Late gadolinium enhancement for re-worsening left ventricular ejection fraction in patients with dilated cardiomyopathy
Abstract
Aims
This study aimed to evaluate the clinical parameters including late gadolinium enhancement (LGE) of cardiovascular magnetic resonance to predict re-worsening of left ventricular ejection fraction (LVEF) in patients with dilated cardiomyopathy (DCM).
Methods and results
We included 138 patients with recent-onset DCM who had an LVEF <45% and underwent LGE of cardiovascular magnetic resonance imaging at diagnosis and echocardiography at the yearly follow-up [median 6 (4–8.3) years]. Initial LVEF recovery was defined as LVEF increase >10% from baseline, resulting in LVEF ≧45% after treatment. The patients were divided into three groups: (i) improved (n = 83, 60%), defined as those with sustained LVEF ≧45%; (ii) re-worsening (n = 39, 28%), those with >5% decrease and LVEF <45% after the initial LVEF recovery; and (iii) not-improved (n = 16, 12%), those without initial LVEF recovery. The primary endpoint was a composite of hospitalization for heart failure or sudden cardiac death. In baseline, LGE was observed in 70 patients. The LGE area was significantly larger in the re-worsening and not-improved groups than that in the improved group (P < 0.001). Loess curves of long-term LVEF trajectories showed that LVEF in the re-worsening group increased in the first 2 years and slowly declined thereafter. Multivariate logistic regression analysis demonstrated that LGE area [odds ratio (OR) 1.09, 95% confidence interval (CI) 1.03–1.16, P = 0.004], B-type natriuretic peptide (OR 1.49, 95% CI 1.05–2.21, P = 0.030) level at the initial recovery, and LVEF (OR 0.91, 95% CI 0.86–0.97, P = 0.004) at the initial LVEF recovery were independent predictors of re-worsening of LVEF. During a median follow-up of 2273 (interquartile range: 1634–3191) days, the primary endpoint was observed in 31 (22%) patients. Univariate Cox proportional hazards analysis demonstrated that the risk of experiencing the primary event in the re-worsening group was significantly higher (hazard ratio: 4.30, 95% CI 1.63–11.31, P = 0.003) than that in the improved group and was lower than that in the not-improved group (hazard ratio: 0.33, 95% CI 0.15–0.72, P = 0.006).
Conclusions
Re-worsening of LVEF was observed in 28% of patients with recent-onset DCM who showed an initial improvement in LVEF. High LGE burden, higher B-type natriuretic peptide level, and lower LVEF at the initial LVEF recovery were independent predictors of re-worsening of LVEF in patients with DCM. Careful observation is recommended for patients with a high risk for re-worsening of LVEF, even in those with an initial LVEF recovery.
Introduction
Dilated cardiomyopathy (DCM) is defined as the presence of left ventricular (LV) or biventricular dilatation and systolic dysfunction in the absence of abnormal loading conditions or coronary artery disease.1 The prognosis of DCM has improved over time owing to the refinement in evidence-based treatment in the recent decades.2 However, the incidence of death from cardiovascular causes remains high in this population.3
Left ventricular ejection fraction (LVEF) has been a standard and widely used parameter for measuring LV function.4 Approximately 30–40% of patients with DCM may experience LVEF recovery within 2 years of follow-up after optimal medical treatment.5 Furthermore, recovered LVEF is associated with a favourable prognosis.6, 7 However, 7–40% of patients had declined LVEF during the follow-up after the initial recovery, and this re-worsening was associated with a higher risk of cardiac events.8-14 Although this re-worsening in LVEF may have a prognostic value, the exact mechanism of this re-worsening remains unknown.
Cardiovascular magnetic resonance (CMR) imaging can provide information on the myocardium with tissue characterization techniques, such as late gadolinium enhancement (LGE).15 For example, LGE of the mid-wall of the intraventricular septum, which corresponds to irreversible replacement fibrosis, is often present in patients with DCM.16 The presence of LGE is associated with both a higher incidence of mortality and the improvement of LVEF after treatment.16, 17 However, the association between myocardial fibrosis detected with LGE-CMR imaging and re-worsening of LVEF is unclear.
Therefore, this longitudinal observational study aimed to identify clinical variables including LGE-CMR that may predict re-worsening LVEF in patients with recent-onset DCM and to compare the long-term risk of clinical events among patients with different trajectories of LVEF.
Methods
Study population
We retrospectively reviewed the medical records of consecutive patients with recent-onset DCM who underwent LGE-CMR imaging between 2007 and 2014 at our hospital. DCM diagnosis was defined according to the current European Society of Cardiology position statement, as follows: the presence of LV systolic dysfunction (LVEF ≦45%) at baseline echocardiography in the absence of coronary heart disease and abnormal loading conditions.1 All patients underwent coronary angiography or coronary computed tomography to exclude coronary artery disease. We excluded patients with haemodialysis therapy, sarcoidosis, acute myocarditis, peripartum cardiomyopathy, alcoholic cardiomyopathy, amyloidosis, observation period <1 year from baseline, low quality of LGE-CMR findings, and patients who underwent LV restoration procedures during the follow-up. The investigation conformed to the principles outlined in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee on Human Investigation at our institution (Kitasato University Medical Ethics Organization).
Study protocol
Baseline data, including laboratory data, electrocardiography findings, and echocardiography results, were obtained at the time of the initial diagnosis during the period of clinical stability. All patients underwent LGE-CMR imaging at the time of diagnosis. The patients were followed up by cardiology specialists and were placed on guideline-directed medical therapy.18 LV function was measured using echocardiography at 6 months from baseline and every year thereafter or according to clinical indications. Measurements of LV diameter, LV function, and diastolic function based on echocardiography conformed to the recommendation of the American Society of Echocardiography.19, 20 LVEF was calculated according to the modified Simpson's method using biplane images from the apex.
We defined initial LVEF recovery as LVEF ≧45% and a >10% increase after treatment. The cohort of patients were then classified into three groups: (i) the improved group, those with initial LVEF recovery and sustained LVEF ≧45%; (ii) the re-worsening group, those with initial LVEF recovery but with >5% decrease in LVEF and LVEF <45% after initial LVEF recovery; and (iii) the not-improved group, those without initial LVEF recovery at any time point.
Endpoint data were obtained from patient's medical records. The primary endpoint was a composite of hospitalization for heart failure (HF) or sudden cardiac death. The secondary endpoint was a composite of death due to pump failure, the implantation of a ventricular assist device, or sudden cardiac death. Sudden cardiac death was defined as unexpected death either within 1 h of the onset of cardiac symptoms in the absence of progressive cardiac deterioration, during sleep, or within 24 h of last being seen alive, as defined by the American Heart Association.21
Cardiovascular magnetic resonance imaging
Cardiovascular magnetic resonance imaging was performed with a 1.5 T (GE Healthcare, Milwaukee, WI, USA) or 3.0 T (Siemens Healthineers, München, Germany) imaging scanner using a standard protocol. LGE images were acquired 10–15 min after the intravenous injection of 0.2 mmol/kg of gadolinium using a segmented inversion recovery fast gradient echo sequence (1.5 T) or true fast imaging with a steady-state free precession sequence (3.0 T). Images were acquired in short-axis and long-axis or four-chamber planes. Inversion times were optimized to null the myocardium. LGE was considered present if observed in both short-axis and long-axis or four-chamber planes. An experienced operator determined the location and pattern of LGE. The location was classified as septal, anterior, lateral, inferior, apex, or occurring in all locations. The pattern was classified as linear mid-wall, focal, or occurring in multiple patterns. LGE area was defined as the area with a signal intensity >5 standard deviations above the mean signal intensity of remote the myocardium in the same short-axis slice and expressed as a percentage of LV mass using a commercially available software (Ziostation2, Ziosoft, Tokyo, Japan).22
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed data or median (25th and 75th percentiles) for non-normally distributed data. Categorical variables are expressed as the number of patients and proportion of the study population. The baseline characteristics of each group according to the change in LVEF were compared by analysis of variance or the Wilcoxon–Mann–Whitney test for continuous variables and the χ2 test for categorical variables. Clinical variables at the initial LVEF recovery were also compared between the improved group and the re-worsening group. Loess curves were plotted for each group to show the trend of LVEF and LV diastolic diameter (LVDD) over time. The Kaplan–Meier curves and Cox regression analysis were used to evaluate the association between the three categories of LVEF trajectories and events. Bivariate and multivariable logistic regression analyses were performed to evaluate the prognostic factors for re-worsening of LVEF between the improved group and the re-worsening group. Variables with a P value <0.05 in the bivariate analysis were included in the multivariable regression analysis. Multiple imputation (R-package ‘mice’; 20 imputed datasets generated) was used to handle missing values in variables that were included in multivariable models.
All significance testing used two-tailed analysis, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the R software (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
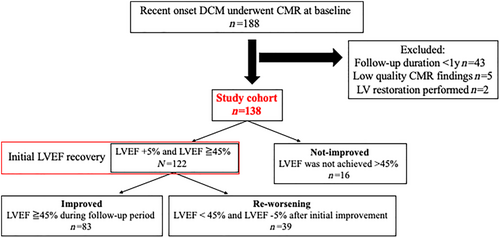
The flow of patients leading to the final cohort is shown in Figure 1. Among the 188 patients who met the inclusion criteria, 50 were excluded according to the aforementioned criteria. Therefore, the remaining 138 patients were analysed. Myocardial biopsy was performed in 124 (90%) patients. Initial recovery of LVEF was observed in 122 (88%) patients. During the 6-year [interquartile range (IQR): 4–8.3] and 7-time (IQR: 5–9) follow-up echocardiography evaluations, re-worsening of LVEF was observed in 39 (28%) patients after the initial recovery. The baseline characteristics are shown in Table 1. No significant differences were observed in age, sex, and B-type natriuretic peptide (BNP) plasma level. The use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, and beta-blockers was not significantly different among the three groups. However, the use of mineralocorticoid receptor antagonists (MRAs) was significantly higher in the re-worsening group and the not-improved group than that in the improved group. LVEF was significantly lower (28.6 ± 7.4% vs. 33.8 ± 7.7%, P < 0.001) and LVDD (63.9 ± 7.9 mm vs. 59.9 ± 9.6 mm, P = 0.009) was significantly larger in patients receiving MRAs than in those not receiving MRAs.
| Missing | All (n = 138) | Improved (n = 83) | Re-worsening (n = 39) | Not-improved (n = 16) | P value | |
|---|---|---|---|---|---|---|
| Age | 0 | 55.7 ± 12.8 | 55.4 ± 12.8 | 57.4 ± 11.8 | 53.4 ± 15.2 | 0.524 |
| Gender, male | 0 | 100 (72.1%) | 60 (72.3%) | 27 (69.2%) | 13 (81.3%) | 0.662 |
| Systolic blood pressure (mmHg) | 0 | 110.8 ±14.4 | 112.8 ± 12.9 | 111.1 ± 15.7 | 100.1 ± 14.5 | 0.005 |
| Heart rate (bpm) | 0 | 72.6 ± 12.7 | 73.8 ± 13.0 | 71.6 ± 11.6 | 69.6 ± 11.7 | 0.384 |
| BNP (pg/mL) | 0 | 158 (76–259) | 141 (56–251) | 200 (100–271) | 270 (89–407) | 0.059 |
| Electrocardiography | ||||||
| Rhythm | 0 | 0.256 | ||||
| Sinus | 113 (81.9%) | 63 (75.9%) | 35 (89.7%) | 15 (93.8%) | ||
| Atrial fibrillation | 24 (18.6%) | 19 (22.9%) | 4 (10.3%) | 1 (6.3%) | ||
| Pacing | 1 (0.7%) | 1 (1.2%) | 0 (0%) | 0 (0%) | ||
| QRS duration (ms) | 0 | 111.2 ± 24.5 | 108.6 ± 23.5 | 116.7 ± 28.0 | 118.3 ± 18.9 | 0.131 |
| LBBB | 0 | 15 (10.9%) | 9 (11.0%) | 4 (10.0%) | 2 (12.5%) | 0.971 |
| Echocardiography | ||||||
| LVEF (%) | 0 | 30.7 ± 7.9 | 30.8 ± 7.5 | 31.0 ± 8.3 | 29.1 ± 9.5 | 0.694 |
| LVDd (mm) | 0 | 62.3 ± 8.8 | 61.3 ± 8.8 | 62.2 ± 8.1 | 67.8 ± 9.1 | 0.024 |
| LVDs (mm) | 0 | 52.7 ± 9.5 | 51.8 ± 9.1 | 51.9 ± 9.1 | 58.7 ± 10.8 | 0.025 |
| LAD (mm) | 1 | 43.3 ± 7.1 | 42.6 ± 7.4 | 44.7 ± 6.0 | 40.8 ± 7.8 | 0.140 |
| E wave (ms) | 21 | 71.0 ± 21.7 | 68.7 ± 21.7 | 77.4 ± 22.0 | 66.5 ± 19.6 | 0.119 |
| E/A | 42 | 1.4 ± 1.0 | 1.3 ± 1.1 | 1.5 ± 0.8 | 1.2 ± 0.7 | 0.722 |
| E′ (ms) | 44 | 5.3 ± 1.7 | 5.4 ± 1.8 | 5.3 ± 1.8 | 5.0 ± 1.4 | 0.836 |
| E/E′ | 29 | 13.8 ± 6.0 | 13.3 ± 5.1 | 14.9 ± 7.9 | 13.2 ± 4.3 | 0.423 |
| MR grade ≧3 | 16 | 33 (27%) | 15 (20.3%) | 10 (29.4%) | 8 (57.1%) | 0.016 |
| TRPG (mmHg) | 14 | 20.5 ± 11.2 | 18.9 ± 11.6 | 24.0 ± 11.1* | 20.9 ± 7.4 | 0.088 |
| Treatment | ||||||
| ACEI/ARB | 0 | 138 (100%) | 83 (100%) | 39 (100%) | 16 (100%) | — |
| Beta-blockers | 0 | 134 (97.1%) | 82 (98.8%) | 36 (92.3%) | 16 (100%) | 0.105 |
| MRA | 0 | 84 (60.9%) | 44 (53.0%) | 28 (75.0%) | 12 (75.0%) | 0.066 |
| Diuretics | 0 | 107 (79.7%) | 64 (81.0%) | 29 (76.3) | 14 (87.5) | 0.627 |
| CRT | 0 | 6 (4.3%) | 4 (4.8%) | 1 (2.6%) | 1 (6.3%) | 0.786 |
| CMR parameter | ||||||
| LGE present | 0 | 70 (50.7%) | 35 (42%) | 22 (56%) | 13 (81.3%) | 0.012 |
| LGE area (%) | 0 | 1.30 (0–9.7) | 0 (0–6.0) | 6.2 (0–11.7)* | 12.3 (3.2–30.5) | <0.001 |
- ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; CRT, cardiac resynchronization therapy; LAD, left atrium diameter; LBBB, left bundle brunch block; LGE, late gadolinium enhancement; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; TRPG, tricuspid regurgitation pressure gradient.
- * P < 0.05 between improved group and re-worsening group.
Late gadolinium enhancement was observed in 70 (50.7%) patients. The LGE area was significantly larger in the re-worsening group and the not-improved group than that in the improved group (P < 0.001). LGE was most frequently located in the septum (53/70, 75%). In the majority of patients (23/35, 65%) in the improved group, LGE was present only in the septum. LGE was also present in the septum in several patients in the re-worsening group (18/22, 82%), few of whom also showed LGE in other locations. The LGE area was significantly larger in patients with multiple patterns than in those with a mid-wall pattern or a focal pattern (P < 0.001). In the improved group, LGE pattern was categorized as a linear mid-wall pattern (49%) or a focal pattern (43%). However, LGE occurring in multiple locations was the predominant feature both in the re-worsening group (55%) and in the not-improved group (85%) (Supporting Information, Figure S1).
Transition of left ventricular ejection fraction
Figure 2 shows the trajectories of LVEF and LVDD among the groups. A marked increase in LVEF was observed within 2 years both in the improved group and in the re-worsening group. However, LVEF gradually declined in the re-worsening group after 2 years. LVDD decreased within 2 years both in the improved group and in the re-worsening group. However, LVDD gradually increased in the re-worsening group after 4 years. The timing of the initial LVEF recovery and re-worsening of LVEF from baseline is shown in Supporting Information, Figure S2. In the most patients, initial LVEF recovery was observed within the first year. No discernible trends in the timing of re-worsening of LVEF were observed.
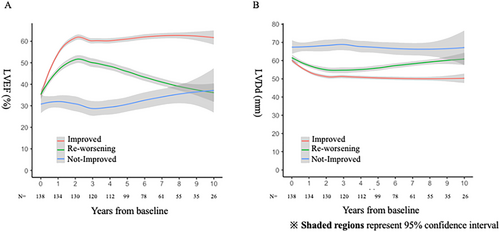
Two patients withdrew from medical therapy, and one patient had new-onset atrial fibrillation, both of which may explain re-worsening of LVEF. However, the majority of patients (36/39, 92%) had re-worsening of LVEF without apparent reasons.
Prognostic variables for re-worsening left ventricular ejection fraction
Table 2 shows the patient characteristics at the initial LVEF recovery in the improved and re-worsening groups. Plasma BNP level was significantly lower and LVEF was significantly higher in the improved group than those in the re-worsening group. There was no significant difference in the treatment approaches for HF between the two groups. Multivariable logistic regression analysis demonstrated that LGE area, plasma BNP level at the initial LVEF recovery, and LVEF at the initial LVEF recovery were independent predictors of re-worsening of LVEF after the initial recovery (Table 3).
| Missing | All (n = 122) | Improved (n = 83) | Re-worsening (n = 39) | P value | |
|---|---|---|---|---|---|
| Timing of initial improvement (years) | 0 | 1.0 ± 1.0 | 1.0 ± 1.1 | 1.0 ± 0.7 | 0.879 |
| BNP (pg/mL) | 0 | 29 (12–76) | 23 (10–57) | 56 (18–141) | 0.010 |
| Echocardiography | |||||
| LVEF (%) | 0 | 55.5 ± 7.6 | 57.1 ± 7.6 | 52.1 ± 6.7 | <0.001 |
| LVDd (mm) | 0 | 54.5 ± 5.8 | 53.4 ± 5.7 | 56.7 ± 5.3 | 0.003 |
| LVDs (mm) | 0 | 38.6 ± 7.0 | 37.2 ± 7.0 | 41.6 ± 5.9 | 0.001 |
| LAD (mm) | 0 | 40.4 ± 6.2 | 40.1 ± 6.5 | 41.2 ± 5.7 | 0.369 |
| E wave (ms) | 6 | 64.1 ± 21.3 | 65.7 ± 22.4 | 60.7 ± 18.7 | 0.244 |
| E/A | 24 | 0.9 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.4 | 0.220 |
| E′ (ms) | 29 | 5.8 ± 2.0 | 6.2 ± 2.0 | 5.1 ± 1.8 | 0.012 |
| E/E′ | 29 | 11.9 ± 5.0 | 11.1 ± 3.8 | 13.5 ± 6.5 | 0.025 |
| MR grade ≧3 | 0 | 4 (4.2%) | 3 (3.6%) | 1 (2.6%) | 1.00 |
| TRPG (mmHg) | 1 | 16.8 ± 10.7 | 16.4 ± 10.2 | 17.5 ± 11.9 | 0.602 |
| Treatment | |||||
| ACEI/ARB | 0 | 124 (100%) | 83 (100%) | 39 (100%) | — |
| Beta-blockers | 0 | 118 (96.7%) | 82 (98.8%) | 36 (92.3%) | 0.096 |
| MRA | 0 | 67 (54.9%) | 44 (53.0%) | 23 (59.0%) | 0.564 |
| Diuretics | 0 | 74 (60.7%) | 51 (61.5%) | 23 (59.0) | 0.844 |
- ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B-type natriuretic peptide; LAD, left atrium diameter; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; TRPG, tricuspid regurgitation pressure gradient.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Age | 1.01 (0.98–1.05) | 0.391 | ||
| Gender, male | 0.86 (0.38–1.98) | 0.729 | ||
| Model 1: Baseline data | ||||
| QRS duration | 1.01 (0.99–1.03) | 0.103 | ||
| LBBB | 0.94 (0.27–3.26) | 0.922 | ||
| Log BNP | 1.50 (0.97–2.32) | 0.055 | ||
| LGE present | 1.77 (0.82–3.83) | 0.143 | ||
| LGE area | 1.09 (1.03–1.16) | <0.001 | 1.09 (1.03–1.16) | 0.004 |
| LVEF | 1.00 (0.95–1.05) | 0.926 | ||
| LAD | 1.04 (0.99–1.10) | 0.134 | ||
| E wave | 1.02 (0.99–1.04) | 0.058 | ||
| E/e′ | 1.04 (0.97–1.12) | 0.225 | ||
| TRPG | 1.04 (1.00–1.08) | 0.031 | 1.04 (0.99–1.08) | 0.056 |
| MR grade ≧3 | 1.64 (0.65–4.15) | 0.298 | ||
| Beta-blockers | 0.15 (0.01–1.46) | 0.101 | ||
| MRA | 2.26 (0.99–5.12) | 0.052 | ||
| CRT | 0.52 (0.06–4.81) | 0.564 | ||
| Model 2: Data at initial LVEF recovery | ||||
| Log BNP | 1.52 (1.10–2.08) | 0.008 | 1.49 (1.05–2.21) | 0.030 |
| LVEF | 0.91 (0.86–0.96) | <0.001 | 0.91 (0.86–0.97) | 0.004 |
| LAD | 1.03 (0.97–1.09) | 0.224 | ||
| E wave | 0.99 (0.97–1.01) | 0.110 | ||
| E/e′ | 1.10 (1.01–1.21) | 0.026 | 1.03 (0.94–1.13) | 0.504 |
| TRPG | 1.01 (0.97–1.05) | 0.597 | ||
| MR grade ≧3 | 0.70 (0.07–6.97) | 0.762 | ||
| Beta-blockers | 0.15 (0.01–1.46) | 0.101 | ||
| MRA | 1.27 (0.59–2.75) | 0.536 | ||
| CRT | 1.86 (0.53–6.52) | 0.331 | ||
- BNP, B-type natriuretic peptide; CI, confidence interval; CRT, cardiac resynchronization therapy; LAD, left atrium diameter; LBBB, left bundle brunch block; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; TRPG, tricuspid regurgitation pressure gradient.
Cardiac events and transition of left ventricular ejection fraction
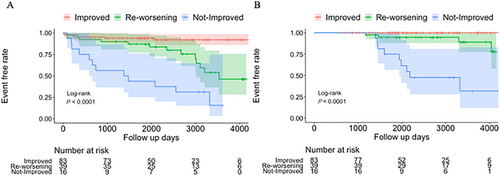
During a median follow-up of 2273 (IQR: 1634–3191) days, the primary endpoint was observed in 31 (22%) patients (hospitalization for HF, 29; sudden death, 2). Figure 3 shows the Kaplan–Meier curves of the primary and secondary endpoints among the three groups. Univariate Cox proportional hazards analysis demonstrated that the risk of experiencing the primary event in the re-worsening group was significantly higher [hazard ratio: 4.30, 95% confidence interval (CI) 1.63–11.31, P = 0.003] than that in the improved group and was lower than that in the not-improved group (hazard ratio: 0.33, 95% CI 0.15–0.72, P = 0.006). In the re-worsening group, one patient experienced the primary event before the initial LVEF recovery, four patients experienced the event concurrently with re-worsening of LVEF, and the remaining eight patients experienced the event 1239 days (IQR: 722–1687) after re-worsening of LVEF. Although most events were observed after 5 years from baseline in the re-worsening group, the primary events in the not-improved group were earlier and more frequently observed than those in the other groups. The secondary endpoints were observed in 13 (10%) patients (three sudden deaths, seven deaths due to HF, and three implantations of a ventricular assist device). The secondary endpoints were not observed in the improved group, whereas these endpoints were observed in four patients in the re-worsening group and in nine patients in the not-improved group. The median duration between re-worsening of LVEF and the occurrence of secondary endpoint was 1880 days (IQR: 534–2830). The event risk of the secondary endpoint was lower in the re-worsening group than that in the not-improved group (hazard ratio: 0.13, 95% CI 0.04–0.44, P = 0.001).
Discussion
Our study demonstrated the following findings: (i) re-worsening of LVEF was observed in 28% of patients with DCM who experienced initial LVEF recovery after treatment; (ii) the larger LGE area, higher BNP plasma level, and lower LVEF at the initial recovery of LVEF independently predicted re-worsening of LVEF; and (iii) event risks in patients with re-worsening LVEF were higher than those in patients with sustained improved LVEF but lower than those in patients without LVEF improvement after treatment.
Late gadolinium enhancement and re-worsening of left ventricular ejection fraction
Late gadolinium enhancement of cardiovascular magnetic resonance visualizes myocardial damage and fibrosis.17 In patients with recent-onset DCM, LGE area was reported to be correlated with the lack of improvement in LVEF within 2 years of treatment.17, 23 Our study adds to this prior finding by demonstrating that LGE area was associated with not only the lack of improvement in LVEF but also re-worsening of LVEF. A damaged myocardium may temporarily respond to treatment for HF but could worsen again despite continuing treatment with longitudinal observation. Therefore, careful observation and intensive therapies may be needed in patients with a high-burden LGE area despite demonstrating an initial improvement in LVEF.
Conversely, the presence of LGE was not a significant predictor for re-worsening of LVEF in the present study. The presence of LGE has been demonstrated as an independent predictor of mortality or HF exacerbation.16, 24 However, the utility of LGE in predicting LVEF improvement remains poorly understood with contradicting studies.25 The degree of myocardial damage is associated with myocardial function including LVEF; however, the presence of LGE alone cannot accurately characterize the degree of myocardial damage. Therefore, quantifying the burden of LGE, rather than binary categorization of its presence or absence, may be valuable in predicting the trajectories of LVEF.
Late gadolinium enhancement in the middle layer of the septum is considered as a typical pattern in patients with DCM.26 Moreover, other heterogeneous patterns of LGE, such as focal and multiple patterns, had also been recognized.27, 28 The differences in LGE patterns may be due to the differences in etiological substrates.27 In the present study, we excluded other types of cardiomyopathy to a reasonable extent, using clinical information including CMR and myocardial biopsy results. However, genetic testing was not performed in our cohort. Therefore, the association between the genetic substrate and LGE patterns remains unclear. We demonstrated that the presence of multiple patterns of LGE was predominant in the re-worsening and not-improved groups. Because the LGE area was also larger in patients with multiple patterns of LGE than in those with other patterns, it remains unclear whether the LGE burden or LGE pattern was associated with re-worsening of LGE. Further studies are needed to investigate the clinical significance of LGE pattern in patients with DCM.
Clinical parameters at the initial left ventricular ejection fraction recovery and re-worsening of left ventricular ejection fraction
We demonstrated that plasma BNP level and LVEF at the time of the initial LVEF recovery were independently associated with re-worsening of LVEF. BNP and amino-terminal pro-BNP are released from the myocardium in response to haemodynamic stress and decrease with increasing LVEF.29 BNP provides prognostic information even in a stable phase after pharmacotherapy in patients with non-ischaemic DCM.30 In the present study, the plasma BNP level decreased at the LVEF recovery from baseline both in the improved group [141 (56–251) pg/dL to 23 (10–57) pg/dL] and in the re-worsening group [200 (100–271) pg/dL to 56 (18–141) pg/dL]. Even in the re-worsening group, the plasma BNP level was relatively low near the reference value, although it was associated with re-worsening of LVEF independent of the LVEF at the initial recovery. Haemodynamic stress indicated by a high BNP level might be related to long-term LVEF decline.
Left ventricular ejection fraction was lower in the re-worsening group than in the improved group. This result is similar to those in previous studies.10, 11 De Groote et al.7, 10 revealed that patients with DCM with an increase of >5% in LVEF and achieving LVEF ≧45% after beta-blocker use had a favourable outcome, whereas the LVEF, after an initial increase, was lower in patients with subsequently declining LVEF during long-term observation than in those who maintained LVEF ≧45%. These observations suggest that the level of LVEF attained after the initial recovery may influence the subsequent trajectories of LVEF. We defined LVEF ≧45% as its improvement because the current European Society of Cardiology statement defined LVEF <45% as systolic dysfunction in patients with DCM.1 Whether the definition of LVEF recovery should be 45%, 50%, or higher is actively debated. Further studies with larger cohorts are needed to rigorously define LVEF improvement.
The use of MRAs was significantly higher in the re-worsening group and the not-improved group than that in the improved group. However, the rates of cardiac events were significantly higher in the re-worsening and not-improved groups than in the improved group. We speculate that baseline status, specifically in LV function, might have been related to the higher incidence of event rates in groups that received a significant number of MRAs. Although the use of MRAs decreases the incidence of cardiovascular events in HF with reduced LVEF in randomized trials, several non-randomized studies indicated that the use of MRAs was neutral or had adverse effects on the incidence of cardiac events.31 One possible reason for this was the severity of LVEF and kidney function with the MRA group in the baseline.32 In fact, LVEF was significantly lower and LVDD was significantly larger in patients receiving MRAs than those in patients not receiving MRAs in the present study.
Clinical implications
The risk of cardiovascular events in the re-worsening group was higher than that in improved group but lower than that in the not-improved group after treatment. These results were similar to those of previous studies.12, 14 Importantly, several cardiac events were observed after 5 years from the initial treatment in the re-worsening group in the present study. The current guideline does not recommend routine echocardiography in patients with HF.33 However, our study suggests that careful long-term observation including echocardiography may be important in patients with high-burden LGE and high BNP level despite LVEF recovery. Additionally, the early detection of re-worsening of LVEF may trigger a more intensive treatment for HF.
Limitations
This was a single-centre retrospective observational study with the following limitations. First, this study comprised a small number of patients in each group according to the change in LVEF, and not all patients were observed systematically during the follow-up period. Second, parametric mapping of CMR was not available. Myocardial T1 mapping is emerging as a method for assessing diffuse myocardial fibrosis that is more sensitive than LGE.34 Therefore, T1 mapping might have an incremental value in predicting re-worsening of LVEF compared with LGE used in isolation. Third, it was difficult to clarify the cause of re-worsening of LVEF in the present study, because information on myocardial tissue characteristics and/or co-morbidities was insufficient when re-worsening of LVEF was observed. Fourth, we did not conduct a genetic study in the present study. Therefore, our study cohort might include a variety of genetic substrates.
Conclusions
Our study showed that substantial myocardial damage detected by LGE-CMR, high plasma BNP level, and low LVEF at the time of initial LVEF recovery were independent predictors for re-worsening of LVEF. Re-worsening of LVEF was observed in 28% of patients with recent-onset DCM who showed an initial improvement in LVEF. Therefore, these findings suggest that careful long-term observations may be warranted in patients with a high risk for re-worsening of LVEF, even in those with an initial LVEF recovery.
Conflict of interest
None declared.
Funding
None.
Author contributions
Takeru Nabeta was the principal investigator and was also responsible for the conception of design, acquisition of data, and data analysis. Kenji Maemura, Takumi Oki, Mayu Yazaki, Yuki, Teppei Fujita, Yuki Ikeda, Shunsuke Ishii, and Takashi Naruke contributed to the acquisition of data. Specifically, Yuki Ikeda and Shunsuke Ishii contributed to drafting the manuscript and critically revising the manuscript for important intellectual content. Takayuki Inomata and Junya Ako contributed to supervising the work and critically revising the manuscript.




