be taken cautiously as a substantial reduction in NT-proBNP levels was observed in both treatment arms and those who were responsive to treatment had superior outcomes to non-responders irrespective of treatment arms.127
Novel biomarkers and future therapeutics for heart failure
As for precise risk stratification of AHF patients, the use of novel molecular biomarkers has been introduced in the recent years. For example, suppression tumourigenicity 2 (ST2) is a protein member of the interleukin-1 receptor family released under conditions of myocardial and vascular strain, and the soluble component (sST2) leads to promoting adverse cardiac remodelling and tissue fibrosis. The prognostic value of sST2 is known to be significant, irrespective of acute or chronic phases and reduced or preserved EF, and is independent of other prognostic indicators such as BNP or NT-proBNP.128, 129 Disease-modifying drugs for HF, including the renin–angiotensin–aldosterone system inhibitors and beta-blockers, can reduce serum concentration of sST2.130, 131 Further, microRNAs (miRNAs), small non-coding RNA molecules that can interfere with gene expression on a post-transcriptional level by binding to messenger RNA, also provide diagnostic and prognostic values in HF patients.132 Individual miRNAs have different pathological characteristics across various phenotypes of HF (i.e. reduced EF vs. preserved EF).133, 134 Recent investigations have shown that miRNAs are valuable to identify biological pathways involved in remodelling and HF progression135; for example, inhibition of miRNA-21 prevented development of HF with preserved EF and was associated with reduced expression of the anti-apoptotic gene Bcl-2 in rats.136 Targeting miRNAs and trying to interfere with their effects might introduce a new potential therapeutic strategy in the future.
Of several novel biomarkers, adrenomedullin, a peptide hormone with vasodilating properties, plays an important role in the preservation of endothelial integrity, and its increased plasma concentration reflects excessive volume overload.137 Importantly, plasma levels of biologically active adrenomedullin remained high after decongestive therapy in patients with residual clinical congestion, while BNP levels decreased in all patients irrespective of the presence and degree of residual congestion.138 Plasma concentration of biologically active adrenomedullin was also independently associated with patient outcomes across a wide clinical spectrum of patients with HF.138, 139 Soluble CD146, cell surface glycoprotein MUC18, is a protein secreted by the vein wall in response to stretch, and its plasma levels are also known to correlate with the presence and grade of clinical congestive signs but are underinvestigated in the context of clinical outcomes.140, 141 Also, lactate might reflect the haemodynamic derangement and organ injury, which can indicate how the therapeutic approach needs to be tailored for AHF patients. On top of its prognostic ability, elevated lactate levels could identify patients without overt but potential tissue hypoperfusion in patients with AHF.142 Thus, lactate may be a possible therapeutic target and inform therapeutic decisions to use inotropes or mechanical circulatory support devices, but there is no evidence to support this approach so far. There are no trials to tailor the treatment based on specific and clinically reliable markers for congestion or tissue hypoperfusion, and thus, a further investigation through sophisticated strategies with biomarkers (by bioprofiling) should enable us to repeatedly assess precise pathophysiological status is warranted in patients with AHF.
Conclusions
This study provides an opportunity for clinicians and interventionalists to recalibrate their knowledge on the clinical management of AHF and its pertinence and research across the entire disease spectrum. Significant variations in HF practice patterns exist between countries, regions, and even institutions. While a standardized approach for the treatment of various AHF phenotypes is warranted, early interventions with an accurate assessment are crucial for the improvement in clinical outcomes, especially for specific phenotypes such as ACPE and cardiogenic shock that encompass time-sensitive and high-morbidity characteristics. Further stratification is warranted to identify the target population that could benefit from a specific treatment and time-sensitive approach in trial settings and clinical practice, which can significantly improve the treatment for AHF.
Conflict of interest
The authors declare that they have no conflicts of interest. Y.S. is affiliated with an endowed department by Nippon Shinyaku Co., Ltd. and received a research grant from the SECOM Science and Technology Foundation and an honorarium from Otsuka Pharmaceutical Co., Ltd.; S.K. received an unrestricted research grant for the Department of Cardiology, Keio University School of Medicine from Bayer Pharmaceutical and Daiichi Sankyo Co., Ltd. and honoraria from Bristol Myers Squibb and Bayer Pharmaceutical Co., Ltd.; and M.K. received a consulting fee from Nihon Kohden, Inc.
Funding
This project was supported by a Grant-in-Aid for Young Scientists (JSPS KAKENHI; No. 18K15860) and Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI; Nos 25460630, 25460777, 16KK0186, and 16H05215), a grant from Japan Agency for Medical Research and Development (AMED; No. JP17ek0210082), a Grant-in-Aid from the Japanese Ministry of Health, Labour and Welfare (No. H29-Refractory Disease-034), and a Grant-in-Aid for Clinical Research from the Japanese Circulation Society to Yasuyuki Shiraishi (2019).
Author contributions
All authors have performed substantial contributions to conception and design. Y.S., M.K., and S.K. collected and interpreted the data and the main writers of the manuscript. J.N., N.S., and K.F. made important intellectual contributions to the manuscript. All authors read and approved the final manuscript.




