Veno-Arterial Extracorporeal Life Support in Heart Transplant and Ventricle Assist Device Centres. Meta-analysis
Abstract
Aims
Because reported mortality on veno-arterial (V-A) extracorporeal life support (ECLS) substantially varies between centres, the aim of the current analysis was to assess the outcomes between units performing heart transplantation and/or implanting ventricular assist device (HTx/VAD) vs. non-HTx/VAD units in patients undergoing V-A ECLS for cardiogenic shock.
Methods and results
Systematic search according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses was performed using PubMed/MEDLINE databases until 30 November 2019. Articles reporting in-hospital/30-day mortality and centre's HTx/VAD status were included. In-hospital outcomes and long-term survival were analysed in subgroup meta-analysis. A total of 174 studies enrolling n = 13 308 patients were included with 20 series performed in non-HTx/VAD centres (1016 patients, 7.8%). Majority of patients underwent V-A ECLS for post-cardiotomy shock (44.2%) and acute myocardial infarction (20.7%). Estimated overall in-hospital mortality was 57.2% (54.9–59.4%). Mortality rates were higher in non-HTx/VAD [65.5% (59.8–70.8%)] as compared with HTx/VAD centres [55.8% (53.3–58.2%)], P < 0.001. Estimated late survival was 61.8% (55.7–67.9%) without differences between non-HTx/VAD and HTx/VAD centres: 66.5% (30.3–1.02%) vs. 61.7% (55.5–67.8%), respectively (P = 0.797). No differences were seen with respect to ECLS duration, limb complications, and reoperations for bleeding, kidney injury, and sepsis. Yet, weaning rates were higher in HTx/VAD vs. non-HTx/VAD centres: 58.7% (56.2–61.1%) vs. 48.9% (42.0–55.9%), P = 0.010. Estimated rate of bridge to heart transplant was 6.6% (5.2–8.3%) with numerical, yet not statistically significant, difference between non-HTx/VAD [2.7% (0.8–8.3%)] as compared with HTx/VAD [6.7% (5.3–8.6%)] (P = 0.131).
Conclusions
Survival after V-A ECLS differed according to centre's HTx/VAD status. Potentially different risk profiles of patients must be taken account for before definite conclusions are drawn.
Introduction
Despite advances in revascularization strategies, operative techniques, and management, as well as heart failure therapy, cardiogenic shock (CS), whether resulting from acute, chronic, or end-stage heart condition, continues to be associated with significant morbidity and mortality.1 Nowadays, veno-arterial (V-A) extracorporeal life support (ECLS) is widely recognized as an efficient and easy to implant mechanical support system, providing efficient, both haemodynamic and respiratory support. It safeguards end-organ perfusion during the shock state and thereby serves as a bridge to recovery of cardiac function, heart transplantation (HTx), or more durable ventricle assist device (VAD).2-5 Despite that, V-A ECLS still represents a resource-consuming modality and is mostly last resort of treatment. In most cases, centres' protocols and resources limit ECLS implementation to highest risk patients who are least likely to benefit from such therapies.4, 6-9 Because CS arises from a plethora of underlying cardiovascular impairment and because conducting a randomized controlled trial in this peculiar setting would raise ethical questions, V-A ECLS's optimal implementation and management in patients with CS has not been well studied or defined. Despite growing worldwide utilization and experience in mechanical support system, outcomes of patients undergoing V-A ECLS, in particular in-hospital mortality, have not shown substantial progress.4, 10 Interestingly, they are fairly different from centre to centre, suggesting that variations in practice patterns in management of CS endure and may play a role in driving the above differences.11 These might include different schemes of timing of CS recognition, tailored escalation to mechanical circulatory support, centralization of care, and haemodynamic monitoring. We hypothesized that readability of well-experienced ECLS teams and potentially shorter bridging times in HTx/VAD units may contribute to improved results. Therefore, we undertook a systematic review and meta-analysis to assess to which extent the in-hospital and remote outcomes differ across patients in CS and receiving V-A ECLS treatment in HTx/VAD as compared with non-HTx/VAD facilities.
Methods
Data sources and search strategy
This systematic review and meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.12 The PRISMA checklist is available in the Supporting Information, Table S1. Relevant studies to be included were searched for until 30 November 2019, through PubMed, EMBASE, CINAHL, the Web of Science, the Cochrane Register of Controlled Clinical Trials (CENTRAL), and Google Scholar. Abstracts were eligible for detailed assessment if available online and reporting outcomes of interest. The search terms were ‘extracorporeal membrane oxygenation’, ‘extracorporeal life support’, and ‘cardiogenic shock’. No language restrictions were imposed. References of original articles were reviewed manually and cross-checked for other relevant reports.
Selection criteria and quality assessment
Studies were included if they met all of the following criteria: (i) human study; (ii) studies assessing survival after ECLS instituted for refractory CS regardless of underlying conditions; and (iii) studies conducted in the setting of V-A ECLS or studies conducted in combined setting of veno-arterial and veno-venous (V-V) ECLS, but reporting at least one of the outcomes of interest for the former group. Studies were only excluded if they are (i) paediatric and congenital heart surgery-related studies, (ii) animal studies, (iii) conducted entirely in the setting of V-V ECLS regardless of underlying conditions, and (iv) studies not reporting survival/mortality rates. Studies were only eligible if reporting the transplant status of the centre; whenever this was not retrievable from the individual study, institutional website was searched for information regarding range of procedures performed. Lack of clear indication whether the centre performs heart transplantation led to exclusion of the study. Similarly, registries incorporating multiple centres but not reporting the status for single facilities were not considered. Studies reporting in both paediatric and adult population were considered only if pertinent data were available for adult subset or in the case in which paediatric population was <15% of the study. Mixed V-A ECLS configurations were also eligible such as standard V-A ECLS, veno-veno-arterial (V-VA), and/or veno-arterial-venous (V-AV) setting. Studies conducted in centres performing VAD only were pooled with those performing HTx and VADs. Reviews and case reports were not considered.
Two independent reviewers (G.M.R and K.Z.) selected the studies for inclusion, extracted studies, as well as patient characteristics of interest and relevant outcomes. Two authors (G.M.R. and K.Z.) independently assessed the trials' eligibility and risk of bias. Risk of bias at the individual study level was assessed using the Risk of Bias in Not-randomized Studies-of Interventions tool.13 Any divergences were resolved by a third reviewer (M.K.) and quantified using the approach of Cohen's kappa.14
Endpoint selection
The primary endpoint was in-hospital mortality. Secondary endpoints were in-hospital neurologic complications, limb complications, bleeding, sepsis, and acute kidney failure with or without continuous veno-venous haemofiltration. Bridging to VAD and/or HTx was analysed as well. Neurological complications encompassed cerebrovascular events, strokes, transient ischaemic attacks, and other ischaemic and haemorrhagic brain complications. A separate analysis of brain death was also conducted. Successful weaning from ECLS was defined as survival after >48 h from decannulation. Long-term survival was assessed at longest ‘out-of’ hospital follow-up available. Clinical outcome definitions were the ones adopted by the investigators of the included studies.
Statistical analysis
Statistical analyses were performed in Comprehensive Meta-Analysis, v. 2 (Biostat, Englewood, NJ). The results are expressed as pooled untransformed proportions (e.g. event rates, %) and means with their 95% confidence intervals. Heterogeneity across studies was evaluated using the I2 test. Whenever a single study reported median values and interquartile ranges instead of mean ± SD, the latter were approximated as described by Wan et al.15 Where available, we digitized Kaplan–Meier curves using Engauge Digitizer 9.5 (Mark Mitchell, Torrance, CA) and reconstructed time-to-event data using the algorithm specified by Guyot et al.16 To control for the anticipated heterogeneity among observational studies, absolute values and means were pooled using random effects models. Studies were stratified a priori based on the centre status (non-HTx/VAD vs. HTx/VAD performing centre); the interaction coefficient (Q value) is provided for the comparison non-HTx/VAD vs. HTx/VAD along with respective Pinteraction. Additionally, we investigated if non-HTx/VAD and HTx/VAD status had influence on ECLS duration, weaning rates, and bridging to HTx/VAD rates and further if ECLS duration and weaning rates in these centres correlated with bridging to HTx/VAD by means of meta-regression analyses. For the analysis of long-term survival, study-level data were analysed according to the original report for which outcome data were available. To account for potential differences in study's duration of follow-up, rate ratios with 95% confidence interval derived from an analysis with adjusted models by person-years, a measure incorporating study duration, were used as summary statistics. Sensitivity analyses were performed by excluding from analyses single studies, one at a time, and repeating the calculations for primary endpoint. In addition, analysis excluding studies conducted in VAD-only implanting centres was performed. Publication bias was assessed (i) by visual approach plotting log event rate against standard error in the funnel plot17 and (ii) by linear regression approach.
Results
Study characteristics
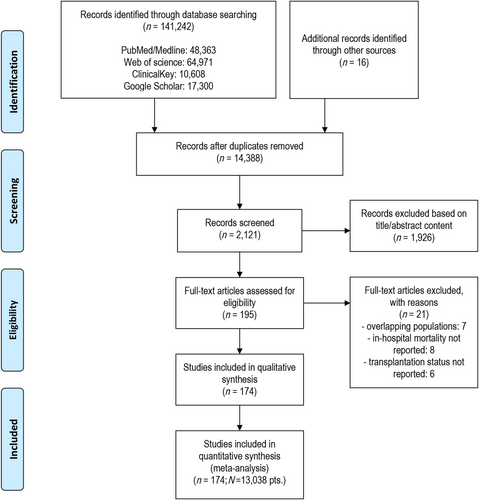
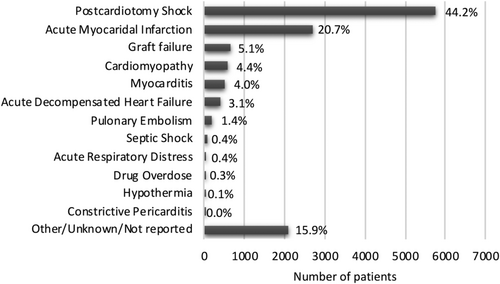
Initial search process yielded 48 226 records; of these, 761 abstracts were retrieved for scrutiny based on the item's title. Registries were excluded because they incorporated both non-HTx/VAD and HTx/VAD centres. Following detailed assessment, 174 studies (n = 13 038) (supplementary references 24–197, conducted between 1990–2019) met inclusion criteria and entered quantitative analyses (Figure 1). Included studies were divided into non-HTx/VAD vs. HTx/VAD centres subgroups: 154 studies including 12 022 (92.2%) patients were conducted in HTx/VAD and 20 studies [1016 patients (7.8%)] in non-HTx/VAD centres. There were six studies enrolling 185 patients (1%) that were conducted in VAD-only centres (Supporting Information, Table S2). Data on patient referrals to HTx/VAD centre were available from 43 studies. Prevalence of ECLS ranged from 0.26% (supplementary reference 190) to 22.6% (supplementary reference 55). Studies were mostly conducted in the setting of post-cardiotomy CS (44.2%) and acute myocardial infarction (20.7%) followed by other indications. Distribution of patients across range of indications for V-A ECLS is detailed in Figure 2. Detailed characteristics of included studies as well as patients' baseline and surgical data are available as Supporting Information, Tables S2 and S3. Publication bias analysis along with reasons for bias risk increase is available as Supporting Information, Table S4. Studies were judged to be moderate to severe risk of bias mainly because none previously compared directly non-HTx/VAD vs. HTx/VAD centres performance; no signs of small/big study effect were seen on visual inspection of funnel plot for primary endpoint (Supporting Information, Figure S1).
Extracorporeal life support strategy
Extracorporeal life support was initiated in the operating room in 43.0% of patients (36.5–49.8%), followed by intensive care unit, cardiac catheterization laboratory, telemetry floor, and emergency department without statistical differences between HTx/VAD, as compared with non-HTx/VAD centres: 43.6% (36.8–50.7%) and 37.3% (19.2–59.8%), respectively (P = 0.589). Sixty-one studies (4610 patients) reported on cannulation technique; rate of peripheral cannulation was estimated at 77.1% (68.3–84.0%) and was non-significantly higher in HTx/VAD [77.6% (68.6–84.5%)] as compared with non-HTx/VAD centres [66.6% (21.5–93.6%)] (P = 0.598). Median reported ECLS duration was 5.1 (interquartile range: 3.5–7.1) days again without apparent differences between HTx/VAD (mean weighted average = 5.95 days) vs. non-HTx/VAD (4.77 days) centres (P = 0.204). Overall, estimated 57.5% (55.2–59.9%) patients were weaned from ECLS with the weaning rates ranging from 0 to 100% in the entire series. These rates were significantly higher in HTx/VAD vs. non-HTx/VAD centres 58.7% (56.2–61.1%) vs. 48.9% (42.0–55.9%), P = 0.010.
Survival and complications following extracorporeal life support institution
Analysis of in-hospital mortality, complications, rate of bridge to VAD/HTx, and remote survival following V-A ECLS institution in non-HTx/VAD vs. HTx/VAD centres is shown in Figure 3. All 174 studies contributed to the analysis of survival. Overall, 5353 patients survived to hospital discharge, which translated to estimated overall in-hospital mortality of 57.2% (54.9–59.4%). Mortality rates were significantly higher in non-HTx/VAD [65.5% (59.8–70.8%)] as compared with HTx/VAD centres [55.8% (53.3–58.2%)], P < 0.001. The detailed estimated rates are reported as Supporting Information, Figure S2. Eighty-seven studies [78 studies in HTx/VAD and nine in non-HTx/VAD centres, 5824 person-years, median follow-up 1.3 years (interquartile range: 1.0–3.0)] were included in the analysis of long-term survival; overall estimated late survival was 61.8% (55.7–67.9%) without significant differences between non-HTx/VAD as compared with HTx/VAD centres: 66.5% (30.3–1.02%) vs. 61.7% (55.5–67.8%), respectively (P = 0.797). The detailed estimated rates are reported as Supporting Information, Figure S3.
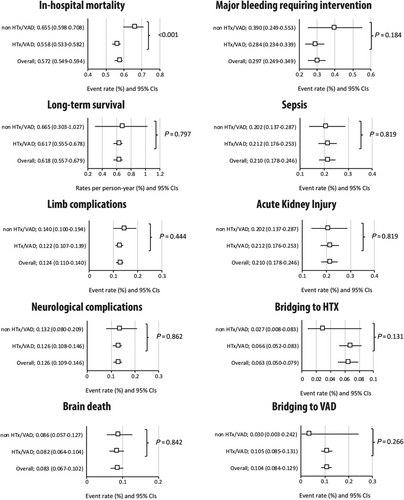
Limb complication incidence was reported in 102 studies (8157 pts). Overall, 1075 patients [12.4% (11.0–14.0%)] had limb complications; in the analysis stratified by centre status, there was no difference between HTx/VAD [12.2% (10.7–13.9%)] and non-HTx/VAD centres [14.0 (10.0–19.4%)] (P = 0.444). Ninety-seven studies enrolling an overall of 7040 patients contributed to the analysis of neurologic complications; 980 patients experienced neurological complications [12.6% (10.9–14.6%)]. Among those, 298 brain deaths [8.3% (6.7–10.2%); 56 studies; 3756 patients] occurred. No marked differences were seen between the rates of neurologic complications and brain deaths in non-HTx/VAD centres as compared with HTx/VAD centres: 13.2% (8.0–20.9%) vs. 12.6% (10.8–14.6%), P = 0.862, and 8.6% (5.7–12.7%) vs. 8.2% (6.4–10.4%), P = 0.842, for neurologic complications and brain deaths, respectively. No further differences were seen between non-HTx/VAD centres as compared with HTx/VAD centres respectively in the analyses of (i) major bleeding requiring intervention [39.0% (24.9–55.3%) vs. 28.4% (23.4–33.9%); P = 0.184]; (ii) sepsis [20.2% (13.8–28.7%) vs. 21.2% (17.6–25.3%); P = 0.819]; and (iii) acute kidney injury [36.4% (25.9–48.4%) vs. 42.8% (39.1–46.5%); P = 0.312]. Detailed analyses of individual studies are available as Supporting Information, Figures S4–S9.
Extracorporeal life support as bridging therapy
Data regarding ECLS bridge to HTx were available from 97 studies that included 7857 patients. In an overall analysis, 474 patients underwent HTx, which translated to an estimated rate of 6.3% (5.0–7.9%). There was numerical, yet not statistically significant, difference between non-HTx/VAD [2.7% (0.8–8.3%)] as compared with HTx/VAD [6.6% (5.2–8.3%)] favouring the latter (P = 0.131) (Figure 3). Similarly, there were no marked differences between non-HTx/VAD centres as compared with HTx/VAD centres with respect to ECLS bridge to VAD. Across 94 studies (7873 patients), 754 underwent bridging to VAD, 10.4% (8.4–12.9%); again, only numerical difference was observed between non-HTx/VAD [3.0% (0.3–24.2%)] and HTx/VAD [10.5% (8.5–13.1%)], P = 0.266 (Figure 3). Bridging rates are reported in two studies from VAD-only implanting centres: Asaumi et al. (supplementary reference 28) report one patient [14% (1/7)] having VAD implanted, and Dobrilovic et al. (supplementary reference 65) report on five [42% (5/12)] having VAD and none having HTx.
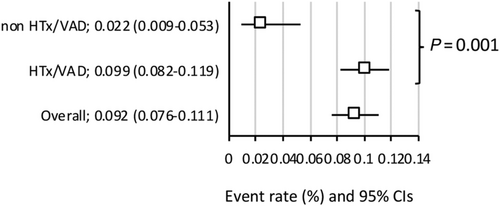
When HTx and VADs were pooled as exploratory single endpoint, the incidence of bridging to HTx and/or VAD was significantly higher in the HTx/VAD centres: 9.9% (8.2–11.9%) vs. 2.2 (0.9–5.3%), P = 0.001 (Figure 4). Meta-regression analyses revealed favourable effect of bridging to HTx {−1.6365 [−2.5066-(−0.7664)]; P < 0.001, Supporting Information, Figure S10}, bridging to VAD {−1.2472 [−1.8574-(−0.6370)]; P < 0.001, Supporting Information, Figure S11} and bridging to either HTx and/or VAD {−1.2860 [−1.7666-(−0.8054)]; P < 0.001, Supporting Information, Figure S12} on in-hospital mortality.
Additional analyses
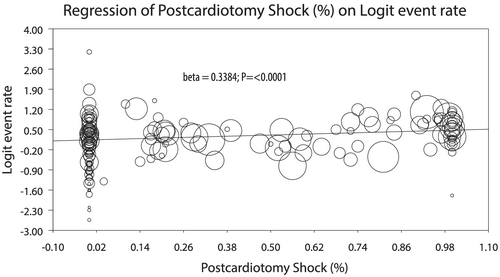
In several conducted meta-regressions counter-opposing logER of in-hospital mortality and percentage of patients undergoing ECLS therapy for different indications, there was higher mortality with increasing (%) post-cardiotomy ECLSs: 0.4214 (0.1843–0.6587), P < 0.001, which was maintained in the subgroup of only HTx/VAD centres [0.4652 (0.2108–0.7197); P < 0.001; Figure 5]. Conversely, lower rates of mortality were observed with increasing (%) of ECLSs for (i) acute myocardial infarction {−0.3765 [−0.7324-(−0.0206)]; P = 0.0381; Supporting Information, Figure S13}; (ii) graft failure {−0.8846 [−1.5415-(−0.2277)]; P = 0.0083; Supporting Information, Figure S14}; (iii) acute decompensated heart failure {−1.7919 [−3.2789-(−0.3049)]; P = 0.0181; Supporting Information, Figure S15}; (iv) myocarditis {−1.5322 [−2.0919-(−0.9726)]; P < 0.001; Supporting Information, Figure S16}; and (v) cardiomyopathy {−1.1074 [−1.7685-(−0.4462)]; P = 0.0010; Supporting Information, Figure S17}, without differences between between non-HTx as compared with HTx/VAD centres. We could not demonstrate any association of logER of in-hospital mortality and percentage of IABP {0.1440 [(−0.2423)-0.5303]; P = 0.4651} or left ventricular venting {−0.2245 [−(0.8856)-0.4367]; P = 0.5058} as addition to ECLS.
In sensitivity analysis for in-hospital mortality, performed deleting single studies, one at a time, and repeating the calculations, no single study effect was seen changing neither direction nor the magnitude of the estimates. Additionally, we repeated the analysis of in-hospital mortality comparing non-HTx/VAD vs. HTx/VAD centres after excluding studies (i) judged to be at critical risk of bias [65.8% (59.8–71.1%) vs. 56.2 (53.7–58.6%); P = 0.001] and those enrolling (ii) <50 patients [70.9% (64.8–76.3%) vs. 58.9 (55.9–61.8%); P = 0.001], (iii) <100 patients [72.4% (67.5–76.8%) vs. 58.0% (54.0–62.0%); P < 0.001], and (iv) those performed in VAD-only implanting centres [mortality rate: 55.7% (53.2–58.1%); P for subgroup difference = 0.002] only to prove maintenance of the effect.
Discussion
The current meta-analysis represents the first attempt to compare, although in indirect fashion, not only in-hospital but also remote outcomes of patients supported with V-A ECLS for refractory CS between HTx/VAD and non-HTx/VAD performing centres. The main findings are as follows: (i) in an overall analysis, in-hospital mortality regardless of the underlying cause of CS remained high and was estimated at 57.2%; (ii) in-hospital mortality rates were significantly higher in non-HTx/VAD (65.5% vs. 55.8%)as compared with HTx/VAD centres; (iii) in the overall analysis, estimated late survival was 61.8% without significant differences between non-HTx/VAD as compared with HTx/VAD centres; (iv) no differences were found between centres with respect to ECLS complications; (v) numerically, more patients were bridged to HTx and VADs in the centres that perform these procedures; and (vi) post-cardiotomy CS yielded higher in-hospital mortality irrespective of centre status and increasing percentage of patients treated with V-A ECLS for CS of other aetiology correlated with lower mortality rates.
In the lack of randomized controlled trials in the area of V-A ECLS, best evidence comes from meta-analyses of observational studies that questioned factors associated with survival including unloading of left ventricle,18 peripheral cannulation,19 or baseline surgical status in post-cardiotomy setting.20 Prolonging of V-A ECLS duration after 7 days was associated with a disproportionate increase in mortality21 (supplementary 63). Consequently, this led to the hypothesis of the present meta-analysis that potentially shorter bridging times in HTx/VAD units due to readability of well-experienced ECLS teams experienced in dealing with both acute, chronic, and end-stage heart failure also with prompt resources availability (medium-term and long-term mechanical circulatory support and heart transplantation) might prevent life-threatening complications and contribute to better results.
The majority of reports came from HTx/VAD centres. Out of the 174 studies included, 154 were conducted in HTx/VAD units, and as a result, 92.2% of reported patients were treated there. This is similar to findings from analysis focused exclusively on post-cardiotomy patients.22 Overrepresentation of reports on V-A ECLS from HTx/VAD centres seem to apply to different aetiologies of CS. Hypotheses explaining this notion might include higher disease burden and subsequent risk of patients treated in HTx/VAD centres, higher preference for ECLS use in the treatment of refrectory CS in this centres, or unexplained underreporting from non-HTx centres.
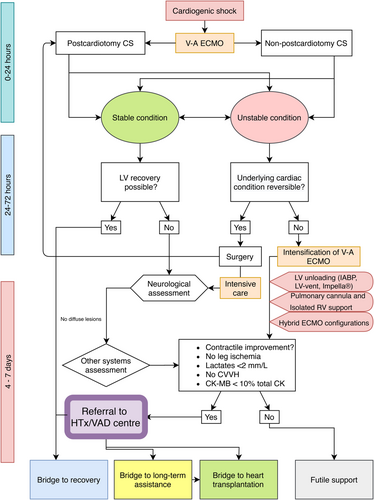
The main finding of present analysis is that 30 day/in-hospital weaning and survival rates were significantly lower in non-HTx/VAD as compared with HTx/VAD centres. This is in contrast to results of analysis focusing exclusively on post-cardiotomy patients where no differences were observed.22 Different risk profiles of post-cardiotomy and non-post-cardiotomy patients must be taken to account. V-A ECLS after adult cardiac surgery had been associated with higher risk of in-hospital mortality compared with other indications (supplementary references 42 and 159). Its exponential increase in use in this setting has not been paralleled by improved results.4 The current meta-analysis demonstrates that post-cardiotomy yielded higher in-hospital mortality irrespective of centre status. Indeed, in the mixed aetiology population, overall 30-day survival was 42.8% and in the post-cardiotomy setting, as shown in previous meta-analysis, it was estimated at 35.3%.22 Negative prognostic effect of post-cardiotomy on survival may be explained by the impact of the underlying cardiac disease with its accompanying risk factors as well as the extent of the surgical procedure and its potential complications. In acute settings such as post-cardiotomy shock, V-A ECLS is mainly instituted as a temporizing measure and a bridge to recovery rather than bridge to VAD or heart transplantation, which are indeed more frequent therapeutic options in chronic heart failure patients (3, 4, and supplementary reference 158). In fact, in the overall analysis, estimated rates of HTx and bridging to VAD were 6.3% (5.0–7.9%) and 10.4% (8.4–12.9%), respectively. On the other hand, in the post-cardiotomy setting, respective estimated rates were 3.5% (1.8–6.6%) and 4.3% (2.8–6.5%).22 We propose an algorithm of patient management in the setting of CS managed with V-A ECMO with respect to patient referral to HTx/VAD performing centre (Figure 6) in which the patient, if stable, may be a candidate for referral between 4 and 7 days of treatment.
Despite difference in short-term outcomes, estimated late survival (5824 person-years) was similar. In fact, long-term survival in both PCS-ECMO and non-PCS-ECMO seems to be strongly influenced by the early hospitalized critical phase, after which the survival slightly drops but remains satisfactory.23 In the study of Smedira et al., survival after being bridged from ECLS to a VAD/HTx vs. after being weaned from ECLS without a bridge was 67% vs. 52% at 30 days and 5-year survival was similar (44% and 40%, respectively) (supplementary reference 156). Profit from potentially shorter bridging times in HTx/VAD units might be more apparent in the early period of intensive treatment characterized by high risk of mortality. Failure to wean from V-A ECLS support or chronic heart disease and thus need of bridging to more durable VAD or HTx might be associated with patient's high disease burden, and thus, effect of such procedure in the long-term might be less evident. Procedures of transition to more durable support or HTx itself may also affect mortality rates in this respect. What could be responsible for observed differences in mortality remains inconclusive. There was no statistically significant difference in rates of HTx and bridging to VAD between non-HTx/VAD as compared with HTx/VAD centres, however numerically higher in the latter. Also, there was no statistical difference between HTx/VAD (mean weighted average = 5.95 days) vs. non-HTx centres (4.77 days) in the median ECLS duration. Unexpected, yet complex finding, we could not demonstrate any difference in complication rates between HTx/VAD and non-HTx/VAD centres, in contrast to previous meta-analysis focusing on post-cardiotomy CS alone, which found that neurological complications occurred less frequently in HTx centres. On one hand, distinct shock protocols, designated, well-trained response teams, daily monitoring, neurovascular assessment, end-organ perfusion and haemodynamics assessment, and daily evaluation for weaning vs. escalation of support could be responsible at least in part for the overall low number of complications and, in particular, in HTx/VAD centres.
Limitations
Current meta-analysis is based on observational, one-arm comparisons and because of that is more prone to confounding as compared with head-to-head comparisons. On the other hand, no randomized controlled trial exists regarding analysed topic and presumably will not be organized due to ethical issues. Information about referrals from non-HTx/VAD facilities to centres performing these procedures and time of referral (prior or past ECLS implantation) is largely missing. One limitation of the study may be underrepresentation of patients treated in non-HTx/VAD institutions. In fact, only centres with the highest ECLS activity may have the opportunity to publish their results. While this may represent a bias of selection when compared with real life, presence of reports of non-favourable outcomes from non-HTx/VAD small volume centres could potentially only increase the difference between estimates. Random effect meta-analysis and inverse variance analysis were used to account for that fact; these methods appoint random weights also in a subgroup analysis, which could overcome discrepancies between population sizes. Another limitation is inclusion by single studies of mixed patient populations; while this was partially accounted for with meta-regression analysis, bias due to inclusion of heterogenous patient populations by single reports remains. There was no standard definition for secondary endpoints or risk of bias of the included studies. Conducting detailed subgroup and inference analyses (such as death within 24 h after implantation of ECMO in OR) was precluded by insufficient data on timing and location of implantation, exact device type, cannulation strategies, left ventricular unloading techniques if any, status and duration of surgical procedure in case of post-cardiotomy shock, and likewise other baseline characteristics.
Conclusions
There was an apparent difference in weaning rates and survival after V-A ECLS implantation for refractory CS between centres that perform heart transplantations and/or implant VAD and those that do not in favour of transplant centres. Potentially different risk profiles of patients in these facilities must be taken account for before definite conclusions are drawn.
Ethics approval and consent to participate
NA.
Consent for publication
NA.
Availability of supporting data
All data are available.
Author contributions
M.K., G.R., J.M., P.S., and R.L. conceptualized and designed the study. M.K., K.Z., M.A., M.G., E.N., E.B., and P.M. acquired the data. M.K., G.R., M.A., S.H., R.S., T.D., R.D., M.v.d.P., and J.W.S. analysed the data. M.K., S.H., R.S., T.D., J.W.S., P.R., J.M., and R.L. interpreted the data. M.K., K.Z., G.R., and R.L. drafted the manuscript. M.A., M.G., E.N., E.B., R.S., T.D., R.D., J.W.S., M.v.d.P., P.R., P.M., J.M., and P.S. participated in the critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
Dr Lorusso is consultant and conducts clinical trial for LivaNova (London, UK), is consultant for Medtronic (Minneapolis, MN), and an Advisory Board member of PulseCath (Arnhem, The Netherlands). The other authors have no conflicts of interest to disclose.
Funding
None.




