Adherence to beta-blockers and long-term risk of heart failure and mortality after a myocardial infarction
Abstract
Aims
The aim of this study is to investigate the association between adherence to beta-blocker treatment after a first acute myocardial infarction (AMI) and long-term risk of heart failure (HF) and death.
Methods and results
All patients admitted for a first AMI included in the nationwide Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies register between 2005 and 2010 were eligible (n = 71 638). After exclusion of patients who died in-hospital, patients with previous HF, patients with unknown left ventricular ejection fraction (EF), and patients who died during the first year after the index event, 38 608 patients remained in the final analysis. Adherence to prescribed beta-blockers was determined for 1 year after the index event using the national registry for prescribed drugs and was measured as proportion of days covered, the ratio between the numbers of days covered by the dispensed prescriptions and number of days in the period. As customary, a threshold level for proportion of days covered ≥80% was used to classify patients as adherent or non-adherent. At discharge 90.6% (n = 36 869) of all patients were prescribed a beta-blocker. Among 38 608 1 year survivors, 31.1% (n = 12 013) were non-adherent to beta-blockers. Patients with reduced EF with and without HF were more likely to remain adherent to beta-blockers at 1-year compared with patients with normal EF without HF (NEF). Being married/cohabiting and having higher income level, hypertension, ST-elevation MI, and percutaneous coronary intervention were associated with better adherence. Adherence was independently associated with lower all-cause mortality [hazard ratio (HR) 0.77, 95% confidence interval [CI] 0.71–0.84] and a lower risk for the composite of HF readmission/death, (HR 0.83, 95% CI 0.78–0.89, P value <0.001) during the subsequent 4 years of follow up. These associations were favourable but less apparent in patients with HFNEF and NEF.
Conclusions
Nearly one in three AMI patients was non-adherent to beta-blockers within the first year. Adherence was independently associated with improved long-term outcomes; however, uncertainty remains for patients with HFNEF and NEF.
Introduction
Beta-blocker therapy after acute myocardial infarction (MI) (AMI) has been shown to improve survival in clinical trials, meta-analyses, and observational studies.1-4 The early randomized controlled trials with beta-blockers showed benefits in high-risk patients, although patients with heart failure (HF) were often excluded, and left ventricular ejection fraction (EF) (LVEF) was not commonly available; therefore, there is a definite knowledge gap regarding the use of beta-blockers in HF post-AMI.5-9 This gap was partially filled later with observed major benefits in patients with chronic HF and reduced LVEF.10-12 However, the use of beta-blockers early post-AMI has remained a controversial field with studies reporting conflicting findings and potentially harmful effect of intravenous (i.v.) beta-blockers in ST-elevation MI (STEMI) patients.13-15 Moreover, the role of long-term treatment especially in patients without reduced LVEF and/or HF post-AMI is uncertain because of a lack of randomized clinical trials in the modern reperfusion era. A large observational study did not find a lower risk of cardiovascular events in these patients.16
Non-adherence to medications is common in patients with cardiovascular diseases.17, 18 Beyond the early discharge period, adherence to prescribed cardio-protective medications (e.g. statins and beta-blockers) progressively decline over time. Studies have shown that the persistent use of beta-blockers after discharge is low both in coronary heart disease patients and in HF patients.19-21 Furthermore, beta-blocker doses used in routine clinical practice are often substantially lower than doses used in the randomized trials that established their efficacy.22, 23 Quality measures of evidence-based medications post-AMI have focused on the prescription at hospital discharge. However, survival benefits of these medications are heavily dependent on sustained therapy, and there are present knowledge gaps with regard to beta-blocker adherence and the effect on outcome in contemporary AMI populations.
We aimed to study adherence to long-term beta-blocker treatment and its association with long-term outcomes in 1 year survivors of AMI with vs. without reduced LVEF or clinical HF during hospitalization, using a cohort of consecutive first time AMI patients admitted to coronary care units in Sweden between 2005 and 2010.
Methods
Study population
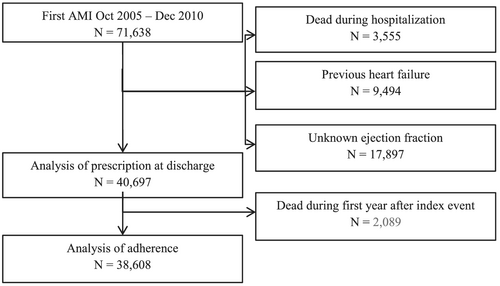
All patients that were admitted for a first diagnosis of AMI between October 2005 and December 2010 and included in the nationwide Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies (SWEDEHEART) registry were eligible. The SWEDEHEART database comprises all Swedish emergency hospitals (n = 72) and enrols all consecutive patients admitted to a coronary care unit or other specialized facility.24 Patients with a previously diagnosed AMI, with a prior history of HF, identified with the SWEDEHEART registry and the National Inpatient Register from International Classification of Diseases (ICD) codes for HF, were excluded from the study (Figure 1). Patients who died during hospitalization and patients with missing data on LVEF were also excluded from the study. In the final analysis of adherence, patients who died during the first year after the index event were excluded. The national registry for prescribed drugs was used to gather data on previously dispensed prescriptions of secondary prevention medications. The study complies with the Declaration of Helsinki and was approved by the regional Human Research Ethics committee in Stockholm, Sweden.
Data sources
Data on baseline characteristics, medication at admission, hospital course variables, and drug prescription at discharge were obtained from the SWEDEHEART register. Information from other relevant registries was obtained using the unique personal identity number assigned to each Swedish resident. Data on previous history of diabetes mellitus, hypertension, MI, or stroke were also obtained from the National Inpatient Register, and death dates from the National Population Registry. Data on socio-economic background was obtained from Statistics Sweden. Data on hospitalization for HF after discharge from the index AMI admission (up to 31 March 2013) were obtained from the National Inpatient Register and defined as a new hospitalization for HF. The discharge codes applied were I50.0-I50.9 (ICD-10). A hospital diagnosis of HF in Sweden has been validated against European Society of Cardiology criteria for the definition of HF25 with a validity of 95% for a principal diagnosis of HF and 82% irrespective of position. Data on drug dispensations were collected from the Swedish Prescribed Drug Register that includes all dispensed prescribed drugs in Sweden since July 2005. Data from the above-mentioned registers were merged into a single database using the Swedish unique personal identification number.
Assessment of heart failure status during hospitalization for an index acute myocardia infarction
In-hospital HF was defined as the presence of pulmonary rales or use of i.v. diuretics or i.v. inotropic drugs during admission.26, 27 Patients were categorized into four groups on the basis of echocardiographic LVEF data during hospitalization: those with signs of HF and normal EF (NEF) (≥50%) (HFNEF), those with signs of HF and reduced EF (REF) (<50%) (HFREF), those with NEF ≥50% without signs of HF, and those with REF <50% without signs of HF.28
Assessment of outcomes
Late onset HF (LOHF) was defined if the patient was rehospitalized and diagnosed to have HF as primary or secondary diagnosis. Data on hospitalization for HF after discharge from the index AMI admission (up to 31 December 2013) were obtained from the national patient register and defined as a new hospitalization for HF (ICD-10). The composite event of LOHF or death was defined as readmission because of HF as primary or secondary diagnosis or death after discharge from the index AMI.
Assessment of prescription and adherence
Patients were considered prescribed beta-blockers if they were on any beta-blocker drug during discharge from the index AMI admission. The information is obtained directly from the SWEDEHEART case report form. Adherence was determined as proportion of days covered (PDC), as the ratio between the numbers of days covered by the dispensed prescriptions divided by the total number of days in the period. As customary, a threshold level for proportion of days covered ≥80% was used to classify patients as adherent or non-adherent.29 All AMI patients who survived the first year after the index admission were included in the adherence analysis.
Statistical analysis
Patient characteristics were described using means and standard deviations for continuous variables and proportions for categorical variables. Logistic regression was used to calculate odds ratios (ORs) for comparisons between HF groups regarding non-prescription and non-adherence using NEF as reference group. We report crude and multivariable ORs with 95% confidence intervals (CIs). Multivariable adjustment was made using three models; in Model 1, OR was adjusted for factors that should affect prescription/compliance to beta-blockers [atrioventricular-block II/III, previous peripheral artery disease (PAD), chronic obstructive pulmonary disease, cardiogenic shock during hospitalization, systolic blood pressure <90 mmHg, heart rate <60 bpm]. In Model 2, we additionally adjusted for factors that, besides HF types, could affect prescription/compliance [age, hypertension, diabetes, chronic kidney disease (CKD) defined as estimated glomerular filtration rate <60 mL/min, PAD, prior stroke, prior cancer within 3 years, newly diagnosed atrial fibrillation at discharge, percutaneous coronary intervention (PCI) during hospitalization, and coronary arterial bypass graft surgery (CABG)]. In Model 3, we added adjustments for gender and socio-economic factors (country of birth, civil status, educational level, and income). Logistic regression analysis was also used to identify independent predictors of good adherence 1 year after discharge from the index AMI.
The cumulative risk of HF readmission or death 1 year after discharge from the index admission based on adherence status to beta-blocker treatment was determined from Kaplan–Meier time to event estimate tables. The long-term risk of all-cause mortality and the composite of LOHF or death 1 year after discharge in relation to status of adherence to beta-blocker treatment and LVEF/HF category were determined using Cox proportional hazard regression analysis showing hazard ratios (HRs) and 95% CIs.
SPSS (IBM, Version 21) was used for all data management and statistical analyses.
Results
Patient characteristics
A total of 71 638 patients were admitted for their first AMI between October 2005 and December 2010. After exclusion of patients who died during hospitalization, patients with previous HF, and patients with unknown LVEF, 40 697 patients remained for the final analysis (Table 1). The proportion of patients in relation to in-hospital HF by signs of HF and normal or reduced LVEF were NEF in 55.1% (n = 22 405), REF in 25.7% (n = 10 481), HFNEF in 7.6% (n = 3082), and HFREF in 11.6% (n = 4729), respectively. Baseline characteristics are presented in Table 1. Patients with HFNEF and HFREF were considerably older compared with patients with NEF and REF. The proportion of women was highest in patients with HFNEF. Comorbidities such as diabetes, hypertension, and CKD were more prevalent in patients with HFNEF and HFREF compared with those in patients with REF and NEF. At time of admission, 25.6% patients were already on beta-blockers.
| All patients (N = 40 697) | NEF (N = 22 405) | REF (N = 10 481) | HFNEF (N = 3082) | HFREF (N = 4729) | P value | |
|---|---|---|---|---|---|---|
| Demographics and socio-economic status | ||||||
| Age, median (IQR), mean (SD) | 66, 18 (67 ± 12) | 65, 17 (65 ± 12) | 69, 17 (68 ± 12) | 74, 16 (72 ± 11) | 75, 16 (72 ± 11) | <0.001 |
| Men, n (%) | 27 169 (66.8%) | 15 243 (68.0%) | 7337 (70.0%) | 1700 (55.2%) | 2889 (61.1%) | <0.001 |
| Country of birth outside Europe | 1410 (3.5%) | 842 (3.8%) | 321 (3.1%) | 106 (3.4%) | 141 (3.0%) | 0.003 |
| Civil status (married/cohabiting) (n = 39 461) | 22 013 (55.8%) | 12 808 (58.0%) | 5667 (55.8%) | 1448 (49.7%) | 2090 (48.4%) | <0.001 |
| Educational level (n = 38 878) | <0.001 | |||||
| Primary | 16 688 (42.9%) | 8594 (39.4%) | 4470 (44.7%) | 1442 (50.6%) | 2182 (51.6%) | |
| Lower secondary | 11 061 (28.5%) | 6432 (29.5%) | 2820 (28.2%) | 742 (26.1%) | 1067 (25.2%) | |
| Upper secondary | 11 129 (28.6%) | 6780 (31.1%) | 2708 (27.1%) | 664 (23.3%) | 977 (23.1%) | |
| Income (quartile) (n = 39 461) | <0.001 | |||||
| 1 (lowest) | 9215 (23.4%) | 4694 (21.3%) | 2377 (23.4%) | 844 (28.9%) | 1300 (30.1%) | |
| 2 | 9354 (23.7%) | 4681 (21.2%) | 2505 (24.7%) | 838 (28.7%) | 1330 (30.8%) | |
| 3 | 10 024 (25.4%) | 5767 (26.1%) | 2609 (25.7%) | 688 (23.6%) | 960 (22.2%) | |
| 4 (highest) | 10 868 (27.5%) | 6931 (31.4%) | 2665 (26.2%) | 546 (18.7%) | 726 (16.8%) | |
| Smoking, n (%; n = 38 456) | 10 333 (26.9%) | 5888 (27.5%) | 2695 (27.1%) | 703 (25.1%) | 1047 (24.3%) | |
| Comorbidities at admission, n (%) | ||||||
| Diabetes mellitus | 7799 (19.2%) | 3657 (16.3%) | 1965 (18.7%) | 808 (26.2%) | 1369 (28.9%) | <0.001 |
| Hypertension | 19 268 (47.3%) | 10 303 (46.0%) | 4699 (44.8%) | 1786 (57.9%) | 2480 (52.4%) | <0.001 |
| eGFR <60 mL/min (n = 39 928) | 8270 (20.7%) | 3298 (15.0%) | 2106 (20.4%) | 1027 (34.0%) | 1839 (39.5%) | <0.001 |
| Peripheral vascular disease | 1196 (2.9%) | 492 (2.2%) | 310 (3.0%) | 144 (4.7%) | 250 (5.3%) | <0.001 |
| Any stroke | 3397 (8.3%) | 1547 (7.0%) | 821 (7.8%) | 421 (13.7%) | 608 (12.9%) | <0.001 |
| COPD | 3061 (7.5%) | 1407 (6.3%) | 726 (6.9%) | 413 (13.4%) | 515 (10.9%) | <0.001 |
| Dementia | 106 (0.2%) | 46 (0.2%) | 31 (0.3%) | 9 (0.3%) | 20 (0.4%) | 0.045 |
| Cancer diagnosis within 3 years | 719 (1.9%) | 343 (1.5%) | 183 (1.7%) | 92 (3.0%) | 101 (2.1%) | <0.001 |
| Dialysis (at any time) | 122 (0.3%) | 52 (0.2%) | 40 (0.4%) | 15 (0.5%) | 15 (0.3%) | 0.024 |
| Hospital course, n (%) | ||||||
| STEMI | 14 411 (35.6%) | 6560 (29.4%) | 4947 (47.5%) | 892 (29.1%) | 2012 (42.8%) | <0.001 |
| PCI during hospitalization | 26 852 (66.0%) | 15 312 (68.3%) | 7370 (70.3%) | 1570 (50.9%) | 2600 (55.0%) | <0.001 |
| CABG during hospitalization | 1390 (3.4%) | 703 (3.1%) | 337 (3.2%) | 139 (4.5%) | 211 (4.5%) | <0.001 |
| Previous bleeding or bleeding during hospitalization | 2207 (5.4%) | 1153 (5.1%) | 532 (5.1%) | 217 (7.0%) | 305 (6.4%) | <0.001 |
| Atrial fibrillation at discharge | 1715 (4.3%) | 520 (2.4%) | 509 (5.0%) | 206 (6.9%) | 480 (10.4%) | <0.001 |
| Medication, n (%) | ||||||
| Aspirin at admission | 9723 (24.0%) | 4894 (21.9%) | 2358 (22.6%) | 1005 (32.9%) | 1466 (31.3%) | <0.001 |
| Aspirin at discharge | 38 569 (94.9%) | 21 557 (96.3%) | 9914 (94.7%) | 2797 (91.3%) | 4301 (91.1%) | <0.001 |
| Other antiplatelet drugs at admission | 1275 (3.1%) | 697 (3.1%) | 282 (2.7%) | 123 (4.0%) | 173 (3.7%) | <0.001 |
| Other antiplatelet drugs at discharge | 33 516 (82.5%) | 19 125 (85.5%) | 8794 (84.0%) | 2213 (72.0%) | 3384 (71.7%) | <0.001 |
| Warfarin at admission | 1159 (2.9%) | 459 (2.1%) | 316 (3.0%) | 150 (5.1%) | 234 (5.0%) | <0.001 |
| Warfarin at discharge | 1987 (4.9%) | 640 (2.9%) | 644 (6.1%) | 236 (7.7%) | 467 (9.9%) | <0.001 |
| Beta-blockers at admission | 10 365 (25.6%) | 5320 (23.9%) | 2445 (23.4%) | 1129 (37.1%) | 1471 (31.6%) | <0.001 |
| Beta-blockers at discharge | 36 869 (90.7%) | 20 142 (90.0%) | 9710 (92.8%) | 2715 (88.4%) | 4302 (91.1%) | <0.001 |
| Calcium channel blocker at admission | 5580 (14.5%) | 2968 (13.3%) | 1413 (13.5%) | 633 (20.8%) | 836 (17.9%) | <0.001 |
| Calcium channel blocker at discharge | 5092 (12.5%) | 2972 (13.3%) | 998 (9.5%) | 600 (19.5%) | 522 (11.1%) | <0.001 |
| Digitalis at admission | 528 (1.3%) | 203 (0.9%) | 133 (1.3%) | 78 (2.5%) | 114 (2.4%) | <0.001 |
| Digitalis at discharge | 751 (1.8%) | 205 (0.9%) | 167 (1.6%) | 108 (3.5%) | 271 (5.7%) | <0.001 |
| ACE/ARB at admission | 9561 (23.6%) | 5044 (22.6%) | 2311 (22.1%) | 935 (30.7%) | 1271 (27.2%) | <0.001 |
| ACE/ARB at discharge | 29 479 (72.5%) | 14 678 (65.6%) | 8835 (84.4%) | 2096 (68.2%) | 3870 (82.0%) | <0.001 |
| Diuretics at admission | 6656 (16.4%) | 3134 (14.0%) | 1569 (15.0%) | 850 (27.9%) | 1103 (23.6%) | <0.001 |
| Diuretics at discharge | 9099 (22.4%) | 3056 (13.7%) | 2018 (19.3%) | 1301 (42.3%) | 2724 (57.7%) | <0.001 |
| Statins at admission | 7229 (17.8%) | 3939 (17.6%) | 1719 (16.5%) | 662 (21.7%) | 909 (19.4%) | <0.001 |
| Statins at discharge | 36 723 (90.4%) | 20 820 (93.0%) | 9570 (91.4%) | 2519 (82.0%) | 3814 (80.8%) | <0.001 |
- ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CABG, coronary arterial bypass graft surgery; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Prescription
Prescriptions of secondary prevention drugs at discharge are presented in Table 1 and show high rates in all groups. Beta-blockers were prescribed to 90.6% (n = 36 869) of all patients.
The distribution of prescription and corresponding ORs for prescription by type of HF are shown in Table 2. Compared with patients with NEF, patients with REF and HFREF were more likely to receive beta-blockers at discharge, while patients with HFNEF received beta-blockers to a similar extent at discharge. These findings were consistent even after adjustment for factors that should affect prescription (possible contraindications) of the drugs (Model 1).
| All patients (N = 40 697) | NEF (N = 22 405) | REF (N = 10 481) | HFNEF (N = 3082) | HFREF (N = 4729) | P value | ||
|---|---|---|---|---|---|---|---|
| Beta-blocker | 36 869 (90.7%) | 20 142 (90.0%) | 9710 (92.8%) | 2715 (88.4%) | 4302 (91.1%) | <0.001 | |
| Crude OR (n = 40 697) | 1.00 (ref) | 1.42 (1.31–1.55) | 0.85 (0.75–0.95) | 1.14 (1.03–1.28) | |||
| Model 1 OR (n = 38 325)a | 1.00 (ref) | 1.41 (1.29–1.55) | 0.92 (0.81–1.05) | 1.19 (1.06–1.34) | |||
| Model 2 OR (n = 36 928)b | 1.00 (ref) | 1.41 (1.29–1.55) | 1.04 (0.91–1.19) | 1.34 (1.18–1.52) | |||
| Model 3 OR (n = 35 329)c | 1.00 (ref) | 1.41 (1.28–1.56) | 1.06 (0.92–1.22) | 1.40 (1.22–1.60) | |||
- HF, heart failure; HFNEF, HF and normal ejection fraction; HFREF, HF and reduced ejection fraction; NEF, normal ejection fraction; OR, odds ratio; REF, reduced ejection fraction.
- In Model 1, OR was adjusted for factors that should affect prescription of beta-blockers: (atrioventricular-block II/III, previous peripheral artery disease, and chronic obstructive pulmonary disease, cardiogenic shock during hospitalization, systolic blood pressure <90 mmHg, and heart rate <60 bpm). In Model 2, in addition, we adjusted for factors that, besides HF classification, could affect prescription/compliance [age, hypertension, diabetes, estimated glomerular filtration rate <60 mL/min, type of myocardial infarction (MI) (STEMI/NSTEMI), peripheral arterial disease, previous stroke, prior cancer within 3 years, atrial fibrillation at discharge, during hospitalization performed percutaneous coronary intervention, and coronary arterial bypass graft surgery]. In Model 3, we also adjusted for gender and socio-economic factors (country of birth, civil status, educational level, and income).
- a Model 1—Adjusted for absolute and relative contraindications that should influence prescription.
- b Model 2—In addition to Model 1 adjusted for factors and comorbidities that could affect prescription or compliance.
- c Model 3—In addition to Models 1 and 2 adjusted for gender and socio-economic factors.
Adherence
Out of all AMI patients alive at 1 year after discharge from the index admission, 68.9% (n = 26 595) reached the adherence level of 80% with beta-blocker treatment. Compared with patients with NEF, patients with REF were more likely to reach an adherence level above 80% in the crude analysis and after adjustment in all models, whereas patients with HFNEF reached the threshold for adherence to a similar extent as patients with NEF. Patients with HFREF were more likely to reach the adherence threshold after adjustment for factors that could affect prescription (Model 2) and socio-economic factors (Model 3) while remaining to a similar extent as patients with NEF after adjustment for possible contraindications to beta-blocker treatment (Model 1) (Table 3).
| All patients (N = 38 608) | NEF (N = 21 780) | REF (N = 9929) | HFNEF (N = 2797) | HFREF (N = 4102) | P value | |
|---|---|---|---|---|---|---|
| Adherent | 26 595 (68.9%) | 14 788 (67.9%) | 7175 (72.3%) | 1822 (65.1%) | 2810 (68.5%) | <0.001 |
| Crude OR (N = 43 473) | 1.00 (ref) | 1.23 (1.17–1.30) | 0.88 (0.81–0.96) | 1.03 (0.96–1.11) | <0.001 | |
| Model 1 (N = 36 493)a | 1.00 (ref) | 1.21 (1.15–1.28) | 0.90 (0.82–0.98) | 1.02 (0.95–1.10) | ||
| Model 2 (N = 35 268)b | 1.00 (ref) | 1.22 (1.16–1.30) | 1.01 (0.92–1.11) | 1.14 (1.05–1.24) | ||
| Model 3 (N = 34 706)c | 1.00 (ref) | 1.24 (1.17–1.31) | 1.05 (0.95–1.15) | 1.16 (1.07–1.27) |
- HFNEF, heart failure and normal ejection fraction; HFREF, heart failure and reduced ejection fraction; NEF, normal ejection fraction; OR, odds ratio; REF, reduced ejection fraction.
- a Model 1—Adjusted for absolute and relative contraindications that should influence prescription.
- b Model 2—In addition to Model 1 adjusted for factors and comorbidities that could affect prescription or compliance.
- c Model 3—In addition to Models 1 and 2 adjusted for gender and socio-economic factors.
Predictors of adherence
Using multivariable analysis, we found higher odds of non-adherence in patients with increasing age, low income level, non-married/non-cohabiting, chronic obstructive pulmonary disease, prior stroke, prior cancer, bleeding, dementia, PAD, and being on dialysis treatment as well as traditional relative contraindications such as heart rate <60/min and high-grade atrioventricular-block during hospitalization. Being married or cohabiting and having higher income level, hypertension, PCI during the index hospitalization, and STEMI were associated with better odds of adherence to beta-blocker treatment (Table 4).
| Demographics | OR (95% CI) | P value |
|---|---|---|
| Age (years) | 0.99 (0.99–0.99) | <0.001 |
| Men | 1.02 (0.97–1.08) | 0.423 |
| Civil status (married/cohabiting) | 1.30 (1.24–1.36) | <0.001 |
| Income (quartile) | ||
| 1 (lowest) | 1.00 (ref) | |
| 2 | 1.07 (1.00–1.14) | 0.071 |
| 3 | 1.17 (1.09–1.25) | <0.001 |
| 4 (highest) | 1.08 (1.00–1.16) | 0.041 |
| Comorbidities at admission, n (%) | ||
| Hypertension | 1.18 (1.12–1.24) | <0.001 |
| Estimated glomerular filtrate rate <60 mL/min | 0.94 (0.88–1.00) | 0.039 |
| Peripheral vascular disease | 0.84 (0.73–0.96) | 0.013 |
| Any stroke | 0.77 (0.70–0.83) | <0.001 |
| COPD | 0.79 (0.72–0.86) | <0.001 |
| Dementia | 0.30 (0.19–0.50) | <0.001 |
| Cancer diagnosis within 3 years | 0.79 (0.66–0.95) | 0.01 |
| Dialysis (at any time) | 0.57 (0.37–0.86) | 0.008 |
| Hospital course, n (%) | ||
| STEMI | 1.10 (1.05–1.16) | <0.001 |
| PCI during hospitalization | 1.34 (1.27–1.41) | <0.001 |
| Previous bleeding or bleeding during hospitalization | 0.86 (0.77–0.95) | 0.003 |
| Atrial fibrillation at discharge | 0.93 (0.83–1.05) | 0.237 |
| Heart failure classification | ||
| NEF without HF | 1.00 (ref) | |
| REF without HF | 1.23 (1.17–1.31) | <0.001 |
| HFNEF | 1.03 (0.94–1.13) | 0.524 |
| HFREF | 1.16 (1.07–1.25) | <0.001 |
| AV blockage II/III | 0.53 (0.44–0.63) | <0.001 |
| Systolic blood pressure <90 mmHg at admission | 0.89 (0.73–1.09) | 0.254 |
| Heart rate <60 at admission | 0.68 (0.64–0.73) | <0.001 |
- AV, atrioventricular; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HF, heart failure; HFNEF, HF and normal ejection fraction; HFREF, HF and reduced ejection fraction; NEF, normal ejection fraction; OR, odds ratio; PCI, percutaneous coronary intervention; REF, reduced ejection fraction; STEMI
Association between prescription of beta-blockers at discharge and long-term outcome
The 2 and 4 years cumulative risk for the composite of LOHF or death were 12.2% and 20.5% for patients prescribed beta-blocker treatment. The respective 2 and 4 years risks for patients who were not prescribed beta-blocker treatment were 16.7% and 24.3%. For HFREF patients the respective 2 and 4 years risks were 40.3% and 51.3% for patients with prescription and 49.4% and 62.1% for patients without prescription. HFREF patients did have the highest cumulative risks in both groups followed by patients with HFNEF, REF, and NEF, respectively (supporting information, Table S1).
Prescription of beta-blocker treatment at discharge was associated with a reduction of all-cause mortality in the crude analysis in all AMI patients as well as both HF groups. After adjustment, a significantly lower risk of all-cause mortality was observed in patients with REF and HFREF, but this association was not seen in patients with NEF and HFNEF. Prescription was associated with a lower crude risk of the composite of LOHF/death in all patients except for patients with NEF, which did not reach statistical significance after adjustment (Table S2).
Adherence to beta-blocker drugs vs. long-term outcomes
The 2 and 4 year cumulative risks for the composite of LOHF or death were 5.4% and 13.3% for adherent patients. The respective risks for non-adherent patients were 7.8% and 18.7%. HFREF patients did have the highest cumulative risks in both groups followed by patients with HFNEF, REF, and NEF, respectively (Table S3), (Figures 2 and 3A–D).
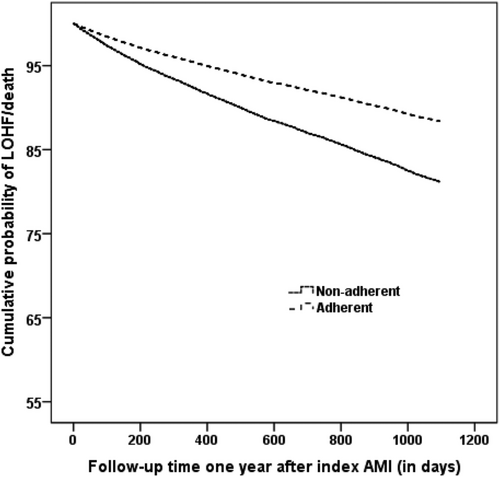
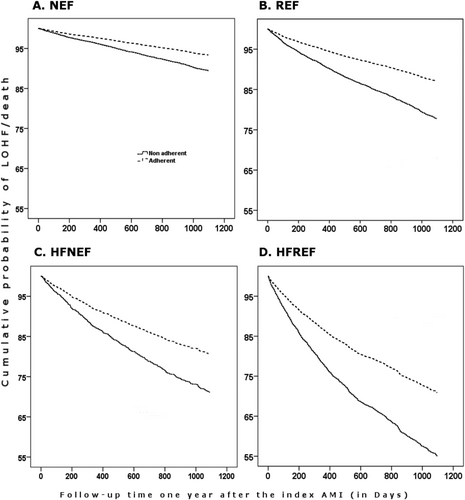
Adherence to beta-blocker treatment was associated with lower all-cause mortality in both the crude and adjusted analysis in all patients. In the adjusted subgroup analysis, adherence to treatment was associated with lower all-cause mortality and the composite endpoint of LOHF/death in all groups except that statistical significance was not reached for patients with NEF without HF and HFNEF although numerical trends towards favourable associations were observed in both groups. Patients with HFREF and REF seem to benefit most from the treatment (Table 5). Interaction tests showed significant P values (<0.05) for all cause-mortality and non-significant P values (>0.05) for the composite endpoint indicating that the association between adherence to beta-blocker treatment and all-cause mortality does differ between the LVEF subgroups (normal LVEF vs. reduced LVEF irrespective of status of HF).
| All patients (N = 38 608) | NEF (N = 21 780) | REF (N = 9929) | HFNEF (N = 2797) | HFREF (N = 4102) | |
|---|---|---|---|---|---|
| Death | |||||
| Crude | 0.51 (0.47–0.55) | 0.58 (0.50–0.66) | 0.45 (0.39–0.52) | 0.59 (0.48–0.73) | 0.46 (0.40–0.54) |
| Adjusted | 0.77 (0.71–0.84) | 0.84 (0.73–0.97) | 0.70 (0.60–0.82) | 0.93 (0.74–1.18) | 0.70 (0.59–0.82) |
| Combined | |||||
| Crude | 0.59 (0.56–0.63) | 0.62 (0.55–0.69) | 0.55 (0.49–0.62) | 0.63 (0.53–0.76) | 0.58 (0.51–0.65) |
| Adjusted | 0.83 (0.78–0.89) | 0.89 (0.78–1.01) | 0.75 (0.66–0.86) | 0.91 (0.74–1.11) | 0.78 (0.68–0.89) |
- CABG, coronary arterial bypass graft surgery; HFNEF, heart failure and normal ejection fraction; HFREF, heart failure and reduced ejection fraction; LOHF, late onset heart failure; NEF, normal ejection fraction; REF, reduced ejection fraction.
- Combined = LOHF or death.
- Variables in the model: age, sex, diabetes, hypertension, chronic kidney disease, prior stroke and drugs (Acetylsalicylic acid, angiotensin-converting enzyme/angiotensin receptor blocker, statins, and beta-blocker on admission), performed percutaneous coronary intervention or CABG during hospitalization, other antiplatelet/anticoagulant at discharge, and adherence to other drugs during first year (Acetylsalicylic acid, angiotensin-converting enzyme/angiotensin receptor blocker, and statins).
Multivariable analysis of predictors for late onset heart failure or death
Adherence to beta-blockers was associated with a lower risk of HF readmission or death (HR 0.83; 95% CI 0.78–0.9) after adjustment. Increased age, CKD, diabetes mellitus, and prior stroke increased the risk whereas female gender, PCI (HR 0.67; 0.61–0.72), CABG (HR 0.56; 0.45–0.70), and statin treatment at discharge (HR 0.70; 0.65–0.75) were associated with a lower risk of LOHF/death. Higher income level (HR 0.89; 0.86–0.96), being married/cohabiting (HR 0.80; 0.75–0.85) and higher educational level (HR 0.94; 0.90–0.99) was associated with a lower risk, whereas country of birth (European vs. non-European) (HR 0.91; 0.70–1.18) showed no associations with risk for LOHF/death (Table S4).
Discussion
The proportion of patients being prescribed secondary preventive medications including beta-blockers after AMI is high in Sweden. Patients with REF and HFREF were more likely to receive beta-blockers at discharge. These patients were also more likely to adhere to treatment. Adherence to beta-blocker treatment was independently associated with a marked reduction of all-cause mortality and the combined endpoint of HF readmission and death in AMI patients, irrespective of HF but may be confined to patients with reduced EF. Socio-economic factors such as being married/cohabiting and higher income level and clinical factors such as hypertension, ST-elevation MI, and percutaneous coronary intervention were associated with better adherence.
Our findings of high prescription frequencies of evidence-based medications in AMI patients at discharge are consistent with the findings of other studies.30-32 This can be largely attributed to the cumulative effect of evidence-based guidelines and various national quality improvement initiatives. Medications do have a pivotal role as secondary prevention measures after AMI. Poor adherence is considered a critical barrier to treatment success and remains one of the leading challenges to healthcare professionals.33 Quality measures of evidence-based medications post-MI have focused on the prescription at hospital discharge. However, the survival benefits of these medications are best realized with persistent therapy, reinforced by the findings of the present study.34 Previous studies have shown that long-term survival advantages associated with improved drug adherence after AMI appear to be class specific, suggesting that adherence outcome benefits are mediated by drug effects and do not merely reflect an epiphenomenon of ‘healthy adherer’ behavioural attributes.35 Non-adherence is also related to the increased risk of hospitalization and cost.36
In clinical practice, some patients might need to discontinue beta-blocker treatment for various medical reasons such as bradycardia or orthostatic hypotension. However, it is highly likely that a sizeable proportion of patients will discontinue the treatment without sufficient reason.
Our study shows that a considerable number of patients discontinue beta-blocker treatment at 1 year compared with a recent study by Puymirat et al., which reported 89% adherence rate at 1 year.37 One reason for the seemingly large difference could be that in the study by Puymirat et al, adherence was assessed by patient report, and not by repeated collection of prescribed drugs as in our study. A few studies have shown that inpatient pharmaceutical counselling linked to a medication and information discharge summary contributed to better drug knowledge and compliance together with reduced unplanned visits to the doctor and re-admissions.38, 39
A higher 2 and 4 years cumulative risk of LOHF or death was seen among patients who were non-adherent to the treatment. As expected, patients with HFREF did have the highest cumulative risks of LOHF/death followed by patients with HFNEF, REF, and NEF, respectively. Adherence to beta-blocker treatment was also associated with a reduction in all-cause mortality and the composite endpoint of LOHF/death both in the adjusted and crude analyses. In the adjusted subgroup analysis, the effect of adherence on the composite of LOHF/death was uncertain in patients with NEF without HF and HFNEF as statistical significance was not reached although a trend towards favourable effect was seen. This is consistent with the finding of previous studies.34 Contrary to the findings of other studies37 and the questioned place of beta-blocker treatment long after AMI in the modern reperfusion era,16, 40 we observed a favourable association with outcome even in patients with NEF without HF as shown by survival curves that continue separating until the end of the follow-up period. The finding that the association between adherence to beta-blocker and long-term outcome in patients with HFNEF was less obvious is also interesting and in line with the fact that patients with HFNEF lack effective evidence-based treatment to date.
Limitations
Our study has limitations. As for all observational studies, we are unable to explore the influence of unmeasured confounders. Meanwhile, although drawing definitive conclusions is difficult, the observed associations reinforce the findings of randomized and observational studies regarding the effectiveness of beta-blockers after AMI on long-term outcomes as well as good tolerability. In the present analysis, we have not accounted for brand or dosage. This will remain an inherent limitation. It is known that dosage of beta-blockers used constitute an important aspect of beta-blocker treatment, as under-dosage of beta-blockers is a common problem.23, 41
Because we use pharmacy data of collected prescriptions, we cannot absolutely be certain whether a patient took the medication or not. However, as a drug prescription in Sweden only covers 3 months at a time, if patients do not refill, we can assume that these patients have discontinued treatment. In addition, our study does not reveal the reasons for non-prescription or discontinuation of the drug, but as in previous studies,42 we can assume that it might because of fear of or actually experienced side effects.
Conclusions
Prescription of beta-blockers was high at discharge from AMI, but nearly one in three patients was non-adherent to beta-blockers within the first year. Adherence to beta-blocker treatment was higher in patients with REF with or without in-hospital HF. Adherence was independently associated with improved long-term outcomes, in particular in patients with reduced EF with or without HF. Uncertainties regarding patients with HFNEF and NEF warrants further controlled studies. Socio-economic and clinical factors widely influenced adherence to beta-blocker treatment. Based on our findings, approaches that improve adherence to secondary prevention drugs are warranted to improve long-term outcomes after AMI.
Acknowledgements
This study would not have been possible without the staff of all hospitals participating in SWEDEHEART in Sweden, who enter data on the coronary care unit patients into the registry over the Internet 24 h a day every day of the year. We are also indebted to Mir Khedri for substantial contribution to developing the semi-automated supervised script.
Conflict of interest
None declared.
Funding
This work was a part of the national TOTAL-AMI project, supported by the Swedish Strategic Research Foundation (SSF), and also supported through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet and the Swedish Heart Lung Foundation. Financial support for the SWEDEHEART registry is provided by the Swedish Association of Local Authorities and Regions and the National Board of Health and Welfare.
Role of the sponsor
Neither the SSF, the Swedish Heart Lung Foundation, nor the Swedish Association of Local Authorities and Regions participated in the design and conduct of the study, in the collection analysis and interpretation of the data, or in the preparation, review, or approval of the manuscript.




