Time-dependent changes in plasma xanthine oxidoreductase during hospitalization of acute heart failure
Abstract
Aims
The aim of present study is to evaluate the clinical significance of the time-dependent changes in xanthine oxidoreductase (XOR) activity during hospitalization for acute heart failure (AHF).
Methods and results
A total of 229 AHF patients who visited to emergency room were prospectively enrolled, and 187 patients were analysed. Blood samples were collected within 15 min of admission (Day 1), after 48–72 h (Day 3), and between Days 7 and 21 (Day 14). The AHF patients were divided into two groups according to the XOR activity on Day 1: the high-XOR group (≥100 pmol/h/mL, n = 85) and the low-XOR group (<100 pmol/h/mL, n = 102). The high-XOR patients were assigned to two groups according to the rate of change in XOR from Day 1 to Day 14: the decreased group (≥50% decrease; n = 70) and the non-decreased group (<50% decrease; n = 15). The plasma XOR activity significantly decreased on Days 3 and 14 [23.6 (9.1 to 63.1) pmol/h/mL and 32.5 (10.2 to 87.8) pmol/h/mL, respectively] in comparison with Day 1 [78.5 (16.9 to 340.5) pmol/h/mL]. A Kaplan–Meier curve indicated that the prognosis, including heart failure (HF) events (all-cause death and readmission by HF) within 365 days, was significantly poorer in the low-XOR patients than in the high-XOR patients and was also significantly poorer in the non-decreased group than in the decreased group.
Conclusions
The plasma XOR activity was rapidly decreased by the appropriate treatment of AHF. Although high-XOR activity on admission was not associated with increased HF events in AHF, high-XOR activity that was not sufficiently reduced during appropriate treatment was associated with increased HF events.
Introduction
Two interconvertible forms of xanthine oxidoreductase (XOR), namely, xanthine oxidase (XO) and xanthine dehydrogenase (XDH), are important enzymes for the metabolism of uric acid (UA).1, 2 Through this metabolic system, via XOR, reactive oxygen species (ROS), such as H2O2 and O2−, are generated and induce increases in oxidative stress.2 These by-products lead to cell damage. From this perspective, an excessive increase in XOR activity would lead not only to serum UA elevation but also to adverse outcomes. Otaki et al. reported that high-XOR activity was associated with a poor prognosis in chronic heart failure (HF).3 However, in the setting of acute HF (AHF), the haemodynamic status and tissue oxygenation can drastically change within a short period of time. It might therefore be important to measure the time-dependent changes of XOR activity rather than the absolute value of XOR for the evaluation of the prognostic value.
We evaluated the XOR activity in AHF patients and suggested that the plasma XOR activity was extremely high in patients with severely decompensated AHF.4 Although the main mechanism of increased XOR activity was the occurrence of tissue hypoxia due to the lactate levels, the long-term prognostic impact of XOR activity on AHF has remained unclear. Furthermore, the time-dependent changes in XOR activity during the treatment of AHF have remained obscure. We hypothesized that rather than the XOR activity on admission, the time-dependent changes of XOR activity during hospitalization might have important prognostic value. We therefore predicted that the insufficient reduction of the XOR activity during appropriate treatment would be associated with a poor outcome. In the present study, we first investigated the time-dependent changes in the XOR activity during hospitalization for AHF and then evaluated the prognostic impact of the XOR activity in the AHF cohort.
Methods
Subjects
A total of 229 consecutive AHF patients who visited the emergency room of Nippon Medical School, Chiba Hokusoh Hospital, between December 2016 and December 2018 and in whom the plasma XOR activity was evaluated within 15 min after visiting the emergency room were prospectively enrolled in this study. Among these, 15 patients who were admitted to a general ward and 27 patients whose samples were not collected at all sampling points (Days 1, 3, and 14) due to missing samples or death within 14 days were initially excluded from this study. As a result, a total of 187 AHF patients who were admitted to the intensive care unit (ICU) were enrolled in the present study.
Acute HF is defined as a gradual or rapid change in HF signs and symptoms requiring urgent therapy. HF was diagnosed by the treating physician at the outpatient clinic according to the European Society of Cardiology guidelines for the diagnosis of HF.5 Physicians first considered HF based on the clinical history (i.e. symptoms, functional limitation, prior cardiac disease, risk factors, exacerbating factors, co-morbidities, and drugs), physical examination (i.e. of vital signs, weight and volume status of the heart, lungs, abdomen, and peripheral vascular regions), and initial investigations [i.e. chest radiography, 12-lead electrocardiography, laboratory measurements of troponins, blood urea nitrogen (BUN), creatinine, sodium, potassium, glucose, liver function, and complete blood count]. Furthermore, evaluations of plasma natriuretic peptide and echocardiography were performed to support the diagnosis of HF. Echocardiography assessed the left/right ventricular systolic and diastolic function and ventricular/atrial volume based on the European Society of Cardiology guideline. All enrolled patients were diagnosed with AHF (either new-onset or decompensated chronic HF with symptoms sufficient to warrant hospitalization) in the emergency department according to the aforementioned procedure within 30 min of admission by the treating physician.
Patients requiring any of the following were admitted to the ICU: (i) high-concentration oxygen inhalation (including mechanical support) to treat orthopnoea; (ii) inotrope or mechanical support due to low blood pressure; or (iii) various types of diuretics to improve general or lung oedema. The treatment strategy was chosen by each physician. In all cases, diuretics or vasodilators were administered to treat AHF.
Xanthine oxidoreductase measurements and comparisons
Blood samples were collected from AHF patients within 15 min after visiting the emergency room (Day 1), on hospital Day 3 (48–72 h after admission), and on hospital Day 14 (between Days 7 and 21). The blood samples were centrifuged within 5 min of collection at 4°C and immediately frozen at −80°C until the analysis. A plasma XOR activity assay was performed using a stable isotope-labelled substrate and liquid chromatography–triple quadrupole mass spectrometry (Sanwa Kagaku Kenkyusho Co., Ltd., Mie, Japan).
To remove small molecules, including hypoxanthine, xanthine, and UA, 100 μL of each plasma sample was purified using a Sephadex G25 column. The eluate was then mixed with 16 μmol/L [13C2, 15N2]-xanthine as the substrate and 16 μmol/L NAD+ and 1 μmol/L [13C2, 15N2]-UA as the internal standard in 250 μL Tris buffer (pH 8.5). Each of the mixtures was incubated at 37°C for 90 min, mixed with 500 μL methanol, and centrifuged at 2000× g for 15 min at 4°C. The supernatants transferred to new tubes were evaporated, reconstituted with 150 μL of distilled water, and filtered through an ultrafiltration membrane before undergoing a liquid chromatography–triple quadrupole mass spectrometry analysis using the Nano Space SI-2 LC system (Shiseido, Ltd., Tokyo, Japan) and a TSQ-Quantum TQM spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with an external systems interface. The amount of [13C2, 15N2]-UA produced was quantified using the calibration curve, with the XOR activity expressed as [C2, 15N2]-UA in pmol/h/mL plasma. The lower limit of detection for XOR activity was 6.67 pmol/h/mL, and the upper limit of detection was 6.670 pmol/h/mL; the inter-detection assay coefficients of variation of pooled human plasma activity were 6.5% and 9.1%, respectively.6 The XOR activity was reported with no addition of NAD+; therefore, it was impossible to measure the actual XO activity. The standard reporting for XO is U/mL of plasma (1 U = 1 μmol of UA formed/min), while that for XOR is pmol/h/mL of plasma (600 pmol/h/mL plasma, which equals 10 μU/mL plasma).
The AHF patients were divided into two groups according to the XOR activity on Day 1: the high-XOR group (≥100 pmol/h/mL, n = 102) or the low-XOR group (<100 pmol/h/mL, n = 85). Because the normal range of XOR activity was not established yet, cut-off value (100 pmol/h/mL) of the present study was defined by the reference of the previous reports in patients with AHF, which the median plasma XOR activity was 77.0 and 104.0 pmol/h/mL, respectively.4, 7 Furthermore, 85 high-XOR patients were assigned to two groups according to the rate of change in XOR from Day 1 to Day 14: the decreased group (≥50% decrease, n = 70) and the non-decreased group (<50% decrease, n = 15). Cut-off value of the decreased or non-decreased group was decided by the median value (52%) of the rate of change in XOR from Day 1 to Day 14. We compared the status and vital signs (age, sex, systolic blood pressure, and heart rate), co-morbidities [diabetes mellitus, hypertension, dyslipidaemia, hyperuricaemia, and chronic kidney disease (CKD)], features of AHF [readmission or new onset, orthopnoea, atrial fibrillation, left ventricular ejection fraction (LVEF) upon admission, and aetiology of HF status], arterial blood gas (pH, PCO2, PO2, HCO3−, SaO2, and lactate), laboratory data [sodium, potassium, BUN, creatinine, total bilirubin, UA, haemoglobin, C-reactive protein, brain natriuretic peptide (BNP), adrenaline, noradrenaline, antidiuretic hormone, and aldosterone], history of XOR inhibitor administration, and in-hospital mortality between each groups.
The long-term prognosis, including all-cause death and HF events (all-cause death or readmission by HF) within 365 days, was evaluated. Patients received clinical follow-up examinations at routine outpatient visits. The prognoses of patients being followed up at other institutes were determined through telephone interviews. The prognosis of HF events was evaluated by Kaplan–Meier curve analysis and a Cox regression hazards model.
Statistical analyses
All of the data were statistically analysed using the SPSS 22.0 J software program (SPSS Japan Institute, Tokyo, Japan). All numerical data were expressed as the median and the 25–75% interquartile range, depending on normality. Normality was assessed using the Shapiro–Wilk W-test. The Mann–Whitney U-test was used to compare two groups. The χ2 test was used for comparisons of proportions. The time-dependent difference on Days 1, 3, and 14 was evaluated by a one-way analysis of variance test. P values of <0.05 were considered to indicate statistical significance.
The prognostic value of the plasma XOR activity on Day 1, and the changes in the plasma XOR activity from Days 1 to 14, was assessed using a Cox regression hazards model. A Cox regression analysis was performed to determine the hazard ratio for HF events. All clinically relevant factors affecting the prognosis, including age (per 1 year increase), systolic blood pressure (per 1 mmHg increase), recurrence of HF (yes), CKD (yes), LVEF (per 1% increase), PaO2/FiO2 ratio (per 1 increase), BNP (per 1 pg/mL increase), and lactate (per 1 mg/dL increase), were selected for inclusion in the multivariate Cox regression hazards model to identify factors associated with HF events. The multivariate Cox regression hazards model was performed by the backward stepwise selection. The cumulative survival rates and HF events in each of the two groups were analysed using Kaplan–Meier curves, and the log-rank test was used to calculate the statistical significance of the differences.
Ethical considerations
The research ethics committee of the Chiba Hokusoh Hospital, Nippon Medical School, approved the study protocol. Written informed consent was obtained from all of the participants before commencing the study.
Results
Patient characteristics, prognoses, and differences in the low-xanthine oxidoreductase and high-xanthine oxidoreductase groups
The AHF patient cohort consisted of 120 (64.2%) male patients, and the median age was 76 years. A total of 119 (63.6%) patients had new-onset HF, and 82 (43.9%) had ischaemic heart disease. Most patients (75.9%) had orthopnoea on admission, and the median LVEF upon admission was 34% (Table 1). Patients with hyperuricaemia were significantly more frequent, and the incidence of CKD was significantly higher in the low-XOR group than in the high-XOR group. As a result, the frequency of XOR inhibitor treatment before admission was significantly increased in the low-XOR group in comparison with the high-XOR group. In the low-XOR group, the haemoglobin levels were significantly decreased while the serum BUN levels were significantly increased in comparison with the high-XOR group (Table 1).
| Overall (n = 187) | High-XOR group (n = 85) | Low-XOR group (n = 102) | P value | ||
|---|---|---|---|---|---|
| General status and vital signs | |||||
| Gender | (male, %) | 120 (64.2%) | 58 (68.2) | 62 (60.8%) | 0.358 |
| Age | (years) | 76 (67–82) | 74 (65–81) | 77 (68–83) | 0.102 |
| BMI | (kg/m2) | 23.0 (20.7–25.7) | 22.3 (20.1–25.5) | 23.2 (21.1–25.8) | 0.101 |
| Systolic blood pressure | (mmHg) | 148 (122–176) | 152 (120–176) | 143 (123–176) | 0.668 |
| Heart rate | (b.p.m.) | 100 (86–117) | 110 (92–133) | 98 (84–110) | 0.001 |
| Co-morbidities | |||||
| Hypertension | (yes, %) | 142 (75.9%) | 60 (70.6%) | 82 (80.2%) | 0.126 |
| Dyslipidaemia | (yes, %) | 101 (54.0%) | 41 (48.2%) | 60 (58.8%) | 0.185 |
| Diabetes mellitus | (yes, %) | 80 (42.8%) | 24 (28.2%) | 56 (54.9%) | <0.001 |
| Hyperuricaemia | (yes, %) | 90 (48.1%) | 33 (38.8%) | 57 (55.9%) | 0.027 |
| CKD | (yes, %) | 88 (47.1%) | 32 (37.6%) | 56 (54.9%) | 0.020 |
| Features of AHF | |||||
| New-onset HF | (yes, %) | 119 (63.6%) | 62 (27.1%) | 57 (44.1%) | 0.022 |
| Orthopnoea | (yes, %) | 142 (75.9%) | 67 (78.8%) | 75 (73.5%) | 0.492 |
| Atrial fibrillation | (yes, %) | 50 (26.7%) | 25 (29.4%) | 25 (24.5%) | 0.508 |
| LVEF | (%) | 34 (24–50) | 30 (21–40) | 40 (30–52) | <0.001 |
| Aetiology of AHF | |||||
| Ischaemic HF | (yes, %) | 82 (43.9%) | 30 (35.3%) | 52 (51.0%) | 0.038 |
| Valvular HF | (yes, %) | 36 (19.3%) | 16 (18.8%) | 20 (19.6%) | 1.000 |
| Hypertensive HF | (yes, %) | 21 (11.2%) | 13 (15.3%) | 8 (7.8%) | 0.162 |
| Cardiomyopathy | (yes, %) | 34 (18.2%) | 17 (20.0%) | 17 (16.7%) | 0.704 |
| Laboratory data | |||||
| Sodium | (mEq/L) | 140 (136–143) | 140 (137–143) | 140 (136–143) | 0.565 |
| Potassium | (mEq/L) | 4.4 (3.9–4.7) | 4.4 (4.0–4.7) | 4.3 (3.9–4.7) | 0.986 |
| BUN | (mg/dL) | 25.6 (19.7–43.0) | 24.6 (19.1–32.8) | 29.3 (20.4–48.3) | 0.041 |
| Creatinine | (mg/dL) | 1.22 (0.94–2.07) | 1.17 (0.90–1.57) | 1.30 (0.98–2.31) | 0.067 |
| Total bilirubin | (mg/dL) | 0.7 (0.5–1.1) | 0.8 (0.5–1.2) | 0.7 (0.4–1.1) | 0.143 |
| AST | (U/L) | 38 (26–66) | 60 (39–167) | 29 (20–39) | <0.001 |
| ALT | (U/L) | 25 (16–52) | 49 (25–98) | 18 (11–28) | <0.001 |
| Uric acid | (mg/dL) | 6.8 (5.6–8.0) | 7.2 (6.1–8.9) | 6.4 (5.4–7.5) | 0.001 |
| Haemoglobin | (mg/dL) | 12.1 (10.3–13.9) | 13.0 (10.7–14.6) | 11.3 (9.7–12.9) | <0.001 |
| HbA1c | (%) | 6.1 (5.7–6.8) | 6.0 (5.7–6.6) | 6.3 (5.7–6.9) | 0.194 |
| CRP | (mg/dL) | 0.90 (0.23–3.99) | 0.84 (0.21–2.95) | 0.92 (0.24–5.53) | 0.181 |
| BNP | (pg/mL) | 916 (501–1486) | 964 (530–1511) | 883 (494–1470) | 0.569 |
| Adrenaline | (ng/mL) | 0.23 (0.10–0.42) | 0.30 (0.17–0.82) | 0.18 (0.07–0.34) | 0.003 |
| Noradrenaline | (ng/mL) | 1.4 (0.8–2.4) | 1.9 (1.1–3.2) | 1.1 (0.7–2.2) | 0.008 |
| ADH | (pg/mL) | 10.0 (4.5–33.1) | 13.8 (5.1–36.3) | 9.7 (3.6–23.2) | 0.072 |
| Aldosterone | (ng/mL) | 16.7 (7.8–32.8) | 24.5 (10.2–50.8) | 107 (7.2–25.0) | 0.003 |
| XOR activity | (pmol/h/mL) | 78.5 (16.9–340.5) | 401.0 (189.0–1700.0) | 21.9 (8.4–44.9) | <0.001 |
| Arterial blood gas | |||||
| pH | 7.39 (7.30–7.44) | 7.37 (7.25–7.44) | 7.40 (7.35–7.44) | 0.036 | |
| PO2 | (mmHg) | 103 (79–151) | 100 (79–144) | 107 (77–156) | 0.679 |
| PCO2 | (mmHg) | 37 (31–47) | 38 (31–55) | 37 (32–43) | 0.452 |
| HCO3 | (mEq/L) | 22.0 (19.3–25.4) | 21.3 (18.5–25.0) | 22.0 (19.3–25.4) | 0.052 |
| BE | (mmol/L) | −2.1 (−5.6 to 1.1) | −2.7 (7.2–1.0) | −1.6 (−4.5 to 1.2) | 0.025 |
| Lactate | (mmol/L) | 1.80 (1.10–3.05) | 2.60 (1.50–4.88) | 1.50 (1.00–2.10) | <0.001 |
| Others | |||||
| XOR inhibitors | (yes, %) | 70 (37.4%) | 20 (23.5%) | 50 (49.0%) | <0.001 |
| Febuxostat | (yes, %) | 64 (34.2%) | 19 (22.4%) | 45 (44.1%) | |
| Allopurinol | (yes, %) | 4 (2.1%) | 0 (0.0%) | 4 (3.9%) | |
| Topiroxostat | (yes, %) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| In-hospital mortality | (%) | 17 (9.1%) | 6 (7.1%) | 11 (10.8%) | 0.450 |
- ADH, antidiuretic hormone; AHF, acute heart failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; CRP, C-reactive protein; HbA1c, haemoglobin A1c; HF, heart failure; LVEF, left ventricular ejection fraction measured by echocardiography; XOR, xanthine oxidoreductase.
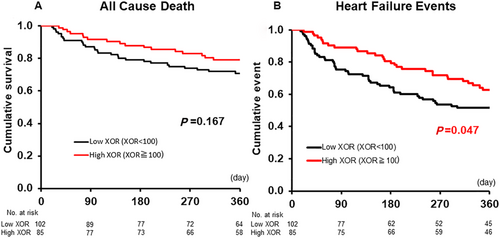
The Kaplan–Meier survival curves, including all-cause death and HF events within 365 days, for XOR activity on admission are shown in Figure 1. Although the all-cause death in the high-XOR group was not statistically different in comparison with the low-XOR group (P = 0.167, Figure 1), the event-free rates in the high-XOR group were significantly lower in comparison with the low-XOR group (P = 0.047, Figure 1).
Time-dependent changes of xanthine oxidoreductase activity and the prognosis
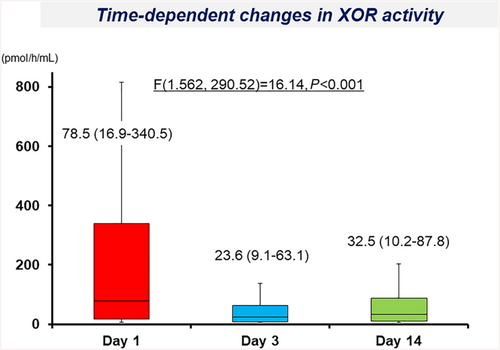
The plasma XOR activity significantly decreased on Day 3 and Day 14 [23.6 (9.1 to 63.1) pmol/h/mL and 32.5 (10.2 to 87.9) pmol/h/mL, respectively] in comparison with the value on Day 1 [78.5 (16.9 to 340.5) pmol/h/mL] (Figure 2).
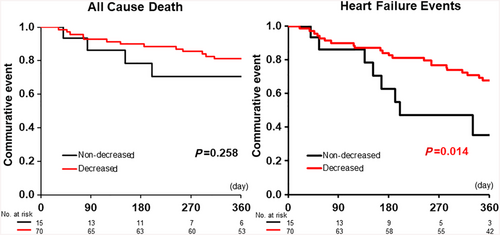
With regard to the clinical findings and patients, on admission, there were almost no significant differences in these parameters between the decreased group and the non-decreased group (Table 2). The Kaplan–Meier curves for the decreased-XOR and non-decreased-XOR groups are shown in Figure 3. The prognosis, including all-cause death within 365 days, was not statistically different between the decreased group and the non-decreased group (P = 0.258, Figure 3). Meanwhile, the prognosis of the decreased group, including HF events within 365 days, was significantly poorer than that of the non-decreased group (P = 0.014, Figure 3). Our multivariate Cox regression model showed that a ≥50% decrease in XOR was an independent predictor of 365 days of HF events (hazard ratio: 0.237; 95% confidence interval: 0.087–0.647, P = 0.005) (Table 3).
| Overall (n = 85) | Decreased group (n = 70) | Non-decreased group (n = 15) | P value | ||
|---|---|---|---|---|---|
| General status and vital signs | |||||
| Gender | (male, %) | 58 (68.2%) | 47 (67.1) | 11 (73.3%) | 0.766 |
| Age | (years) | 74 (65–81) | 74 (67–82) | 72 (51–79) | 0.261 |
| Systolic blood pressure | (mmHg) | 152 (120–176) | 152 (120–176) | 150 (118–177) | 0.953 |
| Heart rate | (b.p.m.) | 110 (92–133) | 110 (91–139) | 110 (99–118) | 0.931 |
| Co-morbidities | |||||
| Hypertension | (yes, %) | 60 (70.6%) | 51 (72.9%) | 9 (60.0%) | 0.357 |
| Dyslipidaemia | (yes, %) | 41 (48.2%) | 35 (50.0%) | 6 (40.0%) | 0.575 |
| Diabetes mellitus | (yes, %) | 24 (28.2%) | 22 (31.4%) | 2 (13.3%) | 0.214 |
| Hyperuricaemia | (yes, %) | 33 (38.8%) | 29 (41.4%) | 4 (26.7%) | 0.386 |
| CKD | (yes, %) | 32 (37.6%) | 24 (34.3%) | 8 (53.3%) | 0.240 |
| Feature of AHF | |||||
| New-onset HF | (yes, %) | 62 (72.9%) | 51 (72.9%) | 11 (73.3%) | 1.000 |
| NYHA IV | (yes, %) | 67 (78.8%) | 58 (82.9%) | 58 (82.9%) | 0.077 |
| Atrial fibrillation | (yes, %) | 25 (29.4%) | 23 (32.9%) | 2 (13.3%) | 0.212 |
| LVEF | (%) | 30 (21–40) | 30 (22–41) | 23 (20–35) | 0.192 |
| Aetiology of AHF | |||||
| Ischaemic HF | (yes, %) | 30 (35.3%) | 25 (35.7%) | 5 (33.3%) | 1.000 |
| Valvular HF | (yes, %) | 16 (18.8%) | 13 (18.6%) | 3 (20.0%) | 1.000 |
| Hypertensive HF | (yes, %) | 13 (15.3%) | 11 (15.7%) | 2 (13.3%) | 1.000 |
| Cardiomyopathy | (yes, %) | 17 (20.0%) | 13 (18.6%) | 4 (26.7%) | 0.487 |
| Laboratory data | |||||
| Sodium | (mEq/L) | 140 (137–143) | 140 (137–143) | 141 (136–144) | 0.972 |
| Potassium | (mEq/L) | 4.4 (4.0–4.7) | 4.4 (4.0–4.8) | 4.1 (4.0–4.8) | 0.171 |
| BUN | (mg/dL) | 24.6 (19.1–32.8) | 23.3 (19.8–32.7) | 23.3 (19.8–32.7) | 0.913 |
| Creatinine | (mg/dL) | 1.17 (0.90–1.57) | 1.16 (0.90–1.56) | 1.35 (0.95–1.72) | 0.430 |
| Total bilirubin | (mg/dL) | 0.8 (0.5–1.2) | 0.8 (0.5–1.4) | 0.6 (0.6–1.0) | 0.803 |
| Uric acid | (mg/dL) | 7.2 (6.1–8.9) | 7.1 (6.0–8.5) | 7.9 (7.0–9.1) | 0.249 |
| Haemoglobin | (mg/dL) | 13.0 (10.7–14.6) | 13.0 (11.1–14.6) | 13.2 (10.2–14.4) | 0.721 |
| CRP | (mg/dL) | 0.84 (0.21–2.95) | 0.87 (0.20–2.91) | 0.64 (0.30–2.36) | 0.972 |
| BNP | (pg/mL) | 964 (530–1511) | 902 (474–1394) | 1235 (785–1656) | 0.067 |
| Adrenaline | (ng/mL) | 0.30 (0.17–0.82) | 0.34 (0.17–1.08) | 0.22 (0.13–0.40) | 0.367 |
| Noradrenaline | (ng/mL) | 1.92 (0.11–3.15) | 2.06 (1.13–4.13) | 1.22 (0.93–1.93) | 0.042 |
| ADH | (pg/mL) | 13.8 (5.1–36.3) | 16.4 (5.9–42.6) | 6.3 (3.9–15.0) | 0.094 |
| Aldosterone | (ng/mL) | 24.5 (10.2–50.8) | 25.3 (11.0–51.6) | 16.4 (7.6–29.7) | 0.348 |
| XOR activity | (pmol/h/mL) | 401.0 (189.0–1700.0) | 568.5 (216.5–2140.0) | 176.0 (131.0–241.5) | 0.001 |
| Arterial blood gas | |||||
| pH | 7.37 (7.25–7.44) | 7.36 (7.21–7.43) | 7.44 (7.39–7.47) | 0.018 | |
| PO2 | (mmHg) | 100 (79–144) | 101 (79–143) | 99 (81–139) | 0.922 |
| PCO2 | (mmHg) | 38 (31–55) | 40 (30–57) | 33 (32–38) | 0.113 |
| HCO3 | (mEq/L) | 21.3 (18.5–25.0) | 21.5 (18.3–25.1) | 21.3 (19.5–23.5) | 0.977 |
| BE | (mmol/L) | −2.7 (−7.2 to 1.0) | −3.5 (−8.2 to 1.0) | −2.0 (−3.4 to 0.6) | 0.387 |
| Lactate | (mmol/L) | 2.60 (1.50–4.88) | 2.93 (1.55–5.05) | 1.50 (0.95–2.60) | 0.022 |
| Others | |||||
| XOI before admission | (yes, %) | 20 (23.5%) | 19 (27.1%) | 1 (6.7%) | 0.107 |
| XOI started during 14 days | (yes, %) | 8 (9.4%) | 7 (10.0%) | 1 (6.7%) | 1.000 |
| In-hospital mortality | (%) | 6 (7.1%) | 4 (5.7%) | 2 (13.3%) | 0.285 |
- ADH, antidiuretic hormone; AHF, acute heart failure; BNP, brain natriuretic peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; CRP, C-reactive protein; HF, heart failure; LVEF, left ventricular ejection fraction measured by echocardiography; NYHA, New York Heart Association; XOR, xanthine oxidoreductase. XOI; Xantine oxidoreductase inhibitors.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| XOR information | ||||||
| ≥50% decrease of XOR | 0.258 | 0.089–0.746 | 0.012 | 0.237 | 0.087–0.647 | 0.005 |
| XOR on admission (per 1 mg/dL increase) | 1.000 | 1.000–1.000 | 0.937 | |||
| Adjusting factors | ||||||
| Age (per 1 year increase) | 1.058 | 1.016–1.102 | 0.006 | 1.057 | 1.019–1.096 | 0.003 |
| SBP (per 1 mmHg increase) | 0.995 | 0.985–1.006 | 0.384 | |||
| Recurrence of HF (yes) | 3.226 | 1.397–7.448 | 0.006 | 3.408 | 1.506–7.713 | 0.003 |
| CKD (yes) | 1.555 | 0.630–3.836 | 0.338 | |||
| LVEF (per 1% increase) | 0.975 | 0.944–1.007 | 0.130 | 0.972 | 0.941–1.003 | 0.080 |
| P/F ratio (per 1 increase) | 0.999 | 0.996–1.001 | 0.354 | |||
| BNP (per 1 pg/mL increase) | 1.000 | 1.000–1.001 | 0.039 | 1.000 | 1.000–1.001 | 0.024 |
| Lactate (per 1 mg/dL increase) | 1.146 | 0.954–1.378 | 0.146 | 1.174 | 1.000–1.379 | 0.050 |
- BNP, brain natriuretic peptide; CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; LVEF, left ventricular ejection fraction measured by echocardiography; P/F ratio, PaO2/FiO2 ratio; SBP, systolic blood pressure; XOR, xanthine oxidoreductase.
Discussion
The XOR activity was rapidly decreased by the treatment of AHF. Furthermore, low-XOR activity on admission was associated with increased HF events within 365 days. Even if the XOR activity was extremely high on admission, an insufficient reduction of the XOR activity within 14 days after admission was also associated with increased HF events. These results underscore the importance of the suppression of XOR activity during treatment in patients with AHF.
Excessively high-xanthine oxidoreductase activity on admission in acute heart failure patients
We previously reported that extremely high levels of plasma XOR activity were present in patients with AHF.4 Lactate is associated with extremely high levels of XOR activity. Tissue hypoxia may be involved in the mobilization of XDH into the circulation and the subsequent conversion of XDH to XO in AHF. Some recent reports have discussed the association between high-XOR activity and cardiac disease (i.e. left ventricular hypertrophy, low LVEF, increased BNP, compensated HF, HF with preserved ejection fraction, (coronary artery spasm (CAS), and ischaemic heart disease).3, 8-12 Although we did not verify the relationship between XOR elevation and right HF in detail, there might be a relationship between liver/intestine oedema by right HF and XOR. Thus, cardiovascular disease itself may increase the XOR activity. A high-XOR activity would be associated with increased ROS. From this perspective, it might be reasonable that high-XOR activity leads to adverse outcomes. However, high-XOR activity was not associated with increased HF events in the present study. The time-dependent changes of the high-XOR activity after admission have a prognostic impact in patients with AHF.
The haemodynamic status and tissue oxygenation can change drastically within a short time after the onset of AHF, particularly in AHF patients who require intensive care. These clinical features were suggested to represent early-onset vascular failure. The majority of patients with vascular failure cannot tolerate their abrupt symptoms for long and thus present to the emergency department immediately. In fact, most patients in our cohort were rushed to the hospital by an ambulance. An arterial blood examination indicated an elevated lactate level, reflecting rapid anaerobic metabolism. Patients with these clinical features were quite frequent in the present study. The concept of acute vascular failure is characterized by a transient volume shift from the peripheral veins to the pulmonary circulation with slight fluid accumulation. They described it as ‘fluid redistribution’, not volume overload. Fluid redistribution leads to systemic hypoxia and metabolic/respiratory acidosis due to hypoperfusion of the major organ. It was sometimes very critical on admission, as a result; XDH was released from the liver and intestine, and XDH was subsequently converted to XO. While the ratio of XDH to XO activity in the blood has not been sufficiently studied,13 the major hypothesis at present is that XDH is quickly converted to XO after being transferred to the bloodstream.14 The measurement of the plasma XOR activity may therefore reflect the amount of XO in the blood. The release of XDH from the liver and/or intestine was therefore a key factor for evaluating the XOR activity and reflected tissue hypoxia or organ damage.
An appropriate clinical approach for the treatment of patients with vascular failure has been established. The earlier initiation of non-invasive positive pressure ventilation and the administration of vasodilator for the afterload reduction are considered important initial managements after patients visit the emergency department.5, 15, 16 The earlier initiation of these managements could be very beneficial. As a result, most patients with vascular failure quickly recovered within several days. These facts may explain the short-term admission to the ICU and the better prognosis of vascular failure. Thus, excessively high-XOR activity did not lead to adverse outcomes in patients with AHF who suddenly presented to the emergency room and required intensive care.
Meanwhile, it was reported that the high-XOR activity was associated with poor outcomes in patients with chronic HF.3 The enhancement of XOR activity is believed to produce ROS and ultimately induce oxidative stress. Oxidative stress would adversely affect patients with HF; thus, high-XOR activity is associated with adverse outcomes in patients with chronic HF. In patients with chronic HF, the high-XOR value might remain high because almost all of these patients had compensated HF. Haemodynamics are not easily changed. In contrast, in patients with AHF, the XOR activity might change dramatically because the haemodynamics of such patients can change in a short period of time. Even though their XOR activity was temporarily increased on admission, in cases in which the rapid reduction of the XOR value is achieved, it might be possible to avoid the enhancement of oxidative stress by increased XOR activity. Thus, a high-XOR value might not be associated with adverse outcomes in patients with AHF.
Time-dependent changes and the prognosis in acute heart failure patients
The time-dependent changes of the XOR activity with the treatment of the disease, particularly HF, have never been investigated. We first reported the time-dependent changes of the XOR activity by the treatment of AHF in the present paper, in which the XOR activity was significantly decreased by the appropriate treatment of AHF. Interestingly, the degree of decrease in the XOR activity varied in each case. Moreover, insufficient reduction of the XOR activity was associated with an increase in 365 days of HF events in patients with AHF.
As mentioned earlier, there were definite differences in the disease conditions of the chronic HF and AHF patients. Because the haemodynamics and clinical features showed dramatic changes within a short time after the onset of AHF, it might be appropriate to evaluate the time-dependent changes of XOR activity rather than the absolute value of XOR activity when we discuss the prognostic value of XOR activity in patients with AHF. We therefore focused on the patients in whom high-XOR activity was prolonged despite optimal therapy. There are several possible explanations for the insufficient decrease in XOR activity. The main reason was considered to be a poor response to treatment for AHF. If the AHF was not sufficiently compensated, despite appropriate treatment/support, the unstable haemodynamics would continue, which would subsequently induce tissue hypoxia or organ damage. This perspective supports the theory that the conditions of severe AHF consequently induce high-XOR activity. Moreover, the insufficient weight loss might be also affected to the prolonged high-XOR activity. Another possible reason might be influenced by the XOR inhibitors which were administered before admission and during the first 14 days. Although the frequency at which XOR inhibitor was initiated within 14 days after admission did not differ between the decreased and non-decreased groups, the frequency at which it was initiated before admission tended to be increased in the decreased group in comparison with the non-decreased group. The efficacy of XOR inhibitors in patients with HF remains controversial.17-19 The present study suggests the importance of the early administration of XOR inhibitors for patients with AHF and demonstrates the prognostic impact of a sufficient decrease in XOR activity during the treatment of AHF. Further interventional trials of XOR inhibitors will be required to investigate the association between the reduction of XOR activity and the prognosis.
Limitations
The present study was associated with some limitations. First, as it was a single-centre study, some patient-related biases might have been included. AHF is recognized as a heterogeneous condition, and its characteristics differ among individuals. We already published that a low plasma XOR activity was associated with malnutrition, renal dysfunction, and aging in AHF.7 These factors might cause underestimation of plasma XOR activities and affect their prognosis. The interaction analysis should be analysed in future studies. Second, our study cohort included patients who were already treated with XOR inhibitors at the time of admission. Sephadex G25 was used to remove small molecules, such as xanthine and hypoxanthine, which are competitive inhibitors of stable isotope-labelled [13C2, 15N2]-xanthine in the XOR activity assay, as well as the interfering drug molecules from plasma samples. However, some patients in the present study were undergoing treatment with medications that decreased UA, including allopurinol, febuxostat, and topiroxostat. If any of these drugs remained in the samples, the XOR activity might have been underestimated. Furthermore, the time after the administration of XOR inhibitors is an important additional consideration. The percentages of these drugs that remained after exclusion with Sephadex G25 have not been reported. Third, because the patients with low-XOR activity were already suffering from hyperuricaemia and CKD on admission in the present cohort, these inhomogeneous differences between the low-XOR and high-XOR groups might be associated with the prognosis. The patients with a low-XOR activity on admission were already taking XOR inhibitors because they were already complicated with hyperuricaemia and CKD before admission. Although low-XOR activity as an effect of XOR inhibitor treatment might lead to better outcomes in AHF patients, it did not do so in the present cohort. Complication with hyperuricaemia and CKD before admission might be associated with the prognosis after the onset of AHF. As the causal relationships among low-XOR activity, XOR inhibitor treatment, the presence of CKD, and the prognosis remained unclear in the present study, further studies will be required. Finally, we did not perform subgroup analysis of plasma XOR activity by each condition (e.g. type of LVEF and clinical profile) because the number of samples was not enough for these analyses. Because AHF is recognized as a heterogeneous cohort, subgroup analysis will be required in future.
Conclusions
Plasma XOR activity was rapidly decreased by the appropriate treatment of AHF. Although high-XOR activity on admission was not associated with HF events (all-cause death or readmission by HF) in AHF, high-XOR activity that was not sufficiently reduced during appropriate treatment was associated with increased HF events.
Acknowledgements
We thank the staff in the ICU at Nippon Medical School Chiba Hokusoh Hospital for collecting the medical data.
Conflict of interest
The authors declare no conflicts of interest in association with the present study.
Funding
This research received no grants from any funding agency in the public, commercial, or not-for-profit sectors.




