Prognostic effects of longitudinal changes in left ventricular ejection fraction with cardiac resynchronization therapy
Abstract
Aims
Left ventricular ejection fraction (LVEF) is considered an indicator of cardiac resynchronization therapy (CRT). Longitudinal studies on the predictive value of LVEF are scarce. We aimed to comprehensively evaluate the prognostic role of LVEF in the outcomes of Chinese patients with CRT.
Methods and results
Three hundred ninety-two patients were divided into three tertiles of LVEF: ≤25%, 25–30%, and 30–35%, and four groups by LVEF changes: <0% (negative response); ≥0% and ≤5% (non-response); >5% and ≤15% (response); and >15% (super-response). One hundred six patients were super-responders. During a median follow-up of 3.6 years, 141 reached the composite endpoint. Odds ratios (ORs) for super-response depicted a reversed U-shaped relationship for baseline LVEF with a peak at 25–30%. Independent predictors of super-response were smaller left atrial diameter [odds ratio 0.897, 95% confidence interval (CI) 0.844–0.955, P = 0.001], smaller left ventricular end-diastolic diameter (OR 0.937, 95% CI 0.889–0.989, P = 0.018), and higher estimated glomerular filtration rate (OR 1.018, 95% CI 1.001–1.035, P = 0.042) in Tertile 1; atrial fibrillation (OR 0.278, 95% CI 0.086–0.901, P = 0.033), left bundle branch block (OR 4.096, 95% CI 1.046–16.037, P = 0.043), and left ventricular end-diastolic diameter (OR 0.929, 95% CI 0.876–0.986, P = 0.016) in Tertile 2; while female sex (OR 2.778, 95% CI 1.082–7.132, P = 0.034) and higher systolic blood pressure (OR 1.045, 95% CI 1.013–1.079, P = 0.006) in Tertile 3. An inverse association with the composite endpoint was found in Tertile 1 vs. Tertile 2 (hazard ratio 1.934, 95% CI 1.248–2.996, P = 0.003). The prognostic effects of CRT response in Tertile 3 and Tertile 1 varied significantly (P for trend = 0.017 and <0.001). Among three tertiles in super-responders, event-free survival was similar (P for trend = 0.143).
Conclusions
Left ventricular ejection fraction of 25–30% is associated with a better prognosis of super-response. Predictors of super-response are different for LVEF tertiles. CRT responses would have better prognostic performance than LVEF tertiles at baseline, which should be considered when clinicians screening eligible patients for CRT.
Introduction
In patients with chronic heart failure (HF), left ventricular ejection fraction (LVEF) is the main factor to evaluate the performance of cardiac resynchronization therapy (CRT). LVEF of 35% was a determined cut-off value for the eligibility of CRT implantation, and it was also used to define the super-response to CRT. Prior studies identified that the incidence of super-response ranges from 9.7% to 37.8%, which was mostly evaluated by LVEF > 50% after 6 months of CRT.1-10 The important MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy) study5 included 752 CRT with defibrillator patients, and 25.4% were super-responders with an LVEF increase of ≥14.5%. Six predictors were identified as follows: female sex, no prior myocardial infarction, QRS duration ≥150 ms, left bundle branch block (LBBB), body mass index, and smaller baseline left atrial volume index. And hypo-response was associated with an increased risk of HF or all-cause mortality. Ypenburg et al.11 indicated that the extent of left ventricular (LV) reverse remodelling that was evaluated by LV end-systolic volume (LVESV) before implantation and after 6 months of CRT was associated with clinical improvement and the long-term follow-up including all-cause mortality and hospitalizations for HF.
To our best knowledge, baseline LVEF has a great deal of effect on outcomes12, 13 so that the effects of LVEF on the prognosis of patients with CRT should also be fully investigated. Agir et al. divided 141 CRT patients into three groups based on the baseline LVEF: 5–15%, 15–25%, and 25–35%, and found that there was no lower limit for baseline LVEF to predict non-response that defined as LVESVi < 10% at 6 months.14 However, whether there is a lower or higher limit to predict the super-response assessed by LVEF is unknown. Meanwhile, in the MADIT-CRT study, patients with baseline LVEF of ≤25% had a higher risk of HF/death than those with LVEF > 25%.15 But few studies illustrate the effects of LVEF at baseline and changes after CRT on the composite outcomes. Therefore, the aims of the current study were to (i) evaluate the association between baseline LVEF and CRT super-response assessed by the LV functional improvement; (ii) explore the predictors of super-response driven by baseline LVEF subgroups; (iii) verify the association between baseline LVEF and the composite endpoint; and (iv) explore the impact of longitudinal LVEF changes on the long-term prognosis.
Methods
Population and design
The records of HF patients who underwent the implantation of CRT from January 2008 to December 2017 in the Cardiac Arrhythmia Center, Fuwai Hospital, were extracted. Patients were recommended for CRT with the evidence of Class I and Class II indications according to the widely accepted guidelines.16-18 The inclusion criteria were as follows: (i) the medical records of the baseline LVEF and the LVEF at 6 months after CRT implantation were available; (ii) patients had pre-implant LVEF ≤ 35%; (iii) patients had de novo CRTs; and (iv) patients had no prior pacemakers or implantable cardioverter defibrillators. Patients who failed the operation, died in hospital, or reached the endpoint within the following 6 months were ruled out. Finally, 392 patients were eligible for analysis (Figure 1). This retrospective study accorded with the Declaration of Helsinki was performed with the written informed consent and was approved by the ethics committee.
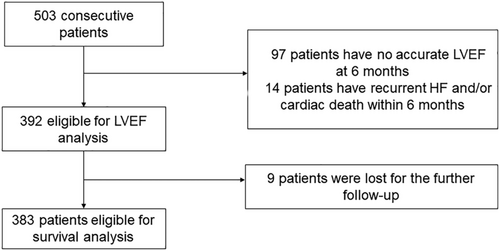
All the patients were subject to the optimal medications before the operation and throughout the follow-up period. Follow-up was based on the outpatient clinics or the medical phone calls, and the last follow-up was in December 2018.
Baseline characteristics included demographic parameters, co-morbidities, vital signs, echocardiographic parameters, and drugs. All patients underwent complete echocardiography examination at baseline and 6 months after CRT device implantation.
Definitions
Left ventricular ejection fraction was immediately estimated when the transthoracic echocardiography conducted by echocardiographers, using the following formula: EF = (EDV − ESV)∕EDV. The biplane method of discs (modified Simpson's rule) is the currently recommended two-dimensional method to assess LVEF in our hospital.19 Pulmonary hypertension (PH) was defined as pulmonary artery systolic pressure ≥35 mmHg. The true complete LBBB was defined as QRS > 120 ms, QS or rS form in V1 and broad R waves with a notch or a slur, without Q waves in the lead I or V6, as well as notched, or slurred R wave in the lead I, aVL, V5, and V6.20 Estimated glomerular filtration rate (eGFR) was calculated using the modified equation for Chinese patients and expressed in mL/min/1.73 m2.21 In this study, patients were divided into three tertiles by baseline LVEF: ≤25%, 25–30%, and 30–35%, and then four groups by LVEF changes after CRT: <0% (negative response); ≥0 and ≤5% (non-response); >5% and ≤15% (response); and >15% (super-response), irrespective of hospitalization for HF and New York Heart Association (NYHA) class. The clinical outcome was a composite endpoint of rehospitalizations for HF or cardiac death.
Device implantation
Cardiac resynchronization therapy device implantation was all performed transvenously, and the LV lead was placed through the coronary sinus. The right ventricular lead and the right atrial lead were implanted in the apex of the right ventricle and the right auricular, respectively. All the CRT devices were programmed to obtain the optimal A–V and V–V intervals according to echocardiography or electrocardiography at discharge.22
Statistical analysis
Categorical variables were expressed using percentages and continuous parameters using mean ± standard deviation or median [inter-quartile range (IQR)]. Comparisons among three tertiles were conducted using the one-way analysis of variance for continuous variables and χ2 test for dichotomous variables. Logistic regression analysis was performed to identify the association between three LVEF tertiles and CRT super-response. To keep the uniformity and reliability of the results, robust subgroup analyses were also performed by smooth spline curves according to sex and the presence of prior atrial fibrillation, myocardial infarction, LBBB, and defibrillator. To identify the predictors of super-response in each LVEF subgroup, logistic regression analysis was also performed. Variables with P value <0.1 in any of the subgroups were listed in the unadjusted model, and variables were entered into the multivariate analysis according to the P value in univariate analysis and the events number. Cox proportional hazard regression was used to explore the predictive effects of baseline LVEF and longitudinal changes in LVEF on the long-term composite endpoint. Event-free survival curves were determined according to the Kaplan–Meier method, with comparisons of cumulative event-free rates by the log-rank test. All analysis were assessed by IBM SPSS Statistics 25.0 software package (SPSS, Inc., Chicago, IL) and R software version 3.6.0 (R Core Team, Vienna, Austria). A two-tailed P value of <0.05 was considered significant.
Results
Baseline characteristics of study patients
A total of 392 HF patients were finally enrolled with a mean age of 58.7 ± 10.9 years, predominantly male (64.8%); 86.5% of patients had no history of myocardial infarction; and 79.3% of patients were diagnosed with dilated cardiomyopathy (DCM). There were significant differences between LVEF tertiles for atrial fibrillation, hyperlipidaemia, PH, systolic blood pressure (SBP), NYHA classification, left atrial diameter (LAD), LV end-diastolic diameter (LVEDD), and baseline LVEF (P < 0.05). The other characteristics did not show statistical significance (P > 0.05). Compared with Tertile 1 and Tertile 2 of LVEF, patients in Tertile 3 were most likely to have the highest percentages of hyperlipidaemia, the highest SBP, the lowest percentages of PH, significant mitral regurgitation and NYHA classification of IV, and the smallest LAD and LVEDD (all P < 0.05) (Table 1).
| Characteristics | LVEF (%) | P value | ||
|---|---|---|---|---|
| Tertile 1 (≤25) | Tertile 2 (25–30) | Tertile 3 (>30) | ||
| N | 143 | 126 | 123 | |
| Male, n (%) | 97 (67.8) | 84 (66.7) | 73 (59.3) | 0.306 |
| Age (years) | 58.0 ± 10.7 | 58.2 ± 10.5 | 60.1 ± 11.4 | 0.235 |
| BMI (kg/m2) | 23.8 (21.7–26.2) | 24.6 (22.5–26.7) | 23.9 (22.1–26.6) | 0.486 |
| DCM | 115 (80.4) | 102 (81.0) | 94 (76.4) | 0.625 |
| Smoking, n (%) | 67 (46.9) | 47 (37.3) | 45 (36.6) | 0.156 |
| MI, n (%) | 19 (13.3) | 15 (11.9) | 19 (15.4) | 0.712 |
| DM, n (%) | 30 (21.0) | 34 (27.0) | 33 (26.8) | 0.424 |
| HTN, n (%) | 43 (30.1) | 38 (30.2) | 49 (39.8) | 0.165 |
| AF, n (%) | 17 (11.9) | 32 (25.4) | 25 (20.3) | 0.016 |
| CLBBB, n (%) | 124 (86.7) | 98 (77.8) | 101 (82.1) | 0.157 |
| HLP, n (%) | 43 (30.1) | 43 (34.1) | 55 (44.7) | 0.040 |
| PH, n (%) | 28 (19.6) | 25 (19.8) | 6 (4.9) | 0.001 |
| SBP (mmHg) | 110.0 (100.0–120.0) | 112.0 (100.0–125.0) | 120.0 (110.0–130.0) | 0.001 |
| DBP (mmHg) | 70.0 (65.0–80.0) | 70.0 (62.5–80.0) | 70.0 (64.0–80.0) | 0.819 |
| NYHA, n (%) | 0.007 | |||
| II | 21 (14.7) | 23 (18.3) | 33 (26.8) | |
| III | 91 (63.6) | 82 (65.1) | 81 (65.9) | |
| IV | 31 (21.7) | 21 (16.7) | 9 (7.3) | |
| Echocardiographic parameters | ||||
| LAD (mm) | 44.4 ± 8.4 | 42.0 ± 9.0 | 40.9 ± 7.9 | 0.003 |
| LVEDD (mm) | 75.0 (70.0–82.0) | 69.0 (62.8–75.3) | 65.0 (60.0–71.0) | <0.001 |
| LVEF (%) | 22.0 (20.0–24.0) | 29.0 (28.0–30.0) | 33.0 (32.0–34.0) | <0.001 |
| Mitral regurgitation, n (%) | <0.001 | |||
| None | 5 (3.5) | 14 (11.1) | 29 (23.6) | |
| Mild | 42 (29.4) | 50 (39.7) | 46 (37.4) | |
| Significant | 96 (67.1) | 62 (49.2) | 48 (39.0) | |
| QRS duration (ms) | 160.0 (152.0–180.0) | 164.0 (152.0–180.0) | 160.0 (152.0–176.0) | 0.165 |
| Creatinine (μmol/L) | 86 (74–103) | 86 (75–99) | 87 (76–101) | 0.972 |
| eGFR (mL/min/1.73 m2) | 97 ± 26 | 97 ± 24 | 96 ± 30 | 0.941 |
| Drugs, n (%) | ||||
| ACEI/ARB | 128 (89.5) | 111 (88.1) | 115 (93.5) | 0.327 |
| Beta-blockers | 134 (93.7) | 122 (96.8) | 114 (92.7) | 0.331 |
| Spironolactone | 120 (83.9) | 111 (88.1) | 108 (87.8) | 0.530 |
| Loop diuretics | 139 (97.2) | 121 (96.0) | 116 (94.3) | 0.492 |
| Statins | 57 (39.9) | 53 (42.1) | 59 (48.0) | 0.395 |
| CRT-D, n (%) | 82 (57.3) | 70 (55.6) | 66 (53.7) | 0.834 |
| LV pacing site, n (%) | ||||
| Lateral or posterior site | 124 (86.7) | 114 (90.5) | 109 (88.6) | 0.627 |
- ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF, atrial fibrillation; BMI, body mass index; CLBBB, complete left bundle branch block; CRT-D, cardiac resynchronization therapy with defibrillator; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HLP, hyperlipidaemia; HTN, hypertension; LAD, left atrial diameter; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; PH, pulmonary hypertension; SBP, systolic blood pressure.
Follow-up after cardiac resynchronization therapy
After a 6 month follow-up, in total patients, LVEF was improved from 28.0% (IQR 24.0–32.0%) to 38.0% (IQR 30.0–47.0%) (P < 0.001). LAD was decreased from 42.5 ± 8.5 to 39.7 ± 9.2 mm (P < 0.001), and LVEDD was decreased from 70.0 mm (IQR 64.0–77.0 mm) to 64.0 mm (IQR 56.3–74.0 mm) (P < 0.001). The QRS duration shortened from 164.0 ms (IQR 152.0–180.0 ms) to 138.0 ms (IQR 132.0–148.0 ms) (P < 0.001). And 106 patients (27.0%) experienced functional super-response to CRT, of whom 34 patients were in Tertile 1 (n = 143), 45 were in Tertile 2 (n = 126), and 27 in Tertile 3 (n = 123). During a median follow-up of 3.6 years (IQR 2.1–5.4 years), 9 (2.3%) patients were lost for visits, and 141 (36.0%) patients reached the composite endpoint (120 suffered rehospitalizations, and 88 suffered cardiac death). Out of the 141 patients, 74 patients (52.5%) were in Tertile 1, 37 patients (26.2%) in Tertile 2, and 30 (21.3%) in Tertile 3. And in the 141 patients, 30 (21.3%) showed negative response to CRT; 48 (34.0%) showed non-response; 53 (37.6%) showed response; and 10 (7.1%) showed super-response.
Association of left ventricular ejection fraction with cardiac resynchronization therapy super-response
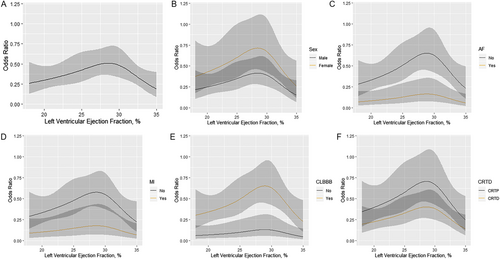
Smoothing spline analysis showed a peak of CRT super-response with LVEF at 25–30% (Figure 2). As seen in Table 2, compared with LVEF of Tertile 2 (25–30%), Tertile 1 (≤25%) had a lower risk of CRT super-response [odds ratio (OR) 0.561, 95% confidence interval (CI) 0.330–0.954; P = 0.033], so did Tertile 3 (>30% and ≤35%) (OR 0.506, 95% CI 0.289–0.887, P = 0.017), leading to a reversed U-shaped relationship between LVEF and CRT super-response, even after adjusting for multiple factors (P for trend = 0.001). Compared with Tertile 2, the other LVEF intervals also had ORs < 1 and remained similar in the multivariable analysis (Table 2).
| Characteristics | Unadjusted OR (95% CI), P value | Adjusted OR (95% CI), P value | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| Age (years) | 0.990 (0.970–1.011), 0.346 | 0.991 (0.971–1.012), 0.397 | 0.998 (0.975–1.022), 0.863 | 0.983 (0.954–1.013), 0.274 |
| Female sex | 1.707 (1.081–2.696), 0.022 | 1.497 (0.895–2.506), 0.124 | 0.607 (0.315–1.169), 0.135 | |
| BMI (kg/m2) | 0.995 (0.943, 1.049), 0.842 | 0.992 (0.937–1.050), 0.992 | 1.010 (0.943–1.082), 0.767 | |
| MI | 0.305 (0.126, 0.736), 0.008 | 0.244 (0.079–0.755), 0.014 | 0.177 (0.050–0.621), 0.007 | |
| HTN | 1.247 (0.782–1.989), 0.353 | 1.444 (0.831–2.509), 0.192 | 1.437 (0.742–2.786), 0.282 | |
| DM | 0.683 (0.396, 1.177), 0.170 | 0.713 (0.381–1.333), 0.289 | 0.616 (0.292–1.301), 0.204 | |
| AF | 0.272 (0.126–0.589), 0.001 | 0.296 (0.124–0.705), 0.006 | 0.246 (0.084–0.717), 0.010 | |
| HLP | 0.837 (0.523–1.340), 0.459 | 0.878 (0.503–1.533), 0.878 | 0.680 (0.340–1.361), 0.276 | |
| PH | 0.318 (0.140–0.725), 0.006 | 0.232 (0.093–0.578), 0.002 | 0.381 (0.133–1.090), 0.072 | |
| CLBBB | 4.709 (1.973–11.239), <0.001 | 26.149 (3.231–211.631), 0.002 | ||
| SBP | 1.028 (1.013–1.042), <0.001 | 1.034 (1.010–1.058), 0.005 | ||
| NYHA | ||||
| II | Reference | Reference | ||
| III | 0.970 (0.551–1.707), 0.916 | 1.767 (0.797–3.918), 0.161 | ||
| IV | 0.677 (0.308–1.488), 0.332 | 1.362 (0.453–4.090), 0.582 | ||
| LAD (mm) | 0.931 (0.905–0.958), <0.001 | 0.940 (0.902–0.981), 0.004 | ||
| LVEDD (mm) | 0.943 (0.918–0.969), <0.001 | 0.927 (0.890–0.965), <0.001 | ||
| LVEF (%) | ||||
| Tertile 1, ≤25 | 0.561 (0.330–0.954), 0.033 | 0.560 (0.329–0.951), 0.032 | 0.460 (0.256–0.826), 0.009 | 0.811 (0.395–1.665), 0.568 |
| Tertile 2, 25–30 | Reference | Reference | Reference | Reference |
| Tertile 3, >30 | 0.506 (0.289–0.887), 0.017 | 0.514 (0.293–0.902), 0.020 | 0.363 (0.194–0.681), 0.002 | 0.258 (0.121–0.548), <0.001 |
| P for trend | 0.015 | 0.017 | 0.001 | 0.001 |
| QRS (ms) | 1.017 (1.004–1.029), 0.008 | 1.022 (1.005–1.039), 0.011 | ||
| eGFR (mL/min/1.73 m2) | 1.004 (0.995–1.012), 0.403 | 0.996 (0.984–1.008), 0.495 | ||
| Drugs | ||||
| ACEI/ARB | 1.436 (0.636–3.240), 0.384 | 0.502 (0.139–1.813), 0.293 | ||
| Beta-blockers | 1.277 (0.459–3.551), 0.640 | 1.328 (0.307–5.755), 0.704 | ||
| Spironolactone | 1.487 (0.735–3.008), 0.270 | 0.798 (0.317–2.013), 0.633 | ||
| CRT-D | 0.595 (0.380–0.932), 0.023 | 0.637 (0.346–1.172), 0.147 | ||
| LV pacing site | 2.167 (0.936–5.015), 0.071 | 1.841 (0.661–5.129), 0.243 | ||
- ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; CLBBB, complete left bundle branch block; CRT-D, cardiac resynchronization therapy with defibrillator; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HLP, hyperlipidaemia; HTN, hypertension; LAD, left atrial diameter; LV, left ventricular; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; OR, odds ratio; PH, pulmonary hypertension; SBP, systolic blood pressure.
- Model 1: adjusted for age. Model 2: adjusted for age, sex, BMI, history of MI, history of HTN, history of diabetes, history of AF, HLP, and PH. Model 3: adjusted for age, sex, BMI, history of MI, history of HTN, history of diabetes, history of AF, HLP, PH, presence of CLBBB, SBP, NYHA classification, LAD, LVEDD, pre-QRS, eGFR, the use of ACEI/ARB, the use of beta-blockers, the use of spironolactone, the presence of affiliated defibrillator, and the site of LV pacing.
Sensitivity analyses
When classified by sex, atrial fibrillation, myocardial infarction, LBBB, and CRT with defibrillation, the unadjusted LVEF ORs maintained a reversed U-shaped relationship with a peak at 25–30% (Figure 2).
Predictors of super-response in left ventricular ejection fraction subgroups
Logistic regression analysis indicated that smaller LAD (OR 0.897, 95% CI 0.844–0.955, P = 0.001) and LVEDD (OR 0.937, 95% CI 0.889–0.989, P = 0.018) and higher eGFR (OR 1.018, 95% CI 1.001–1.035, P = 0.042) were independent predictors of super-response in patients with LVEF of ≤25%. The absence of atrial fibrillation (OR 0.278, 95% CI 0.086–0.901, P = 0.033), the presence of complete LBBB (CLBBB) (OR 4.096, 95% CI 1.046–16.037, P = 0.043), and smaller LVEDD (OR 0.929, 95% CI 0.876–0.986, P = 0.016) were significantly associated with super-response in Tertile 2 of LVEF; while female sex (OR 2.778, 95% CI 1.082–7.132, P = 0.034) and higher SBP (OR 1.045, 95% CI 1.013–1.079, P = 0.006) were identified to independently predict super-response in patients with LVEF higher than 30% (Table 3).
| Unadjusted model | Adjusted model | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR | P value | |
| Tertile 1/super-response (n = 143/34) | ||||
| Female sex | 1.011 (0.444–2.304) | 0.979 | — | — |
| AF | 0.392 (0.085–1.807) | 0.230 | — | — |
| CLBBB | 6.527 (0.838–50.841) | 0.073 | — | — |
| SBP (mmHg) | 1.018 (0.993–1.044) | 0.167 | — | — |
| LAD (mm) | 0.895 (0.847–0.946) | <0.001 | 0.897 (0.844–0.955) | 0.001 |
| LVEDD (mm) | 0.921 (0.877–0.967) | 0.001 | 0.937 (0.889–0.989) | 0.018 |
| QRS (ms) | 1.010 (0.990–1.029) | 0.341 | — | — |
| eGFR (mL/min/1.73 m2) | 1.014 (0.999–1.030) | 0.064 | 1.018 (1.001–1.035) | 0.042 |
| Tertile 2/super-response (n = 126/45) | ||||
| Female sex | 2.145 (0.998–4.611) | 0.051 | — | — |
| AF | 0.250 (0.089–0.706) | 0.009 | 0.278 (0.086–0.901) | 0.033 |
| CLBBB | 6.250 (1.768–22.092) | 0.004 | 4.096 (1.046–16.037) | 0.043 |
| SBP (mmHg) | 1.029 (1.005–1.052) | 0.016 | 1.023 (0.996–1.051) | 0.098 |
| LAD (mm) | 0.940 (0.898–0.983) | 0.007 | 0.989 (0.937–1.045) | 0.699 |
| LVEDD (mm) | 0.932 (0.887–0.979) | 0.005 | 0.929 (0.876–0.986) | 0.016 |
| QRS (ms) | 1.018 (0.997–1.040) | 0.098 | — | — |
| eGFR (mL/min/1.73 m2) | 0.993 (0.977–1.008) | 0.336 | — | — |
| Tertile 3/super-response (n = 123/27) | ||||
| Female sex | 2.652 (1.107–6.358) | 0.029 | 2.778 (1.082–7.132) | 0.034 |
| AF | 0.115 (0.015–0.896) | 0.039 | 0.134 (0.017–1.075) | 0.059 |
| CLBBB | 3.289 (0.718–15.073) | 0.125 | — | — |
| SBP (mmHg) | 1.046 (1.016–1.077) | 0.003 | 1.045 (1.013–1.079) | 0.006 |
| LAD (mm) | 0.954 (0.902–1.010) | 0.103 | — | — |
| LVEDD (mm) | 0.944 (0.887–1.005) | 0.073 | — | — |
| QRS (ms) | 1.024 (0.998–1.051) | 0.065 | — | — |
| eGFR (mL/min/1.73 m2) | 1.004 (0.990–1.018) | 0.600 | — | — |
- AF, atrial fibrillation; CI, confidence interval; CLBBB, complete left bundle branch block; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; OR, odds ratio; SBP, systolic blood pressure.
The composite endpoint-free survival according to left ventricular ejection fraction
The composite endpoint-free survival rates in total patients were 87.9%, 79.4%, 71.0%, and 53.6% at 1, 2, 3, and 6 years, respectively. In Tertile 1, Tertile 2, and Tertile 3, event-free survival at 1 year was 82.9%, 91.8%, and 89.9%, respectively; at 2 years were 68.1%, 85.9%, and 86.3%, respectively; at 3 years were 58.1%, 76.1%, and 81.9%; and at 6 years were 41.2%, 58.4%, and 65.5%, respectively. When divided by the extent of response to CRT, the composite endpoint-free survival rates in patients with negative response were 64.6%, 53.4%, 43.7%, and 24.9% at 1, 2, 3, and 6 years, respectively; in patients with non-response were 79.6%, 63.4%, 47.1%, and 28.0%; in patients with response were 92.7%, 83.8%, 73.9%, and 54.3%; and in patients with super-response were 98.1%, 97.1%, 97.1%, and 85.3%.
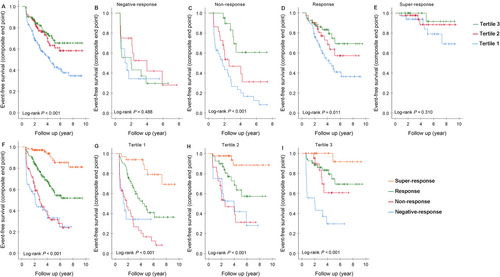
A significant difference was found in the composite endpoint (Figure 3, log-rank P < 0.001) among the three tertiles. CRT patients in Tertile 1 had the poorest outcomes of the composite endpoint. Concerning the subgroups of response to CRT, there was a significant difference in event-free survival among the four groups (Figure 3, log-rank P < 0.001), and patients with super-response had the best survival free from the composite endpoint. Separate comparisons revealed that patients with response and super-response had significant differences from patients with negative response (log-rank P values <0.001), except for patients with non-response (P = 0.448).
Baseline left ventricular ejection fraction and changes of left ventricular ejection fraction in relation to composite endpoint
Baseline LVEF as a continuous variable was strongly and inversely related to the composite endpoint [hazard ratio (HR) 0.941, 95% CI 0.905–0.978, P = 0.002]. In the categorical analysis, the unadjusted HR for composite endpoint in Tertile 1 vs. Tertile 2 was 1.854 (95% CI 1.249–2.751, P = 0.002) and in Tertile 3 vs. Tertile 2 was 0.824 (95% CI 0.509–1.334, P = 0.432), which remained similar when minimally adjusted for possible confounders (Tertile 1 vs. Tertile 2: HR 1.907, 95% CI 1.281–2.840, P = 0.001; Tertile 3 vs. Tertile 2: HR 0.868, 95% CI 0.535–1.408, P = 0.567). The inverse relationship between baseline LVEF and the composite endpoint was also found in the fully adjusted model when comparing Tertile 1 with Tertile 2 (HR 1.934, 95% CI 1.248–2.996, P = 0.003). However, this relationship was not seen in Tertile 3 vs. Tertile 2 (HR 1.359, 95% CI 0.797–2.319, P = 0.260) (Table 4).
| Number of composite endpoint/total number | Unadjusted HR (95% CI), P value | Minimally adjusted HR (95% CI), P valuea,b | Fully adjusted HR (95% CI), P valuec | |
|---|---|---|---|---|
| LVEF at baseline (%) | ||||
| LVEF (continuous) | 141/383 | 0.933 (0.905–0.961), <0.001 | 0.933 (0.905–0.962), <0.001 | 0.941 (0.905–0.978), 0.002 |
| LVEF tertiles | ||||
| Tertile 1 | 74/140 | 1.854 (1.249–2.751), 0.002 | 1.907 (1.281–2.840), 0.001 | 1.934 (1.248–2.996), 0.003 |
| Tertile 2 | 37/122 | Reference | Reference | Reference |
| Tertile 3 | 30/121 | 0.824 (0.509–1.334), 0.432 | 0.868 (0.535–1.408), 0.567 | 1.359 (0.797–2.319), 0.260 |
| P for trend | — | 0.513 | 0.683 | 0.095 |
| Longitudinal changes in LVEF (%) | ||||
| <0 (NEG) | 30/48 | Reference | Reference | Reference |
| ≥0, ≤5 (NON) | 48/80 | 0.833 (0.528–1.315), 0.433 | 0.654 (0.410–1.042), 0.074 | 0.759 (0.460–1.254), 0.282 |
| >5, ≤15 (RESP) | 53/151 | 0.350 (0.223–0.549), <0.001 | 0.246 (0.152–0.400), <0.001 | 0.396 (0.226–0.694), 0.001 |
| >15 (SUPER) | 10/104 | 0.081 (0.039–0.166), <0.001 | 0.059 (0.028–0.123), <0.001 | 0.100 (0.045–0.222), <0.001 |
| P for trend | — | <0.001 | <0.001 | <0.001 |
| Subgroup analysis | ||||
| Longitudinal changes of LVEF in patients with NYHA IV | ||||
| <0 (NEG) | 11/14 | Reference | Reference | — |
| ≥0, ≤5 (NON) | 13/18 | 0.732 (0.327–1.642), 0.449 | 0.628 (0.267–1.477), 0.286 | — |
| >5, ≤15 (RESP) | 9/16 | 0.450 (0.185–1.094), 0.078 | 0.284 (0.107–0.757), 0.012 | — |
| >15 (SUPER) | 3/13 | 0.137 (0.038–0.500), 0.003 | 0.102 (0.027–0.392), 0.001 | — |
| P for trend | — | 0.001 | <0.001 | — |
| Tertile 1 with NEG | 9/14 | Reference | Reference | Reference |
| Tertile 1 with NON | 29/35 | 1.005 (0.474–2.132), 0.989 | 0.897 (0.418–1.926), 0.781 | 0.801 (0.353–1.817), 0.595 |
| Tertile 1 with RESP | 30/58 | 0.376 (0.177–0.800), 0.011 | 0.285 (0.129–0.631), 0.002 | 0.416 (0.168–1.028), 0.057 |
| Tertile 1 with SUPER | 6/33 | 0.096 (0.034–0.274), <0.001 | 0.067 (0.023–0.197), <0.001 | 0.081 (0.023–0.288), <0.001 |
| P for trend | — | <0.001 | <0.001 | <0.001 |
| Tertile 3 with NEG | 12/18 | Reference | Reference | Reference |
| Tertile 3 with NON | 6/23 | 0.285 (0.107–0.761), 0.012 | 0.301 (0.104–0.874), 0.027 | 0.274 (0.065–1.157), 0.078 |
| Tertile 3 with RESP | 11/53 | 0.193 (0.085–0.442), <0.001 | 0.236 (0.093–0.598), 0.002 | 0.290 (0.074–1.136), 0.076 |
| Tertile 3 with SUPER | 1/27 | 0.032 (0.004–0.248), 0.001 | 0.038 (0.005–0.308), 0.002 | 0.071 (0.007–0.693), 0.023 |
| P for trend | — | <0.001 | <0.001 | 0.017 |
| Tertile 1 with SUPER | 6/33 | Reference | Reference | Reference |
| Tertile 2 with SUPER | 3/44 | 0.524 (0.130–2.112), 0.364 | — | — |
| Tertile 3 with SUPER | 1/27 | 0.245 (0.029–2.036), 0.193 | — | — |
| P for trend | — | 0.143 | — | — |
- AF, atrial fibrillation; CI, confidence interval; CLBBB, complete left bundle branch block; CRT-D, cardiac resynchronization therapy with defibrillator; HR, hazard ratio; LAD, left atrial diameter; LVEDD, left ventricular end-diastolic diameter; MI, myocardial infarction; NEG, negative response; NON, non-response; NYHA, New York Heart Association; RESP, response; SBP, systolic blood pressure; SUPER, super-response.
- a Adjusted for age, gender, AF, and MI.
- b For longitudinal changes also adjusted for baseline LVEF.
- c All confounders in the minimally adjusted model and SBP, LAD, LVEDD, CLBBB, QRS duration at baseline, NYHA classification, mitral regurgitation, and CRT-D.
Given that the implantation of CRT could make a big difference in cardiac function improvement, we further analysed whether longitudinal changes in LVEF could predict the composite endpoint. Similarly, a significant and reverse relationship was depicted in Table 4: compared with patients with negative response to CRT, HRs were 0.759 (95% CI 0.460–1.254, P = 0.282) in patients with response, 0.396 (95% CI 0.226–0.694, P = 0.001) in patients with response, and 0.100 (95% CI 0.045–0.222, P < 0.001) in patients with super-response after fully adjusted for confounders, including baseline LVEF.
Composite endpoint in subgroup analysis
The earlier relationship was also tested in a subgroup of individuals with NYHA IV; compared with those who showed negative response to CRT, patients with response to CRT had an HR of 0.284 (95% CI 0.107–0.757, P = 0.012) for the composite endpoint, and patients who had super-response had an HR of 0.102 (95% CI 0.027–0.392, P = 0.001). Moreover, when compared with negative-response patients in Tertile 1, only patients with super-response had an HR of 0.081 (95% CI 0.023–0.288, P < 0.001); in patients with Tertile 3, similarly, only those who showed super-response had an HR of 0.071 (95% CI 0.007–0.693, P = 0.023). In crude analysis, we found no relation between LVEF tertiles and composite endpoint in patients with super-response, when comparing Tertile 2 and Tertile 3 with Tertile 1 (Table 4).
In the Kaplan–Meier analyses, when comparing the survival among patients with three tertiles of baseline LVEF stratified by CRT responses, we found that there was no significant difference in patients with negative response (Figure 3, log-rank P = 0.488) and those with super-response (Figure 3, log-rank P = 0.310). However, significant differences were seen in patients who had non-response and who had response to CRT (Figure 3 and 3, both log-rank P values <0.05). And significant differences in event-free survival were found among the four groups of CRT response either in Tertile 1, or in Tertile 2, or in Tertile 3 (Figure 3, all log-rank P values <0.001).
Discussion
We believe that this study will deepen the understanding of the role of echocardiographic LVEF playing in the short-term and long-term prognosis after the CRT implantation. The findings in the current study can be summarized as follows: (i) there was a reversed U-shaped relationship between baseline LVEF tertiles and the 6 month CRT super-response, and LVEF at 25–30% was associated with the highest response rate; (ii) the independent predictors were LAD, LVEDD, and eGFR in the lowest tertile of LVEF; were atrial fibrillation, CLBBB, and LVEDD in the middle tertile of LVEF; and were female sex and SBP in the highest tertile; (iii) the highest LVEF level of 30–35% alone was not a significant predictor of event-free survival, but when combined with super-response, it was statistically related to the low risk of composite endpoint; and (iv) the predictive performance might be more reliable in CRT responses than the baseline LVEF tertiles.
Predictors of super-response in cardiac resynchronization therapy patients
Blanc et al.23 evaluated 29 HF patients with non-ischaemic DCM, sinus rhythm, and LBBB with a follow-up of 1 year. Five patients (17%) demonstrated both LVEF > 50% and clinical improvement of NYHA class, 6 min walk distance, and peak VO2, and no baseline features were found to predict super-response. Castellant et al.4 investigated 84 CRT patients with DCM, sinus rhythm, and LBBB in NYHA Class III and IV. During a follow-up of 37 ± 141 months, 11 patients (13%) were hyper-responders defined as functional recovery and LVEF ≥ 50%. All responders suffered non-ischaemic DCM. In our study, considering the recovery of LVEF being affected by the baseline status, like MADIT-CRT study,5 super-response was defined as an absolute increase of >15% in LVEF at 6 months after CRT relative to that at baseline, instead of reaching a certain fixed value of LVEF. Super-responders accounted for 27% among total patients.
Several studies have indeed investigated the impact of baseline LVEF on response to CRT in different LVEF groups. But most attention within this field was given to LVEF beyond 35%,15, 24, 25 so that we have rare knowledge of the effects of LVEF tertiles under 35% on the CRT response. In a previous study,14 141 patients with LVEF under 35% were observed and the investigators found no statistically significant relation between CRT response and LVEF groups because of similar response rate (67% vs. 75% vs. 70%, P > 0.05). It is not known whether the difference would be significant as its sample size increased.
Our study with a larger sample size revealed that the super-response rate in Tertiles 1, 2, and 3 were 23.8%, 35.7%, and 22.0% (P = 0.027), respectively. Moreover, we further found a non-linear relationship between baseline LVEF tertiles and super-response, and patients with LVEF of 25–30% had the highest probability of super-response, which was because the lower level is likely to increase.24 By contrast, patients with very low LVEF had the worst cardiac systolic reserve and exhibited lower rate of super-response, which was similar with some previous reports about the effect of CRT.15 The physiological mechanisms need to be deeply explored. Gasparini et al.10 found that higher ejection fraction tertile (30–35%) could predict HF remission, which seems not to be in line with our data. But in fact, the investigators defined the HF remission as LVEF ≥ 50% with NYHA class reduction to ≤II. That is to say, to achieve the HF remission, the LVEF needed to be at least increased by 26% in patients with Tertile 1 (<24%); by 21–26% in patients with Tertile 2 (24–29%); and by 15–20% in patients with Tertile 3 (30–35%). Thus, the two results were both reasonable. It is unexpected and interesting that in our study, patients in Tertile 2 (25–30%) with more frequent atrial fibrillation had a higher rate of super-response. This could be explained by that patients with atrial fibrillation in Tertile 2 were actually more likely to be non-super-responders (84.4%) (P = 0.006).
Although the definitions of CRT super-response in prior studies varied differently, they all could reflect to varying degrees the improvement of cardiac function. Several common predictors of CRT super-response have been identified: female sex,5, 26 non-ischaemic DCM,4, 5, 10, 26 LBBB,1, 5 wider pre-QRS duration,5, 26 smaller left atrial volume index,5 smaller left atrial volume,8 and smaller LVEDD/LVEDV.3, 10 Our study verified that no prior myocardial infarction, LBBB, smaller LAD, smaller LVEDD, and wider pre-QRS were all independently predictors of super-response. Moreover, no atrial fibrillation and higher SBP at baseline were also significant predictors in the fully adjusted model. Further subgroup analysis showed that smaller LAD, smaller LVEDD, and higher eGFR were independent predictors in patients with the lowest LVEF; atrial fibrillation, CLBBB, and smaller LVEDD were independent predictors in patients with the middle LVEF; and female sex and higher SBP were independent predictors in patients with the highest LVEF.
Impact of longitudinal changes in left ventricular ejection fraction on composite endpoint
Left ventricular ejection fraction is an indicator that clinicians rely on most to make decisions, and usually, it is widely recognized that HF patients with higher levels of LVEF have lower risks of all-cause mortality.27 But there is something to be worthy of discussing after the implantation of CRT. In total, baseline LVEF was significantly associated with composite endpoint. Similar to the prior study,15 an inverse and strong relationship was found in Tertile 1 vs. Tertile 2, but not found in Tertile 3 vs. Tertile 2. In the prior part of our study, we re-evaluated the association between baseline LVEF and HF remission after CRT, that is, super-response, and found a reversed U-shaped relationship, even after adjustment for different demographic and clinical variables, which could explain why the highest level of LVEF at baseline was not significantly associated with the lowest risk of the composite endpoint. Although patients in Tertile 2 had lower LVEF levels than those in Tertile 3, they showed higher super-response to CRT, which is also a strong predictor of a good prognosis. Even in patients with the lowest levels of LVEF who had the poorest prognosis, if they showed super-response at 6 months, they would have a better prognosis than those who had worse response consequently, so would patients with the highest level of LVEF. However, in patients with super-response, LVEF tertiles did not show prognostic effects on the composite endpoint. Overall, the impact of baseline LVEF on prognosis would be affected by CRT implantation and the predictive performance might be more reliable in the extent of CRT response than the baseline LVEF tertiles after CRT. Certainly, a combination of LVEF at baseline and CRT response would be better for the prognostic prediction. To our best knowledge, this is a comprehensive study evaluating the relationship between the baseline LVEF with the longitudinal changes and the prognosis.
Limitations
This is a retrospective study, and patients with incomplete echocardiography parameters after 6 months of CRT were excluded from the analysis. In clinical, there may be some artificial bias in the measurements of LVEF across the several years. However, we divided LVEF into three tertiles to decrease this deviation as much as possible. Another possible limitation is that some patients died within the 6 month follow-up. Then we had a relatively limited sample size. This focuses us to combine endpoints from rehospitalization and cardiac death, and not all the values with P < 0.1 could be included in the multivariable analysis. Our results did not show a significant association between three LVEF tertiles and the composite endpoint in patients with super-response, which may be related to the limited number of the composite endpoint. The same reason can explain the fact that we did not perform the adjusted regression analysis among the three tertiles of patients in super-response subgroup. Finally, the patient population does not reflect the real world of CRT population as it includes predominantly patients with idiopathic DCM and patients with an upgrade to CRT from a pacemaker or an implantable cardioverter defibrillator were not enrolled. For these special patients, the results need to be verified.
Conclusions
We demonstrated that there is a reversed U-shaped association between baseline LVEF tertiles and super-response defined as the remission of LV systolic dysfunction 6 months after CRT. There was a little difference in predictors of super-response in three LVEF tertiles. Baseline LVEF and CRT responses to some extent are significantly associated with the composite endpoint, but the latter would be more reliable in the predictive performance. We may suppose that HF patients with LVEF > 35% beyond the eligibility criteria would also benefit from the CRT implantation if they have the most improved LVEF. Further studies need to be designed to identify that kind of patients and test the idea.
Conflict of interest
None declared.
Funding
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2017-I2M-1-009).
Author contributions
N.Z. contributed in the conceptualization, methodology, software, formal analysis, data curation, investigation, writing of the original draft, writing of the review, and editing of the study; M.C. in the software, formal analysis, writing of the review, editing, and data curation of the study; W.H. in the resources, supervision, funding acquisition, writing of the review, and editing of the study; Y.H. in the data curation, writing of the review, and editing of the study; H.N. in the resources, supervision, writing of the review, and editing of the study; C.C. in the resources of the study; M.G. in the resources and supervision of the study; and S.Z. in the project administration of the study.




