Biventricular pacemaker and defibrillator implantation in patients with chronic heart failure in China
Abstract
Aims
This study aims to investigate the current status of biventricular pacemaker and defibrillator implantation in chronic heart failure (CHF) patients with indications for primary prevention of sudden cardiac death (SCD) in China and the effects of cardiac resynchronization therapy (CRT)-pacemaker (P) and CRT-defibrillator (D) implantation on the clinical prognosis of CHF among patients undergoing CRT.
Methods and results
Overall, 798 consecutive patients who had devices implanted (implantable cardioverter defibrillator: 199, CRT-D: 362, and CRT-P: 237) from May 2012 to July 2013 in POSCD-China, a multicentric prospective cohort study, were enrolled. The primary endpoint was all-cause death, and the secondary endpoint was SCD. In total, 71.3% of patients had non-ischaemic CHF. The mean follow-up time was 27.7 ± 12.0 months, and death occurred in 158 cases, with 35 cases of SCD. CHF was the main cause of death (68.4%), followed by sudden death (22.2%). In the CRT-P group, the SCD rate was 8.0%, which was much higher than that in the CRT-D (3.3%) and implantable cardioverter defibrillator (2.0%) groups. No significant differences were identified in the all-cause death rate between the CRT-D and CRT-P groups (CRT-D vs. CRT-P, 20.4% vs. 19.4%, P = 0.840).
Conclusions
In China, among CHF patients with indications for primary prevention of SCD who received device implantation, non-ischaemic CHF was the main aetiology, and the most important cause of death was heart failure. No differences in all-cause death were observed between the CRT-D and CRT-P groups, but the CRT-D group had a lower SCD rate than the CRT-P group.
Introduction
According to the Report on Cardiovascular Diseases in China (2015), 544 000 cases of sudden cardiac death (SCD) occur in China each year, but only 3200 patients undergo implantation of a defibrillator.1 The European and American guidelines recommend primary prevention of SCD for a left ventricular ejection fraction of less than or equal to 35%, which is considered a Class I indication for use of an implantable cardioverter defibrillator (ICD)/cardiac resynchronization therapy (CRT) implantable cardioverter defibrillator (CRT-D).2, 3 Although the European and American guidelines are currently used in China and recommend primary prevention of SCD in high-risk patients by device implantation,4 there is no evidence from Chinese clinical research to support these recommendations. Moreover, limited data have shown controversial results for use of the Multicentre Automatic Defibrillator Implantation Trial II (MADIT II) criterion in East Asian populations to guide ICD implantation.5, 6 Additionally, with regard to device treatment for chronic heart failure (CHF), the effects of the selection of a cardiac resynchronization therapy pacemaker (CRT-P) or CRT-D on the clinical prognosis for different patients with QRS widening remain unclear.
This study investigated (i) the current status of biventricular pacemaker and defibrillator implantation in CHF patients with indications for primary prevention of SCD in China and the risk of SCD of these patients and (ii) the effects of CRT-P and CRT-D implantation on the clinical prognosis of CHF in patients undergoing CRT who have QRS widening (QRS ≥ 120 ms).
Methods
Study setting and design
The Primary Prevention of Sudden Cardiac Death in Patients with Chronic Heart Failure-Multicentre Prospective Registry in China (POSCD-China Study) is a prospective, multicentre, registry project supported by the National Science and Technology Pillar Program during the 12th Five-year Plan Period (2011BAI11B11, Beijing, China). The research protocol has been previously published.7 This study included consecutive CHF patients with indications for primary prevention of SCD who received device implantation (including ICD, CRT-P, and CRT-D). The study was conducted from May 2012 to July 2013 at 58 tertiary hospitals in 22 provinces or municipalities in China. The inclusion and exclusion criteria are shown in Table 1. The implantation of an ICD or CRT-P/D for CHF patients was carried out in accordance with the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities.8 Before device implantation, all enrolled patients were treated with optimized medication for at least 3 months based on the AHA guidelines for CHF,9 including diuretics, beta-blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and mineralocorticoid receptor antagonists. The formulation and implementation of this study were completed by the POSCD-China Study steering committee. This study was in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of West China Hospital of Sichuan University. Informed consent was obtained from each patient participant.
| Inclusion criteria |
|---|
| Regarding the following inclusion criteria, Items 1–3 were mandatory, and either Item 4 or 5 must have been satisfied: |
| 1. Written informed consent was provided. |
| 2. Age 18 years or older. |
| 3. Expected survival time longer than 1 year. |
| 4. CHF patients with Class I or IIa indications who underwent successful implantation of an ICD or CRT-P/D during hospitalization in accordance with the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities.8 |
| Recommendations for CRT |
| Class I |
| a. For patients who have LVEF less than or equal to 35%, a QRS duration greater than or equal to 0.12 s, and sinus rhythm, CRT with or without an ICD is indicated for the treatment of NYHA functional Class III or ambulatory Class IV heart failure symptoms with optimal recommended medical therapy. |
| Class IIa |
| a. For patients who have LVEF less than or equal to 35%, a QRS duration greater than or equal to 0.12 seconds, and AF, CRT with or without an ICD is reasonable for the treatment of NYHA functional Class III or ambulatory Class IV heart failure symptoms on optimal recommended medical therapy. |
| b. For patients with LVEF less than or equal to 35% with NYHA functional Class III or ambulatory Class IV symptoms who are receiving optimal recommended medical therapy and who have frequent dependence on ventricular pacing, CRT is reasonable. |
| Recommendations for ICD |
| Class I |
| a. ICD therapy is indicated in patients with LVEF less than or equal to 35% due to prior MI who are at least 40 days post-MI and are in NYHA functional Class II or III. |
| b. ICD therapy is indicated in patients with non-ischaemic DCM who have an LVEF less than or equal to 35% and who are in NYHA functional Class II or III. |
| c. ICD therapy is indicated in patients with LV dysfunction due to prior MI who are at least 40 days post-MI, have an LVEF less than or equal to 30%, and are in NYHA functional Class I. |
| Class IIa |
| a. ICD implantation is reasonable for non-hospitalized patients awaiting transplantation. |
| 5. CHF patients who had previously received a conventional cardiac pacemaker (single or dual-chamber pacemaker) for other reasons and met the ICD or CRT-P/D implantation indication and were successfully upgraded to an ICD or CRT-P/D. |
| Exclusion criteria |
|---|
| The following patients were excluded: |
| 1. Those in whom an ICD or CRT-D was used for secondary prevention of SCD, that is, with a history of cardiac arrest, persistent ventricular tachycardia, or ventricular fibrillation. |
| 2. Patients with previous placement of an ICD or CRT-P/D who were hospitalized for ICD or CRT-P/D replacement. |
| 3. Patients with congenital ion channel diseases such as long QT syndrome, short QT syndrome, and Brugada syndrome. |
| 4. Patients with CHF due to valvular or congenital heart disease. |
| 5. Patients with unexplained syncope. |
| 6. Patients with psychiatric disorders. |
| 7. Pregnant women. |
| 8. Patients who could not complete follow-up. |
- AF, atrial fibrillation; CHF, chronic heart failure; CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; DCM, dilated cardiomyopathy; ICD, implantable cardioverter defibrillator; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association.
Baseline characteristics at implant
Demographic and clinical characteristics of the patients and the procedure and technical data for implantation were obtained from the electronic medical records of the participating centres. These data are electronically transmitted to the database of the data management centre every 3 months at regular intervals. Records of complications include infection, electrode displacement, venous thrombosis or embolism, bleeding, heart failure (HF), fever, arrhythmia, pericardial effusion or pericardial tamponade, pneumothorax, phrenic nerve stimulation, and death. The medicines patients were prescribed upon discharge from the hospital included beta-blockers, antiarrhythmic drugs, digoxin, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, aldosterone receptor antagonists, diuretics, anticoagulants, and antiplatelet drugs. Pacemaker and defibrillator programming was performed by the researchers at each centre with the goal of achieving optimal biventricular pacing (more than 95%), minimizing the extent of right ventricular pacing, and avoiding inappropriate and unnecessary shock.
Follow-up and outcomes
All patients were scheduled for follow-up at 3, 6, 12, 18, and 24 months after discharge until the end of the study on 31 December 2015. At each visit, the patient underwent a clinical examination, electrocardiogram, transthoracic ultrasound, and device interrogation. In addition, any events that occurred during the period were recorded (e.g. patients were hospitalized). All data from the time the device was implanted until the end of the study or patient death were systematically collected and recorded at each follow-up evaluation. The definition of endpoint events is listed in Supporting Information, Appendix S2. Deaths that occurred unexpectedly in a previously stable patient were adjudicated as witnessed or unwitnessed cardiac death. Cardiovascular death consisted of SCD, HF, and other cardiovascular deaths, including myocardial infarction, acute aortic syndrome, stroke, and pulmonary embolism. Fatal arrhythmias in end-stage HF were classified as non-SCD. Deaths due to other causes, such as infectious disease, respiratory disease, renal failure, or tumour, were classified as non-cardiovascular. When inadequate data were available, the cause of death was classified as unknown. The determination of all endpoint events was considered by the study's Event Adjudication Committee. The primary endpoint was all-cause death, and secondary endpoints included SCD, cardiovascular death, electrical therapy for ICD or CRT-D (antitachycardia pacing and shock), and rehospitalization. Other definitions of the terms are listed in Appendix S2.
Statistical analysis
This report was prepared in compliance with the STROBE checklist for cohort studies.10 Baseline demographics and clinical characteristics among patients were compared and categorized by the types of devices implanted in the three groups: ICD, CRT-D, and CRT-P. Because of the incomparability between the ICD group and the CRT group, baseline characteristics and clinical events were compared between the CRT-D and CRT-P groups, while those of the ICD group were described separately. Continuous variables are expressed as the means ± standard deviation, and categorical variables are reported as the counts and percentages. Analyses using the t-tests and χ2 tests were conducted to assess differences between groups for continuous and categorical variables, respectively. Outcomes were analysed with the use of time-to-event methods. Kaplan–Meier plots for total mortality were prepared, and cumulative incidence curves were generated for events. Comparisons of clinical events between the CRT-D group and the CRT-P group were examined using the log-rank test. Cox proportional hazards regression models were also employed to investigate the independent effect of defibrillation on SCD in patients with CRT. Predictors of outcomes with statistical significance (P < 0.05) in univariate analyses or based on expert experience or previous publications were further entered into multivariate Cox regression analysis. Adjustments were made for the possible confounding effects of sex, body mass index, QRS duration, left ventricular ejection fraction, complete left bundle branch block, New York Heart Association (NYHA) class, cause of HF, and antiarrhythmic drugs. Two-sided P values of less than 0.05 indicated statistical significance. All analyses were performed with STATA software (Version 13.0).
Results
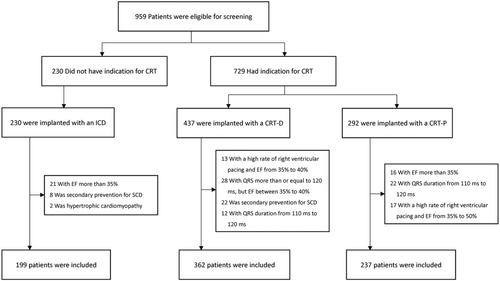
In total, 798 patients were included in the POSCD-China Study (Figure 1). Among them, the average age was 60.6 ± 11.7 months, and men accounted for 70.8%. Overall, 79.1% had an NYHA functional classification of III or above, and 71.3% had non-ischaemic CHF. Of the patients, 70.3% received a defibrillator, and 75.1% received CRT. The baseline characteristics of the three groups (ICD n = 199, CRT-D n = 362, and CRT-P n = 237) are shown in Table 2. There were significantly more non-ischaemic CHF than ischaemic CHF cases for which device implantation was performed.
| Total | ICD | CRT | Pa | ||
|---|---|---|---|---|---|
| (N = 798) | (N = 199) | CRT-D (N = 362) | CRT-P (N = 237) | ||
| Median age (SD) | 60.6 (11.7) | 62.2 (11.5) | 60.0 (12.1) | 60.3 (11.5) | 0.720 |
| Male, n (%) | 565 (70.8) | 164 (82.4) | 270 (74.6) | 131 (55.27) | <0.001 |
| Body mass index (IQR) | 23.5 (3.7) | 24.0 (3.6) | 23.6 (3.7) | 23.0 (3.7) | 0.072 |
| Median blood pressure (mmHg) | |||||
| Systolic blood pressure | 118.2 (18.3) | 121.5 (19.0) | 117.7 (18.3) | 116.7 (17.6) | 0.485 |
| Diastolic blood pressure | 74.4 (12.5) | 76.2 (12.2) | 73.7 (11.9) | 73.8 (13.7) | 0.947 |
| Median QRS duration (IQR) | 147.3 (33.7) | 108.6 (21.8) | 157.8 (27.0) | 163.6 (24.9) | 0.008 |
| Complete LBBB, n (%) | 465 (58.3) | 12 (6.0) | 260 (71.8) | 193 (81.4) | <0.001 |
| LVEF (IQR) | 27.9 (5.3) | 28.6 (5.1) | 27.3 (5.2) | 28.1 (5.4) | 0.085 |
| NYHA, n (%) | 0.186 | ||||
| II | 167 (20.9) | 54 (27.1) | 53 (14.6) | 51 (21.5) | |
| III | 478 (59.9) | 122 (61.3) | 223 (61.6) | 133 (56.1) | |
| IV | 153 (19.2) | 20 (10.1) | 82 (22.6) | 51 (21.5) | |
| Coexisting conditions, n (%) | |||||
| Hypertension | 200 (33.4) | 85 (42.7) | 123 (34.0) | 77 (32.5) | 0.706 |
| Diabetes | 138 (17.3) | 48 (24.1) | 50 (13.8) | 40 (16.9) | 0.305 |
| Atrial fibrillation | 96 (12.0) | 29 (14.6) | 37 (10.2) | 30 (12.7) | 0.355 |
| Cause of heart failure, n (%) | 0.021 | ||||
| Non-ischaemic | 569 (71.3) | 119 (59.8) | 260 (71.8) | 190 (80.2) | |
| Ischaemic | 229 (28.7) | 80 (40.2) | 102 (28.2) | 47 (19.8) | |
| Medications, n (%) | |||||
| Antiplatelets | 231 (29.0) | 78 (39.2) | 89 (24.6) | 64 (27.0) | 0.507 |
| Anticoagulants | 51 (6.4) | 23 (11.6) | 16 (4.4) | 12 (5.1) | 0.715 |
| Statins | 258 (32.3) | 85 (42.7) | 98 (27.1) | 75 (31.7) | 0.227 |
| Beta-blocker | 682 (85.4) | 159 (79.9) | 316 (87.3) | 207 (87.3) | 0.986 |
| ACEIs | 477 (59.8) | 111 (55.8) | 216 (59.7) | 150 (63.3) | 0.374 |
| ARBs | 175 (21.9) | 46 (23.1) | 80 (22.1) | 49 (20.7) | 0.678 |
| ACEIs or ARBs | 643 (80.6) | 156 (78.4) | 292 (80.7) | 195 (82.8) | 0.620 |
| MRAs | 615 (77.1) | 149 (74.9) | 278 (76.8) | 188 (79.3) | 0.466 |
| Digoxin | 395 (49.5) | 88 (44.2) | 191 (52.8) | 116 (49.0) | 0.361 |
| Diuretics | 682 (85.5) | 162 (81.4) | 303 (83.7) | 198 (83.5) | 0.959 |
| Antiarrhythmics | 171 (21.4) | 51 (25.6) | 90 (24.9) | 30 (12.7) | <0.001 |
- ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; ICD, implantable cardioverter defibrillator; IQR, interquartile range; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MRAs, mineralocorticoid receptor antagonists; SD, standard deviation.
- Data are expressed as means ± SD or counts and percentages, as appropriate.
- a Comparison between the CRT-D group and the CRT-P group.
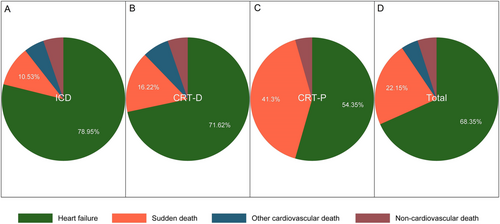
The 798 patients were followed for an average of 27.7 ± 12.0 months (completed in 92.4% of patients), among whom 158 died (resulting in an overall annual mortality rate of 8.6%). The causes of death were analysed (Figure 2); HF was the main cause of death (68.4%), followed by sudden death (22.2%). In the CRT-P group, the sudden death rate was 8.0%, which was much higher than that in the CRT-D (3.3%) and ICD (2.0%) groups (Figure 1 and Table 3).
| Outcomes | Total (N = 798) | ICD (N = 199) | CRT-D (N = 362) | CRT-P (N = 237) | HR (95% CI) unadjusted | Pa | HR (95% CI) adjustedb | Pa |
|---|---|---|---|---|---|---|---|---|
| All-cause death | 158 (19.8) | 38 (19.1) | 74 (20.4) | 46 (19.4) | 1.04 (0.72–1.50) | 0.840 | 0.88 (0.60–1.30) | 0.533 |
| Cardiac death | 150 (18.8) | 36 (18.1) | 70 (19.3) | 44 (18.6) | 1.03 (0.70–1.50) | 0.891 | 0.87 (0.70–1.62) | 0.549 |
| Sudden cardiac death | 35 (4.4) | 4 (2.0) | 12 (3.3) | 19 (8.0) | 0.41 (0.20–0.84) | 0.015 | 0.35 (0.16–0.75) | 0.007 |
| Rehospitalization | 229 (28.7) | 73 (36.7) | 106 (29.3) | 50 (21.1) | 1.39 (0.99–1.94) | 0.058 | 1.25 (0.88–1.77) | 0.221 |
| Electric therapy (first) | 44 (5.5) | 19 (9.6) | 25 (6.9) | n/a | n/a | n/a | n/a | n/a |
| Device infection | 6 (0.8) | 0 | 6 (1.7) | 0 | n/a | n/a | n/a | n/a |
| Lead dislocation | 12 (1.5) | 0 | 9 (2.5) | 3 (1.3) | n/a | n/a | n/a | n/a |
- CI, confidence interval; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; HR, hazard ratio; ICD, implantable cardioverter defibrillator; n/a, not available.
- a Comparison between the CRT-D group and the CRT-P group. The CRT-P group is control group.
- b Adjusted for sex, body mass index, QRS duration, left ventricular ejection fraction, complete left bundle branch block, New York Heart Association class, cause of heart failure, and antiarrhythmic drugs.
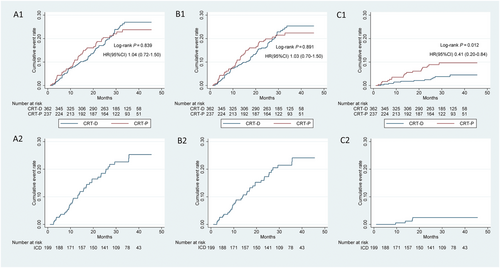
The all-cause death rate of the ICD group was 19.1% (8.3 events per 100 person-years), whereas the all-cause death rates in the CRT-D and CRT-P groups were 20.4% (8.7 events per 100 person-years) and 19.4% (8.4 events per 100 person-years), respectively. No significant differences were identified between the CRT-D and CRT-P groups (CRT-D vs. CRT-P, P = 0.840) (Figure 3, Panels A1 and A2; Table 3). The SCD rate for the ICD group was 2.0% (0.7 events per 100 person-years). The SCD rates for the CRT-D and CRT-P groups were 3.3% (1.4 events per 100 person-years) and 8.0% (3.5 events per 100 person-years), respectively. Moreover, the SCD rate of the CRT-D group was significantly lower than that of the CRT-P group (CRT-D vs. CRT-P, P = 0.012) (Figure 3, Panels C1 and C2; Table 3).
Discussion
The POSCD-China Study is a multicentre, prospective registry of CHF device implantation therapy, with the largest sample size and the most participating centres in China. This study is the first to show the current real-world practice of device implantation for CHF to prevent SCD in China. According to the results of this study, (i) patients receiving device implantation therapy in China mainly had non-ischaemic CHF; (ii) although patients with indications for primary prevention of SCD who received device implantation had been given optimized drug treatments and underwent device implantation recommended by the guidelines, CHF patients still had high all-cause mortality, and the most common cause of death was HF; and (iii) no differences in all-cause death were observed between the CRT-D and CRT-P groups, but the rate of SCD was lower in the CRT-D group than in the CRT-P group.
In previous randomized controlled trials reported in Western countries, non-ischaemic CHF accounted for approximately 30–48% of cases.11-14 However, in our study, non-ischaemic CHF accounted for 71.3% of the patients who received device implantation therapy for CHF and/or prevention of SCD, which is similar to the data reported by the Chinese Ministry of Health and much greater than the reports in studies carried out in Western countries.1 Nonetheless, according to the China Heart Failure Registration Study, approximately 49.4% of hospitalized CHF patients in China have coronary heart disease.15 Therefore, it is speculated that device treatment of ischaemic CHF in China may be insufficient, although the relevant reasons are still unclear.
This study showed that the mortality rate of CHF patients in China with device implantation (8.6% person-years) was higher than that of previous reports from Western countries (mortality rate person-years, 4.0% in the DANISH study16 and 3.0% in the MADIT CRT study17), and the cause of death was mainly HF. One possible reason is that the baseline heart function of CHF patients receiving device implantation in China is poor. In this study, the proportion of NYHA Class III or IV patients was near 80%; in contrast, it was only 46.0% in the DANISH study and 10% in the MADIT CRT study. However, the proportion of NYHA Class III or IV patients in the French CeRtiTuDe registration study was 83.0%, and the overall annual mortality rate was 8.4%,18 which was similar to the results of our study. These results revealed that more patients in late-stage CHF receive device implantation in real-world clinical practice compared with randomized controlled trials. This finding suggests that for the practice of CHF device implantation in China, it is necessary to optimize the indications for patient selection and to perform device implantation at an earlier stage of CHF.
The role of an ICD in the primary prevention of SCD in patients with CHF has been well documented,11, 19-21 but its effect on all-cause death has been shown to be different in ischaemic and non-ischaemic cases. The early MADIT I study,21 MADIT II study,19 and MUSTT study20 showed that an ICD can reduce all-cause mortality in patients with CHF after myocardial infarction. In the subsequent DEFINITE study22 and SCD-HeHF study,11 ICD implantation tended to reduce all-cause mortality in non-ischaemic cases. In contrast, the recently published DANISH study failed to confirm the hypothesis that an ICD reduces all-cause mortality in non-ischaemic cases. In the current study, the SCD rate was significantly lower in the CRT-D group than in the CRT-P group, but no significant reduction in all-cause mortality was observed. One possible explanation is that non-ischaemic cases constituted as many as 80% of all the patients included in this study and that those with device implantation but without ischaemia had a relatively small absolute mortality benefit from ICD therapy among medically treated settings for primary prevention.23 Therefore, it is difficult to detect a difference in mortality between the CRT-D and CRT-P groups. In addition, patients enrolled in Chinese studies were more seriously ill (the proportions of NYHA Class IV patients were 10.1% in the ICD group and 22.6% in the CRT-D group), and most died of HF. Defibrillation had a preventive effect on SCD, but a reduction in all-cause mortality was not clear.
Currently, for CHF patients with CRT-P indications, recommendations regarding whether a defibrillator should be implanted are controversial.2, 3 The only randomized trial, the COMPANION study, did not show that CRT-D was superior to CRT-P in reducing endpoint events.12 Nevertheless, observational studies reported different results: two observational studies, an Italian registration study based on Class Ia ECS indications and another US-based registration study, showed that CHF patients receiving CRT-D had better survival benefits than patients receiving CRT-P.24, 25 In contrast, real-world studies in France showed that the increased death rate in the CRT-P group compared with the CRT-D group was mainly due to non-SCD, suggesting that the additional defibrillator did not significantly improve benefits for patients undergoing CRT-P implantation according to the current guidelines.18 The results of this study also suggest that among Chinese CHF patients receiving device implantation for primary prevention of SCD, CRT-D effectively prevented SCD but did not significantly improve the prognosis of all-cause mortality compared with CRT-P. With respect to the current guidelines-based clinical practice in China, the results of this study indicate that the selection of patients receiving a defibrillator is reasonable but that a defibrillator does not increase the total clinical benefit for patients who meet CRT-P indications. Further studies with new inclusion criteria may be needed to identify CRT-P patients who might obtain additional benefits from a defibrillator.
Strengths and limitations
This registry study includes the largest sample of CHF patients with indications for primary prevention of SCD in China. The results of this study reflect the current state of treatment of CHF with device implantation and show the clinical features of patients undergoing device implantation in China. This study provides clinical evidence for the practice of device implantation in China. However, this study has certain limitations. First, it is a registration study, and thus, it is difficult to completely avoid various biases and confounding factors. Second, although resynchronization therapy reduces all-cause mortality, it is not considered ‘primary prevention therapy’ as a defibrillator therapy. Regardless, this was an observational study. The study protocol did not interfere with the treatment decision of patients or doctors; indeed, some patients received the CRT-P and not CRT-D based on their wishes or doctors' decisions. This study presented China's real-world situation of device therapy for primary prevention therapy in 2012. Third, the sample size was relatively small, the follow-up time was relatively short, and the baseline cardiac function was also relatively poor (the proportions of NYHA Class III and IV patients were near 80%). Most CHF patients died of HF and not SCD, which is not conducive to finding differences in all-cause mortality between groups. Furthermore, the small sample size limited further subgroup analysis, such as the effect of CRT on outcomes in patients with atrial fibrillation. Fourth, although SCD was defined in detail (in Appendix S2), adjudication to SCD compared with non-SCD is often very difficult, and potential adjudication biases could have influenced the reliability of the conclusion. Fifth, population heterogeneity should be considered because upgrades from pacemakers were allowed in this study. Nonetheless, the proportion of patients with pacemaker cardiomyopathy was very low (only 27 patients), which would not have had a significant impact on the results or conclusions of this study. Finally, for some results in this study, such as the lower proportion of patients with ischaemic CHF who received device implantation, this study cannot explain these findings due to the limitations of the original design. These questions are expected to be answered by further studies in the future.
Conclusions
For Chinese CHF patients with indications for primary prevention of SCD who received device implantation, the aetiology was mainly non-ischaemic CHF. Although these patients received optimal medical therapy and underwent device implantation as recommended in the guidelines, all-cause mortality was still high, and the most important cause of death was HF. No differences in all-cause death between the CRT-D and CRT-P groups were observed, but the CRT-D group had a lower SCD rate than the CRT-P group.
Conflict of interest
The authors declare that they have no competing interests.
Funding
This study was supported by the National Science and Technology Program during the Twelfth Five-year Plan Period (2011BAI11B11, Beijing, China).




