Prognostic value of cardiac 123I-metaiodobenzylguanidine imaging for predicting cardiac events after transcatheter aortic valve replacement
Abstract
Aims
In patients with aortic valve stenosis (AS), cardiac sympathetic nervous (CSN) dysfunction and its improvement after transcatheter aortic valve replacement (TAVR) have been reported. The prognostic impact of CSN function remains unclear. This study investigated the prognostic value of cardiac 123I-metaiodobenzylguanidine (MIBG) imaging for predicting cardiac events after TAVR.
Methods and results
This single-centre prospective observational study enrolled patients with AS between July 2017 and May 2019. MIBG scintigraphy was performed before and soon after TAVR to evaluate the late heart–mediastinum ratio (L-H/M). Patients were classified into three pairs of groups based on the baseline and post-TAVR L-H/M (≥2.0 or <2.0) and on the presence of TAVR-related improvement in L-H/M. The study endpoint was the occurrence of major adverse cardiac events (MACE), defined as a composite of all-cause death, non-fatal myocardial infarction, and hospitalization due to heart failure. Among the 187 consecutive patients who underwent TAVR, 107 (27 men; median age: 86 years) were evaluated. Over a median follow-up of 366 days, 15 (14.0%) patients had MACE. The incidence of MACE was significantly low in patients with L-H/M improvement and/or high post-TAVR L-H/M (≥2.0). Baseline L-H/M and frailty were associated with poor response of L-H/M to TAVR treatment. TAVR-related improvement in L-H/M had significant effects on MACE, with an adjusted hazard ratio of 0.233 (95% confidence interval, 0.064–0.856; P = 0.028).
Conclusions
TAVR-related improvement in L-H/M was an independent predictor of cardiac events, 1 year after TAVR. Cardiac MIBG imaging is useful for predicting cardiac events after TAVR.
Introduction
Cardiac sympathetic nervous (CSN) dysfunction is associated with a high incidence of unfavourable cardiac events, such as fatal arrhythmias and sudden cardiac death.1-4 Cardiac 123I-metaiodobenzylguanidine (MIBG) imaging is a useful diagnostic tool for assessing CSN function and has been recommended for assessing the severity of heart failure (HF) and prognosis of HF patient.5 Regardless of the HF aetiology, reduced late heart–mediastinum ratio (L-H/M) has been reported to indicate poor cardiac survival.6 Thus, cardiac MIBG imaging is helpful for better risk stratification and prediction of cardiac events.
Recently, aortic valve stenosis (AS) has become one of the most important cardiac diseases causing HF among elderly patients.7 In patients with AS, CSN overactivation associated with decreased cardiac index and increased left ventricular volume or left ventricular hypertrophy has been reported in previous studies.8-10 Furthermore, prior studies9, 10 have suggested that transcatheter aortic valve replacement (TAVR), a less invasive treatment option for patients at intermediate or high operative risk,11 can improve CSN function in patients with AS. In our previous study,10 we demonstrated that CSN function denoted by L-H/M, rapidly improved within 2 weeks after TAVR. However, the association between this early improvement in CSN function and patients' prognosis following TAVR was not evaluated owing to the absence of post-procedural prognostic data. Nonetheless, as a previous study showed that the improvement in MIBG parameters with medical therapy (e.g. beta-blockers) could be an indicator of better prognosis in patients with chronic HF,12 we hypothesized that MIBG parameters before and soon after TAVR or the difference between them might serve as potential prognostic indicators in patients who underwent TAVR. Therefore, we aimed to investigate the prognostic value of cardiac MIBG imaging before and soon after TAVR for predicting cardiac events after TAVR.
Methods
Study population
This single-centre prospective observational study involved patients who underwent TAVR for severe AS between July 2017 and May 2019. Severe AS was defined as the presence of degenerative AS with mean aortic valve pressure gradient (mPG) > 40 mmHg or peak velocity (Vmax) > 4.0 m/s and/or aortic valve area (AVA) < 1.0 cm2 (or effective orifice area index < 0.6 cm2/m2). MIBG scintigraphy was performed at baseline and soon after TAVR to evaluate the L-H/M; differences between baseline and post-TAVR L-H/M were analysed. The first MIBG scintigraphy was performed at the time of admission for TAVR, that is, a few days before TAVR; and the second MIBG scintigraphy was performed 3–21 days after TAVR, when the patient's general condition was stable, in order to avoid the influence of procedure or postoperative infection. Patients were classified into three pairs of groups based on the baseline and post-TAVR L-H/M (≥2.0 or <2.0) and on the presence of TAVR-related improvement in L-H/M [i.e. ΔL-H/M (post-TAVR − baseline) > 0 or ≤0]. The cut-off value of 2.0 for L-H/M was set based on the results of a previous study, which reported that the threshold of the standardized heart–mediastinum ratio could be converted to 2.0 for an institution in which a medium-energy general-purpose collimator is used.5 Data on laboratory findings [i.e. B-type natriuretic peptide (BNP) level] and echocardiographic parameters [i.e. AVA, Vmax, mPG, and left ventricular ejection fraction (LVEF)] were also collected. Measurements of echocardiographic parameters were performed according to the American Society of Echocardiography recommendations. LVEF was estimated using the biplane Simpson method.
The exclusion criteria were as follows: (i) active cancer and/or Parkinson's disease; (ii) ongoing therapy with medications known to interact with MIBG (e.g. labetalol, reserpine, tricyclic antidepressants, sympathomimetic amines, and serotonin–norepinephrine reuptake inhibitors)13; (iii) unfeasibility of MIBG scintigraphy owing to severe dementia and/or mental disorder; (iv) utilization of trans-apical or trans-aortic TAVR; (v) utilization of valve-in-valve TAVR for structural valve deterioration of bioprosthetic aortic valve; (vi) unstable preprocedural conditions (e.g. cardiogenic shock) treated with intravenous catecholamine administration; (vii) severe periprocedural complications (e.g. left ventricular perforation, annulus rupture, and coronary obstruction); and (viii) insufficient MIBG imaging quality. The study complied with the Declaration of Helsinki (1964) and was approved by the ethics committee of the Kyoto Prefectural University of Medicine. All patients provided written informed consent prior to study participation.
Transcatheter aortic valve replacement
The indication, valve type, and approach for TAVR were determined based on the consensus reached by a heart team consisting of cardiologists, cardiovascular surgeons, anaesthesiologists, and imaging specialists. All patients underwent TAVR using the Edwards SAPIEN 3 transcatheter heart valve (Edwards Lifesciences, Irvine, CA, USA) or the Medtronic CoreValve System (Medtronic, Minneapolis, MN, USA). TAVR was performed using the transfemoral approach under general anaesthesia in almost all patients. Among the patients, three were treated using the trans-subclavian approach, and one was treated under local anaesthesia owing to severe respiratory function impairment.
Cardiac 123I-metaiodobenzylguanidine imaging
A dose of 111 MBq of MyoMIBG (FUJIFILM RI Pharma Co. Ltd., Tokyo, Japan) was injected intravenously. Anterior planar images were acquired at 15 min (early image) and 240 min (late image) after MIBG injection using a large-field-of-view gamma camera (Discovery NM/CT 670; GE Healthcare, Waukesha, WI, USA) equipped with a medium-energy collimator. An experienced radiology technician blinded to the patients' information analysed the images according to the region of interest using the smart MIBG software (Fujifilm RI Pharma Co., Tokyo, Japan) to obtain semi-quantitative parameters for tracer distribution. The smart MIBG software was developed to semi-automatically determine the heart–mediastinum ratio and correct it to standard medium-energy collimator conditions.14 Heart–mediastinum ratio was determined by measuring the average counts in each region, whereas the washout rate (WR) from the heart was calculated as the time decay-corrected difference between early and late images. MIBG scintigraphy was performed at rest after HF stabilization.
Follow-up and endpoints
All patients were routinely followed up at 1, 6, and 12 months after TAVR in our institution. Beyond 1 year, clinical outcomes were confirmed by patient medical records or through telephone interviews. The study endpoint was the occurrence of major adverse cardiac events (MACE), defined as a composite of all-cause death, non-fatal myocardial infarction, and HF hospitalization until the date of the last follow-up. Furthermore, the aetiology of death was assessed. Cardiovascular death and procedural complications were defined according to Valve Academic Research Consortium-2 definitions.15 Although there were no predefined criteria for HF hospitalization, the consensus indication included patients exhibiting HF symptoms and objective signs of volume overload, in which the administration of intravenous diuretic therapy should be considered.
Statistical analysis
Continuous variables were presented as medians and interquartile ranges (Quartiles 1–3). Categorical variables were expressed as counts and percentages, because all variables, except haemoglobin, Vmax, mPG, L-H/M, WR, post-TAVR LVEF, ΔVmax (post-TAVR − baseline), ΔmPG (post-TAVR − baseline), ΔLVEF (post-TAVR − baseline), ΔL-H/M (post-TAVR − baseline), and ΔWR (post-TAVR − baseline), did not follow a normal distribution. With respect to normally distributed variables, the median and mean values were nearly identical. Differences in continuous and categorical variables between the MACE and non-MACE groups were compared using the Wilcoxon rank-sum and χ2 tests, respectively. The rate of freedom from MACE was estimated using the Kaplan–Meier method; the difference between the two groups was evaluated using the log-rank test.
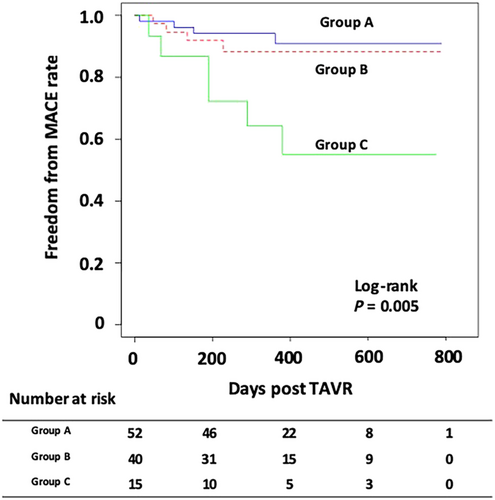
We classified our study participants into three pairs of groups based on the baseline L-H/M (≥2.0 or <2.0), post-TAVR L-H/M (≥2.0 or <2.0), and presence or absence of TAVR-related improvement in L-H/M [ΔL-H/M (post-TAVR – baseline) > 0 or ≤0]. In addition, we classified the study population into the following three groups based on the presence of L-H/M improvement [ΔL-H/M (post-TAVR − baseline) > 0 or ≤0] and the post-TAVR L-H/M level (<2.0 vs. ≥2.0): Group A, L-H/M improvement (+) and post-TAVR L-H/M ≥ 2.0; Group B, L-H/M improvement (+) or post-TAVR L-H/M ≥ 2.0; and Group C, L-H/M improvement (−) and post-TAVR L-H/M < 2.0.
We conducted multiple regression analysis to identify the independent determinants of a poor response of CSN function after TAVR, that is, low post-TAVR L-H/M (<2.0) with no TAVR-related improvement in L-H/M [ΔL-H/M (post-TAVR − baseline) ≤ 0]. The following variables were included as possible confounders: Canadian Study of Health and Aging Clinical Frailty Scale (CSHA-CFS) ≥ 6, history of hypertension, history of HF hospitalization, baseline albumin level, and baseline L-H/M. Additionally, we compared the impact of TAVR-related improvement in L-H/M [ΔL-H/M (post-TAVR − baseline) > 0 or ≤0] and the impact of low post-TAVR L-H/M (<2.0) on MACE using univariate and multivariate Cox regression models. The following variables were included as possible confounders: age, gender, body mass index (BMI), baseline LVEF, CSHA-CFS ≥ 6, classical low-flow low-gradient AS, post-TAVR BNP level, and paravalvular leak (≥moderate). Among variables with P-values < 0.05 in the univariate analysis of each model, clinically relevant variables with lower P-values were treated as confounders, considering the number of endpoints and multicollinearity. Statistical analyses were performed using the R software package (Version 3.3.2; R Development Core Team, Vienna, Austria). The significance level for statistical hypothesis testing was set at 0.05, and the alternative hypothesis was two-sided.
Results
Among 187 consecutive patients who were scheduled for TAVR, 107 were evaluated (Figure 1). Follow-up was completed until death or the end of the study for 101 (94.4%) patients. Although follow-up was incomplete for six patients because they have been unreachable on telephone, we included their data in the final analyses because they had enough follow-up, that is, >1 year, between TAVR and final visits. MACE were identified in 14.0% (15/107) of patients during a median follow-up period of 366 days (range: 210–552 days). There were nine (8.4%) all-cause deaths, including four (3.7%) cardiovascular deaths, and six (5.6%) HF hospitalizations were also observed.

Baseline data of the study participants
The study population's baseline characteristics, procedural information, and post-TAVR data including laboratory, echocardiographic, and MIBG imaging findings are summarized in Table 1. The median age of the study population was 86 years, and 27 (25.2%) patients were male. Patients with MACE were more likely to have a history of HF hospitalization and chronic atrial fibrillation as well as frequently receive diuretic therapy than those without MACE. Classical low-flow low-gradient AS was more frequent in the MACE group than in the non-MACE group. No significant difference was observed in baseline laboratory findings, echocardiographic findings, MIBG parameters, and procedural information between the two groups. The median period between TAVR and post-procedural MIBG scintigraphy was 6 days, which was comparable in both groups. Regarding the post-procedural MIBG parameters, post-TAVR L-H/M was significantly lower and post-TAVR WR was significantly higher in the MACE group than in the non-MACE group. The frequencies of L-H/M improvement tended to be lower in the MACE group than in the non-MACE group; however, this comparison failed to meet statistical significance.
| Parameters | Total n = 107 | MACE (+) n = 15 | MACE (−) n = 92 | P-value |
|---|---|---|---|---|
| Baseline characteristics of the study population | ||||
| Age, years | 86 (84 to 89) | 87 (84 to 89) | 86 (84 to 89) | 0.943 |
| Male, n (%) | 27 (25.2) | 5 (33.3) | 22 (23.9) | 0.523 |
| BMI, kg/m2 | 21.5 (19.1 to 23.8) | 20.0 (19.0 to 22.6) | 21.6 (19.3 to 24.0) | 0.257 |
| STS score | 6.2 (4.8 to 8.6) | 6.7 (5.7 to 9.3) | 6.2 (4.8 to 8.5) | 0.270 |
| CSHA-CFS ≥ 6, n (%) | 40 (37.7) | 9 (60.0) | 31 (34.1) | 0.055 |
| NYHA class III/IV, n (%) | 36 (33.6) | 6 (40.0) | 30 (32.6) | 0.574 |
| Current smoking, n (%) | 5 (4.7) | 1 (6.7) | 4 (4.3) | 0.537 |
| Past medical history, n (%) | ||||
| Hypertension | 79 (73.8) | 10 (66.7) | 69 (75.0) | 0.532 |
| Dyslipidaemia | 45 (42.1) | 4 (26.7) | 41 (44.6) | 0.193 |
| Diabetes mellitus | 19 (17.8) | 1 (6.7) | 18 (19.6) | 0.299 |
| Chronic atrial fibrillation | 11 (10.3) | 5 (33.3) | 6 (6.5) | 0.008 |
| Previous PCI | 37 (34.6) | 5 (33.3) | 32 (34.8) | 0.913 |
| Previous CABG | 5 (4.7) | 0 (0.0) | 5 (5.4) | 1.000 |
| Previous MI | 9 (8.4) | 0 (0.0) | 9 (9.8) | 0.354 |
| Previous CVD | 15 (14.0) | 3 (20.0) | 12 (13.0) | 0.439 |
| Pacemaker implantation | 13 (12.2) | 2 (13.3) | 11 (12.0) | 1.000 |
| History of HF hospitalization, n (%) | 49 (45.8) | 12 (80.0) | 37 (40.2) | 0.004 |
| Medications, n (%) | ||||
| ACEIs/ARBs | 48 (44.9) | 9 (60.0) | 39 (42.4) | 0.204 |
| Beta-blockers | 50 (46.7) | 7 (46.7) | 43 (46.7) | 0.996 |
| Diuretics | 59 (55.1) | 14 (93.3) | 45 (48.9) | 0.001 |
| Categories of AS, n (%) | ||||
| High-gradient AS | 88 (82.2) | 9 (60.0) | 79 (85.9) | 0.026 |
| Classical low-flow low-gradient AS | 10 (9.4) | 4 (26.7) | 6 (6.5) | 0.032 |
| Paradoxical low-flow low-gradient AS | 7 (6.3) | 2 (13.3) | 5 (5.4) | 0.254 |
| Normal-flow low-gradient AS | 2 (1.9) | 0 (0.0) | 2 (2.2) | 1.000 |
| Laboratory data on admission | ||||
| Hb, g/dL | 10.9 (10.1 to 12.3) | 10.7 (10.2 to 12.3) | 10.9 (9.9 to 12.3) | 0.924 |
| eGFR, mL/min/1.73 m2 | 51.4 (34.0 to 64.6) | 50.5 (35.3 to 66.5) | 52.0 (33.8 to 64.3) | 0.932 |
| BNP, pg/mL | 290 (133 to 520) | 417 (288 to 644) | 256 (124 to 504) | 0.069 |
| TTE data before TAVR | ||||
| Vmax, m/s | 4.5 (4.1 to 5.0) | 4.3 (3.8 to 4.9) | 4.5 (4.1 to 5.0) | 0.109 |
| mPG, mmHg | 48 (39 to 63) | 44 (33 to 54) | 48 (40 to 63) | 0.139 |
| AVA, cm2 | 0.6 (0.4 to 0.7) | 0.5 (0.4 to 0.7) | 0.6 (0.5 to 0.7) | 0.578 |
| LVESV, mL | 27 (20 to 41) | 28 (22 to 53) | 27 (20 to 41) | 0.475 |
| LVEDV, mL | 74 (56 to 90) | 88 (66 to 100) | 71 (54 to 88) | 0.209 |
| LVEF, % | 60 (51 to 69) | 60 (38 to 71) | 60 (51 to 67) | 0.961 |
| MIBG data before TAVR | ||||
| Early H/M | 2.98 (2.57 to 3.25) | 2.72 (2.40 to 3.15) | 3.02 (2.60 to 3.25) | 0.199 |
| Late H/M | 2.53 (2.04 to 2.84) | 2.19 (1.94 to 2.62) | 2.57 (2.12 to 2.86) | 0.177 |
| Washout rate, % | 31.9 (26.6 to 37.3) | 33.9 (30.7 to 40.8) | 31.6 (26.5 to 37.1) | 0.214 |
| Period between TAVR and 2nd MIBG (days) | 6 (4 to 8) | 6 (3 to 8) | 6 (4 to 8) | 0.985 |
| Procedural information | ||||
| Valve type, n (%) | ||||
| Edwards SAPIEN 3 | 53 (47.7) | 6 (37.5) | 47 (49.5) | 0.375 |
| Medtronic CoreValve Evolut R/Pro | 54 (50.5) | 9 (60.0) | 45 (48.9) | 0.426 |
| Approach, n (%) | ||||
| Transfemoral | 104 (97.2) | 15 (100) | 89 (96.7) | 1.000 |
| Trans-subclavian | 3 (2.8) | 0 (0.0) | 3 (3.3) | 1.000 |
| Anaesthesia, n (%) | ||||
| General anaesthesia | 106 (99.1) | 15 (100) | 91 (98.9) | 1.000 |
| Local anaesthesia | 1 (0.9) | 0 (0.0) | 1 (1.1) | 1.000 |
| Procedure time (min) | 92 (80 to 114) | 92 (89 to 102) | 92 (79 to 116) | 0.893 |
| Procedural complications | ||||
| Permanent PM implantation, n (%) | 9 (8.4) | 1 (6.7) | 8 (8.7) | 1.000 |
| Post-TAVR CLBBB, n (%) | 15 (14.0) | 2 (13.3) | 13 (14.1) | 1.000 |
| Bleeding complication, n (%) | ||||
| Life threatening | 2 (1.9) | 1 (6.7) | 1 (1.1) | 0.262 |
| Major | 5 (4.7) | 1 (6.7) | 4 (4.3) | 0.537 |
| Minor | 3 (2.8) | 0 (0.0) | 3 (3.3) | 1.000 |
| Laboratory data after TAVR | ||||
| Hb, g/dL | 10.2 (9.5 to 11.2) | 10.3 (9.0 to 10.6) | 10.2 (9.5 to 11.2) | 0.237 |
| eGFR, mL/min/1.73 m2 | 59.1 (46.3 to 75.8) | 54.8 (43.3 to 75.3) | 59.8 (46.6 to 75.5) | 0.737 |
| BNP, pg/mL | 140 (78 to 275) | 210 (107 to 446) | 136 (78 to 219) | 0.096 |
| ΔBNP (post-TAVR − baseline), pg/mL | −105 (−327 to 2) | −164 (−389 to 21) | −102 (−327 to 7) | 0.982 |
| TTE data after TAVR | ||||
| Vmax, m/s | 2.0 (1.7 to 2.4) | 2.0 (1.8 to 2.2) | 2.1 (1.7 to 2.4) | 0.496 |
| mPG, mmHg | 9 (6 to 12) | 8 (6 to 10) | 9 (7 to 12) | 0.185 |
| AVA, cm2 | 1.4 (1.2 to 1.7) | 1.5 (1.4 to 1.5) | 1.4 (1.2 to 1.7) | 0.951 |
| LVESV, mL | 27 (18 to 37) | 27 (21 to 51) | 27 (18 to 35) | 0.272 |
| LVEDV, mL | 79 (62 to 90) | 83 (66 to 93) | 76 (62 to 88) | 0.453 |
| LVEF, % | 64 (56 to 70) | 64 (48 to 69) | 64 (56 to 70) | 0.429 |
| Paravalvular leak (≥moderate) | 4 (3.7) | 2 (13.3) | 2 (2.2) | 0.099 |
| ΔVmax (post-TAVR − baseline), m/s | −2.4 (−1.8 to −3.0) | −2.1 (−1.6 to −3.1) | −2.4 (−1.8 to −3.0) | 0.303 |
| ΔmPG (post-TAVR − baseline), mmHg | −38 (−28 to −54) | −37 (−22 to −50) | −39 (−29 to −54) | 0.289 |
| ΔLVESV (post-TAVR − baseline), mL | −2 (−9 to 3) | −6 (−10 to 5) | −2 (−9 to 2) | 0.993 |
| ΔLVEDV (post-TAVR − baseline), mL | 2 (−12 to 14) | −1 (−19 to 13) | 3 (−12 to 14) | 0.426 |
| ΔLVEF (post-TAVR − baseline), % | 5 (−2 to 11) | 0 (−5 to 8) | 6 (−1 to 12) | 0.550 |
| LVEF improvement | 67 (62.6) | 7 (46.7) | 60 (65.2) | 0.152 |
| MIBG data after TAVR | ||||
| Early H/M | 2.89 (2.54 to 3.12) | 2.72 (2.47 to 3.05) | 2.91 (2.54 to 3.11) | 0.292 |
| Late H/M | 2.61 (2.18 to 2.90) | 2.20 (1.89 to 2.57) | 2.63 (2.27 to 2.95) | 0.039 |
| Washout rate, % | 27.2 (22.5 to 36.7) | 34.7 (26.0 to 38.6) | 27.0 (22.2 to 35.3) | 0.031 |
| ΔLate H/M (post-TAVR − baseline) | 0.07 (−0.15 to 0.22) | −0.06 (−0.17 to 0.15) | 0.09 (−0.13 to 0.25) | 0.090 |
| ΔWR (post-TAVR − baseline) | −3.1 (−8.3 to 2.3) | −0.8 (−5.8 to 4.4) | −3.2 (−8.6 to 2.2) | 0.201 |
| Late H/M improvement, n (%) | 60 (56.1) | 5 (33.3) | 55 (59.8) | 0.056 |
| WR improvement, n (%) | 65 (60.7) | 8 (53.3) | 57 (62.0) | 0.526 |
- Categorical and continuous variables are presented as number (percentage) and median (25th to 75th percentile), respectively.
- ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; AS, aortic valve stenosis; AVA, aortic valve area; BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CLBBB, complete left bundle branch block; CSHA-CFS, Canadian Study of Health and Aging Clinical Frailty Scale; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; Hb, haemoglobin; HF, heart failure; H/M, heart–mediastinum ratio; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MACE, major adverse cardiac events; MI, myocardial infarction; MIBG, 123I-metaiodobenzylguanidine; mPG, mean aortic valve pressure gradient; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PM, pacemaker; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiography; Vmax, peak velocity; WR, washout rate.
The incidence of major adverse cardiac event after transcatheter aortic valve replacement
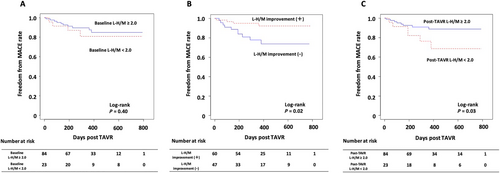
The Kaplan–Meier curves for MACE after TAVR are shown in Figure 2. Patients with baseline L-H/M ≥ 2.0 were less likely to experience MACE than those with baseline L-H/M < 2.0; nevertheless, this difference was not significant. The incidence of MACE was significantly lower in patients with an improvement in L-H/M than in those without improvement. Moreover, patients with post-TAVR L-H/M ≥ 2.0 had significantly better prognosis than those with post-TAVR L-H/M < 2.0. To better clarify the association of L-H/M value with prognosis, we compared the incidence of MACE among the three groups: Group A, L-H/M improvement (+) and post-TAVR L-H/M ≥ 2.0; Group B, L-H/M improvement (+) or post-TAVR L-H/M ≥ 2.0; and Group C, L-H/M improvement (−) and post-TAVR L-H/M < 2.0. There was a significant difference in MACE among the three groups, with Group C showing the highest incidence of MACE (Figure 3).
Predictors of poor response of cardiac sympathetic nervous function after transcatheter aortic valve replacement and predictors of major adverse cardiac event after transcatheter aortic valve replacement
In the multivariate analysis, baseline L-H/M and CSHA-CFS ≥ 6 were independent predictors of being Group C with poor response of CSN function after TAVR treatment [i.e. low post-TAVR L-H/M (<2.0) with no TAVR-related improvement in L-H/M] (Table 2). Furthermore, L-H/M improvement after TAVR [ΔL-H/M (post-TAVR − baseline) > 0] was an independent predictor of MACE after TAVR (adjusted hazard ratio, 0.233; 95% confidence interval, 0.064–0.856; P = 0.028) (Table 2). The comparison of each endpoint (i.e. all-cause death, cardiovascular death, non-fatal myocardial infarction, and HF hospitalization) according to the presence or absence of L-H/M improvement after TAVR is presented in Table 3. The incidence of all-cause death and cardiovascular death was comparable between the two groups. In contrast, the incidence of HF hospitalization was significantly lower in patients with L-H/M improvement after TAVR than in those without L-H/M improvement (P = 0.045).
| Predictors of poor response to TAVR treatment (i.e. low post-TAVR L-H/M with no TAVR-related improvement in L-H/M) | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |||
| Age | −0.102 (−0.236 to 0.029) | 0.124 | — | — | ||
| Gender (male) | 0.813 (−0.375 to 1.947) | 0.163 | — | — | ||
| BMI | −0.088 (−0.277 to 0.082) | 0.335 | — | — | ||
| Baseline LVEF | −0.007 (−0.046 to 0.036) | 0.748 | — | — | ||
| CSHA-CFS ≥ 6 | 1.403 (0.280 to 2.643) | 0.018 | 1.564 (0.125 to 3.164) | 0.040 | ||
| Hypertension | −1.085 (−2.221 to −0.063) | 0.059 | — | — | ||
| History of HF hospitalization | 0.9999 (−0.115 to 2.232) | 0.089 | — | — | ||
| Baseline albumin level | −1.183 (−2.408 to −0.007) | 0.049 | −0.751 (−2.504 to 0.866) | 0.373 | ||
| Baseline L-H/M | −2.425 (−3.833 to −1.270) | <0.001 | −2.668 (−4.277 to −1.390) | <0.001 | ||
| Predictors of MACCE after TAVR | ||||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate (Model 1) | Multivariate (Model 2) | ||||
| HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | |
| Age | 0.962 (0.851 to 1.088) | 0.541 | ||||
| Gender (male) | 1.723 (0.577 to 5.144) | 0.329 | ||||
| BMI | 0.942 (0.802 to 1.107) | 0.468 | ||||
| Baseline LVEF | 0.987 (0.949 to 1.027) | 0.519 | ||||
| CSHA-CFS ≥ 6 | 3.260 (1.092 to 9.730) | 0.034 | ||||
| Classical low-flow low-gradient AS | 4.304 (1.348 to 13.740) | 0.014 | 2.145 (0.578 to 7.967) | 0.254 | 2.445 (0.617 to 9.689) | 0.203 |
| Post-TAVR BNP | 1.001 (1.000 to 1.002) | 0.018 | 1.001 (1.00001 to 1.0022) | 0.047 | 1.001 (0.9997 to 1.002) | 0.180 |
| Paravalvular leak (≥moderate) | 5.646 (1.255 to 25.400) | 0.024 | ||||
| L-H/M improvement after TAVR | 0.285 (0.089 to 0.908) | 0.034 | 0.233 (0.064 to 0.856) | 0.028 | ||
| Post-TAVR L-H/M < 2.0 | 2.911 (1.044 to 8.122) | 0.041 | 2.620 (0.906 to 7.578) | 0.075 | ||
- In the multivariate model for predicting poor response to TAVR treatment, the adjusted OR for patients with post-TAVR L-H/M < 2.0 and no L-H/M improvement [ΔL-H/M (post-TAVR − baseline) ≤ 0] compared with that for patients with post-TAVR L-H/M ≥ 2.0 and/or L-H/M improvement [ΔL-H/M (post-TAVR − baseline) > 0] was calculated by adjusting for CSHA-CFS ≥ 6, baseline albumin level, and baseline L-H/M. In the multivariate model for predicting MACE after TAVR, the adjusted HR for patients with L-H/M improvement after TAVR compared with that for patients without L-H/M improvement after TAVR was calculated by adjusting for classical low-flow low-gradient AS, and post-TAVR BNP level (Model 1). In addition, the adjusted HR for patients with post-TAVR L-H/M < 2.0 compared with that for patients with post-TAVR L-H/M ≥ 2.0 was calculated by adjusting for classical low-flow low-gradient AS and post-TAVR BNP level (Model 2).
- AS, aortic valve stenosis; BMI, body mass index; BNP, B-type natriuretic peptide; CI, confidence interval; CSHA-CFS, Canadian Study of Health and Aging Clinical Frailty Scale; HF, heart failure; HR, hazard ratio; L-H/M, late heart–mediastinum ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events; OR, odds ratio; TAVR, transcatheter aortic valve replacement.
| Parameters | Total n = 107 | L-H/M improvement after TAVR (+) n = 60 | L-H/M improvement after TAVR (−) n = 47 | P-value |
|---|---|---|---|---|
| All-cause death, n (%) | 9 (8.4) | 4 (6.7) | 5 (10.6) | 0.502 |
| Cardiovascular death, n (%) | 4 (3.7) | 2 (3.3) | 2 (4.3) | 1.000 |
| Non-cardiovascular death, n (%) | 5 (4.7) | 2 (3.3) | 3 (6.4) | 0.652 |
| Non-fatal myocardial infarction, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| HF hospitalization, n (%) | 6 (5.6) | 1 (1.7) | 5 (10.6) | 0.045 |
| Major adverse cardiac events, n (%) | 15 (14.0) | 5 (8.3) | 10 (21.3) | 0.056 |
- Categorical variables are presented as number (percentage).
- HF, heart failure; L-H/M, late heart–mediastinum ratio; TAVR, transcatheter aortic valve replacement.
Discussion
In this study, we investigated the prognostic value of cardiac MIBG imaging for predicting cardiac events after TAVR. Patients with TAVR-related improvement in L-H/M [ΔL-H/M (post-TAVR − baseline) > 0] showed a significantly lower incidence of MACE than those without. Patients with high post-TAVR L-H/M (≥2.0) had significantly better prognosis than those with low post-TAVR L-H/M (<2.0). In contrast, patients with poor response of CSN function after TAVR (i.e. low post-TAVR L-H/M with no TAVR-related improvement in L-H/M) showed the highest incidence of MACE. Baseline L-H/M and CSHA-CFS were associated with poor response of L-H/M after TAVR. Moreover, TAVR-related improvement in L-H/M was an independent predictor of MACE after TAVR. As no studies have shown that CSN function assessment using MIBG imaging is helpful for risk prediction in patients with AS undergoing TAVR, our study could provide physicians with new insights into this field.
Impact of transcatheter aortic valve replacement-related improvement in late heart–mediastinum ratio on cardiac prognosis after transcatheter aortic valve replacement
Patients with TAVR-related improvement in L-H/M [ΔL-H/M (post-TAVR − baseline) > 0] showed a significantly lower incidence of MACE than those without such improvement. In our previous study, we reported that the CSN function as denoted by L-H/M significantly improved shortly after TAVR.10 We speculated that such rapid improvement was accomplished because TAVR can immediately relieve left ventricular pressure overload and markedly improve haemodynamics, avoiding the negative effects of open-heart procedures.10 However, the potential relationship between TAVR-related improvement in CSN function and patients' prognosis following TAVR was not evaluated. Therefore, this study investigated the clinical benefit of early improvement of CSN function in patients who underwent TAVR. Our results indicated that the presence of TAVR-related improvement in L-H/M was associated with better prognosis after TAVR. While previous studies showed that the improvement in CSN function with medical therapy (e.g. beta-blockers) could be an indicator of better prognosis in patients with chronic HF, our study demonstrated for the first time that the improvement in CSN function by TAVR was related to better clinical outcome in patients with severe AS. Notably, compared with medical therapy, TAVR achieved such prognostic benefits within a very short time. Thus, evaluation of serial changes in CSN function represented by MIBG imaging before and after TAVR provides essential information about the patient's prognosis.
Predictors of major adverse cardiac event after transcatheter aortic valve replacement
Patients with L-H/M improvement after TAVR showed a significantly better prognosis than those without L-H/M improvement. Moreover, L-H/M improvement after TAVR was an independent predictor of MACE after TAVR. Previous studies have reported several prognostic predictors after TAVR, such as the CSHA-CFS, a diagnosis of low-flow low-gradient severe AS, and post-TAVR BNP level at discharge.16-18 Our data from univariate analysis seem to be consistent with these results. Moreover, according to the results of our multivariate analysis, L-H/M improvement in MIBG imaging was considered as one important predictor for cardiac events after TAVR, in addition to other previously reported predictors. We previously reported that MIBG parameters could reflect not only the haemodynamic severity of AS but also the severity of frailty or HF symptoms.10 This finding indicated that MIBG parameters could be a more comprehensive measure of severity in patients with AS. Hence, MIBG parameters might be a more reliable prognostic indicator after TAVR.
Baseline late heart–mediastinum ratio and frailty as predictors of poor response of cardiac sympathetic nervous function after transcatheter aortic valve replacement
Patient with poor response of CSN function after TAVR (i.e. low post-TAVR L-H/M with no TAVR-related improvement in L-H/M) showed the highest incidence of MACE. Moreover, baseline L-H/M and CSHA-CFS were associated with poor response of L-H/M after TAVR. From the viewpoint of better selection of suitable candidates, it would be ideal if the baseline L-H/M value could be a strong predictor of post-TAVR cardiac events. Although patients with low baseline L-H/M tended to experience MACE than those with high baseline L-H/M, baseline L-H/M (<2.0 or ≥2.0) was not directly associated with MACE after TAVR in the present study population. However, multivariate analysis revealed patients' baseline L-H/M and CSHA-CFS to be independent predictors of poor response of CSN function after TAVR, suggesting that patients with severe impairment in CSN function and/or advanced frailty gained little prognostic benefit from TAVR. Thus, we believe that preprocedural MIBG imaging help in identifying suitable candidates who may benefit the most from TAVR.
Clinical implications
Most TAVR candidates may have multiple risk factors that can potentially affect prognosis. Thus, a simple and comprehensive risk stratification tool is needed to aid physicians in managing their patients. In daily clinical practice, CSN function can be easily assessed using MIBG scintigraphy. Our results suggest that assessment of CSN function as denoted by L-H/M is useful for outcome prediction in patients undergoing TAVR. Patients with poor response in CSN function to TAVR treatment should be carefully monitored to avoid future cardiac events, particularly HF hospitalization. Additionally, it may be necessary to aim at further CSN function improvement for such patients by strengthening medical treatment, including beta-blockers and angiotensin-converting enzyme inhibitors.
Limitations
The present study has several limitations. First, owing to the relatively small sample size and relatively short follow-up period, it might be insufficient to completely understand the influence of CSN function on prognosis in patients who underwent TAVR. It should be noted that although the difference in the incidence of MACE arose from the difference in HF hospitalization, the absolute number of HF hospitalizations was very low. Because of this low number of endpoints, potential clinical confounding factors, such as age, gender, LVEF, and BMI, were not included in the multivariate analysis. A larger study population and longer follow-up period may be necessary to confirm the impact of CSN function on prognosis after TAVR. Second, the time between TAVR and the second MIBG scintigraphy varied from 3 to 21 days in this study, and this difference might affect the improvement of MIBG parameters. However, the median period between TAVR and post-procedural MIBG scintigraphy was comparable in MACE and non-MACE groups and was not associated with the response of CSN function to TAVR treatment in our analysis. Thus, we believe that the difference between the duration of TAVR and the second MIBG scintigraphy had no significant impact on the results of the current study. Third, because this is a prospective observational study, determining whether patients required hospitalization due to HF worsening was based on the attending physician's judgement without any predefined criteria. Fourth, preprocedural MIBG scintigraphy could not be performed in patients with unstable general conditions; thus, most of the critically ill candidates were excluded from the study population. Fifth, because this study included only Japanese patients, our results cannot be generalized to other ethnicities.
Conclusions
TAVR-related improvement in L-H/M was an independent predictor of cardiac events 1 year after TAVR. CSN function assessment using MIBG imaging before and after TAVR provides essential information about the patient's prognosis following TAVR.
Acknowledgements
None.
Conflict of interest
None declared.
Funding
None.




