Prognostic impact of 6 min walk test distance in patients with systolic heart failure: insights from the WARCEF trial
Abstract
Aims
This study aimed to investigate the impact of baseline 6 min walk test distance (6MWTD) on time to major cardiovascular (CV) events in heart failure with reduced ejection fraction (HFrEF) and its impact in clinically relevant subgroups.
Methods and results
In the WARCEF (Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction) trial, 6MWTD at baseline was available in 2102 HFrEF patients. Median follow-up was 3.4 years. All-cause death and heart failure hospitalization (HFH) exhibited a significant non-linear relationship with 6MWTD (P = 0.023 and 0.032, respectively), whereas a significant association between 6MWTD and CV death was shown in a linear model [hazard ratio (HR) per 10 m increase, 0.989; P = 0.011]. In linear splines with the best cut-off point at 200 m, the positive effect of a longer 6MWTD on all-cause death and HFH was only observed for 6MWTD > 200 m (HR per 10 m increase, 0.987; P = 0.0036 and 0.986; P = 0.0022, respectively). The associations between 6MWTD and CV outcomes were consistent across clinical subgroups; for age, a significant relationship between 6MWTD and HFH was observed in patients ≥60 years (HR per 10 m increase, 0.98; P < 0.001), but not in patients <60 years (HR per 10 m increase, 1.00; P = 0.98; P = 0.02 for the interaction).
Conclusions
In HFrEF, 6MWTD is independently associated with all-cause death, CV death, and HFH. 6MWTD of 200 m is the best cut-off point for predicting these adverse events. The prognostic impact of 6MWTD for HFH was only observed in older patients.
Introduction
The 6 min walk test (6MWT) provides a reliable and inexpensive way to measure exercise capacity in patients with heart failure (HF) with reduced ejection fraction (HFrEF).1, 2 A number of studies have shown that low 6MWT distance (6MWTD) is associated with increased total mortality and more numerous hospital admissions for HF.3-19 However, the full prognostic impact of 6MWTD has not been completely elucidated. While it is well known that for 6MWTD ‘the lower, the worse’, the optimal cut-off value for predicting adverse events is still unclear.3 It is also unclear whether the relationship of 6MWTD with outcome is linear or non-linear.4-8 Although 6MWTD is frequently treated as a continuous variable in clinical studies,4, 5, 9-12 a non-linear relationship between 6MWTD and clinical events was indicated in several articles.5, 6 Moreover, while patients with HFrEF are diverse in terms of demographics, co-morbidities, aetiology of HF, and other factors,20 it is unclear whether the prognostic impact of 6MWT differs among particular subgroups stratifying by clinical characteristics including age, sex, and co-morbidities.3
Previous studies were limited by relatively small sample sizes6, 7, 11-17 and short follow-up duration.4, 6, 12-14, 17, 18 The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial,21 with its larger cohort and longer follow-up, allows a more detailed investigation of the prognostic impact of 6MWT and its clinical utility. The primary aim of this exploratory analysis is to investigate the impact of baseline 6MWTD on time to five major cardiovascular (CV) events in the HFrEF patients enrolled in WARCEF: all-cause death, CV death, myocardial infarction (MI), ischaemic stroke, and HF hospitalization. The secondary aim was to investigate the prognostic impact of 6MWTD in clinically relevant subgroups.
Methods
Study patients
The design of WARCEF trial has been described previously.21 In brief, 2305 patients with HFrEF were enrolled at 168 centres in 11 countries from October 2002 to January 2010. Eligible patients were >18 years of age and had normal sinus rhythm, no contraindication to warfarin therapy, a modified Rankin score of 4 or less, left ventricular ejection fraction ≤35% within 3 months before randomization, and optimal medical therapy of HFrEF including angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, and beta-blocker. Patients who had a clear indication for warfarin or aspirin, or a condition that conferred a high risk of cardiac embolism, were ineligible. Patients were randomly assigned to either adjusted-dose warfarin with target international normalized ratio of 2.75 (acceptable range: 2.0–3.5) or aspirin 325 mg daily in a double-blind, double-dummy design.
For each participant, 6MWT was administered by study coordinators who measured distance walked over a 6 min period following a standardized protocol22 at each study site. Instructions to patients included standardized phrasing (e.g. ‘Walk for 6 minutes around this course, covering as much ground as possible … keep going … don't worry if you have to slow down and rest …’) and consistent timing of encouragement (1 min intervals).
The investigation conforms to the Declaration of Helsinki. The study was approved by institutional review boards at the coordinating centres of all sites, and all subjects provided informed consent.
Follow-up and outcome events
Follow-up was performed monthly by telephone or in person. A follow-up assessment in person was conducted quarterly for a clinical evaluation and annually for a detailed examination. The primary outcome of the trial was the time to the first event in a composite endpoint of ischaemic stroke, intracerebral haemorrhage, or death from any cause. The present study focuses on individual CV outcomes, which were defined as follows. Sudden death was defined as (i) death witnessed or occurring within 15 min of observed collapse or new cardiac symptoms, without preceding other modes of death, or (ii) death unwitnessed but known to have occurred in the prior 72 h in the absence of other modes of death or (iii) patient resuscitated from cardiac arrest and dying within 24 h or prior to discharge, in case neurological function was not restored. CV death included sudden death, documented ventricular tachycardia or fibrillation, documented bradyarrhythmia, MI, and circulatory failure. The diagnosis of MI was based on two of the following: (i) typical cardiac pain or its equivalent, (ii) electrocardiogram evidence of acute MI, or (iii) positive cardiac biomarkers. Stroke was defined as a clinically relevant new lesion detected on computed tomography or magnetic resonance imaging or, in its absence, clinical findings consistent with clinical stroke and lasting over 24 h. HF hospitalizations during the follow-up were defined as admissions with typical symptoms; intravenous diuretics, vasodilator, or inotropic therapy; and at least 24 h of hospital stay. Median follow-up of the patients was 3.4 years (inter-quartile range: 2.0–5.0 years).
Statistical analysis
Mean values ± standard deviation (SD) for continuous variables and number/total number (%) for categorical variables known to affect 6MWTD or CV events were presented and compared using Jonckheere–Terpstra's trend test by quantiles of 6MWTD. Kaplan–Meier survival curves for each of the outcome measures listed earlier were generated in quantiles of 6MWTD. Univariable and multivariable Cox proportional hazards regression analyses were performed to estimate the magnitude of changes in risk for all-cause death, CV death, MI, stroke, and HF hospitalization conferred by unit changes in 6MWTD. The multivariable models were then adjusted for patient characteristics selected by stepwise forward–backward selection, with entry and removal criteria at P = 0.05. All baseline covariates (Table 1) were eligible for selection. Non-linear relationship between 6MWTD and the aforementioned clinical outcomes was assessed with restricted cubic splines. If a trend of non-linearity was found (P < 0.05), we further considered the use of linear splines to assess the association between 6MWTD and clinical outcomes. Choices of knots used in the linear splines include following prespecified clinically important cut-off points: (i) the third quartile,7, 10 (ii) 1 SD above the mean,5 and (iii) other popular cut-offs, including 200, 300, 400, and 500 m.6, 7, 11, 12, 14, 15 The best cut-off point was determined by model fit statistics including Akaike's information criterion; the one with the lowest model statistics was chosen.
| Variable | 6MWTD | P-valuea | |||
|---|---|---|---|---|---|
| 1st quartile (≤252 m) (n = 524) | 2nd quartile (253–354 m) (n = 523) | 3rd quartile (355–438 m) (n = 523) | 4th quartile (>439 m) (n = 522) | ||
| Age (years) | 62.9 ± 11.2 | 61.4 ± 12.2 | 59.9 ± 10.7 | 59.1 ± 11.0 | <0.0001 |
| Male sex | 393 (75.0) | 415 (79.3) | 426 (81.5) | 444 (85.1) | <0.0001 |
| Race or ethnic group | <0.0001 | ||||
| Non-Hispanic White | 383 (73.1) | 368 (70.4) | 414 (79.2) | 446 (85.4) | |
| Non-Hispanic Black | 85 (16.2) | 88 (16.8) | 62 (11.9) | 35 (6.7) | |
| Hispanic | 46 (8.8) | 51 (9.8) | 31 (5.9) | 24 (4.6) | |
| Other | 10 (1.9) | 16 (3.1) | 16 (3.1) | 17 (3.3) | |
| Body mass index (kg/m2) | 29.7 ± 6.4 | 29.5 ± 6.0 | 28.8 ± 5.6 | 28.5 ± 5.4 | 0.0018 |
| Education level | 0.0073 | ||||
| <High school | 257 (49.0) | 222 (42.4) | 218 (41.9) | 220 (42.2) | |
| High school graduate or some college | 204 (38.9) | 216 (41.3) | 208 (40.0) | 210 (40.3) | |
| College graduate or postgraduate | 63 (12.0) | 85 (16.3) | 94 (18.1) | 91 (17.5) | |
| Alcohol consumption | 0.78 | ||||
| Current consumption (>2 oz/day) | 121 (23.1) | 129 (24.7) | 126 (24.1) | 145 (27.8) | |
| Previous consumption (>2 oz/day) | 132 (25.2) | 115 (22.0) | 114 (21.8) | 87 (16.7) | |
| Never consumed alcohol | 271 (51.7) | 279 (53.3) | 282 (54.0) | 290 (55.6) | |
| Smoking status | 0.53 | ||||
| Current smoker | 89 (17.0) | 104 (19.9) | 100 (19.2) | 84 (16.1) | |
| Former smoker | 257 (49.0) | 253 (48.4) | 282 (54.0) | 271 (51.9) | |
| Never smoked | 178 (34.0) | 166 (31.7) | 140 (26.8) | 167 (32.0) | |
| Clinical characteristics | |||||
| Heart rate (b.p.m.) | 73.4 ± 13.0 | 71.5 ± 11.0 | 71.3 ± 12.0 | 71.0 ± 11.6 | 0.0043 |
| Systolic blood pressure (mmHg) | 124.4 ± 19.1 | 123.9 ± 20.3 | 123.3 ± 17.5 | 123.7 ± 17.4 | 0.60 |
| Diastolic blood pressure (mmHg) | 74.9 ± 11.7 | 73.5 ± 11.5 | 74.2 ± 11.7 | 74.3 ± 10.8 | 0.70 |
| NYHA Class III or IV | 274 (52.4) | 180 (34.4) | 126 (24.1) | 63 (12.1) | <0.0001 |
| LV ejection fraction (%) | 24.3 ± 7.3 | 24.6 ± 7.3 | 24.7 ± 7.3 | 25.4 ± 8.0 | 0.061 |
| Intracardiac defibrillator | 101 (19.3) | 124 (23.8) | 131 (25.1) | 122 (23.4) | 0.093 |
| Baseline MMSE score | 28.1 ± 2.5 | 28.4 ± 2.1 | 28.6 ± 1.8 | 28.9 ± 1.7 | <0.0001 |
| Diabetes mellitus | 197 (37.7) | 150 (28.7) | 151 (28.9) | 143 (27.4) | 0.0007 |
| Hypertension | 343 (67.9) | 329 (64.9) | 305 (59.2) | 263 (52.2) | <0.0001 |
| Atrial fibrillation | 24 (4.6) | 20 (3.8) | 21 (4.0) | 14 (2.7) | 0.14 |
| Ischaemic cardiomyopathy | 241 (46.2) | 226 (43.3) | 219 (42.0) | 219 (42.0) | 0.16 |
| Coronary artery disease | 336 (64.2) | 300 (57.6) | 284 (54.4) | 294 (56.4) | 0.0058 |
| Peripheral vascular disease | 73 (13.9) | 65 (12.4) | 53 (10.1) | 36 (6.9) | 0.0001 |
| Prior stroke or TIA | 89 (17.0) | 74 (14.1) | 58 (11.1) | 40 (7.7) | <0.0001 |
| Blood chemistry | |||||
| White blood cell count (× 109/L) | 7.5 ± 2.1 | 7.6 ± 2.0 | 7.6 ± 2.1 | 7.3 ± 1.9 | 0.32 |
| Haemoglobin (g/dL) | 13.8 ± 1.7 | 14.1 ± 1.5 | 14.1 ± 1.5 | 14.4 ± 1.4 | <0.0001 |
| Haematocrit (%) | 41.4 ± 5.1 | 41.8 ± 4.3 | 41.9 ± 4.2 | 42.6 ± 4.1 | <0.0001 |
| Creatinine (mg/dL) | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | <0.0001 |
| BUN (mg/dL) | 25.1 ± 13.7 | 23.1 ± 11.4 | 23.5 ± 12.8 | 23.5 ± 12.5 | 0.061 |
| eGFR (mL/min/1.73 m2) | 65.6 ± 21.6 | 66.6 ± 19.6 | 69.0 ± 20.3 | 71.6 ± 19.1 | <0.0001 |
| Serum sodium (mEq/L) | 139 ± 3.6 | 139 ± 3.3 | 140 ± 3.0 | 140 ± 6.7 | 0.051 |
| Medications | |||||
| Aspirin | 299 (61.9) | 297 (61.4) | 274 (55.9) | 265 (54.6) | 0.0065 |
| Warfarin or other oral anticoagulant | 48 (9.2) | 28 (5.4) | 51 (9.8) | 35 (6.7) | 0.57 |
| ACE inhibitor or ARB | 513 (98.1) | 514 (98.5) | 517 (99.2) | 514 (98.8) | 0.18 |
| Aldosterone blocker | 185 (67.3) | 209 (64.1) | 196 (59.4) | 161 (48.9) | <0.0001 |
| Beta-blocker | 457 (87.4) | 487 (93.1) | 477 (91.7) | 477 (91.6) | 0.041 |
| Nitrates | 167 (32.0) | 132 (25.2) | 112 (21.5) | 85 (16.3) | <0.0001 |
| Calcium channel blocker | 51 (9.8) | 44 (8.4) | 42 (8.1) | 37 (7.1) | 0.13 |
| Diuretics | 445 (85.1) | 443 (84.7) | 412 (79.1) | 389 (74.8) | <0.0001 |
| Statin | 306 (85.2) | 348 (85.9) | 321 (79.9) | 312 (79.0) | 0.0038 |
- 6MWTD, 6 min walk test distance; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; LV, left ventricular; MMSE, Mini-Mental State Examination; NYHA, New York Heart Association; TIA, transient ischaemic attack.
- Mean ± standard deviation were calculated for continuous variables and number/total number (%) for categorical variables.
- a P-values were calculated using Jonckheere–Terpstra's trend test.
Additionally, to assess the potential effect modification on the association of 6MWTD with outcomes in HF, we selected covariates that were shown to be associated with both 6MWTD and prognosis in HF patients in the previous publications.4, 12, 16, 19, 23, 24 Candidate variables for subgroup analysis include age, sex, estimated glomerular filtration rate (eGFR), body mass index (BMI), haemoglobin, hypertension, diabetes mellitus, and ischaemic cardiomyopathy. Each of these models included terms for 6MWTD, one candidate variable, and the interaction of that candidate variable with 6MWTD. The candidate variable was considered to be an effect modifier if its interaction with 6MWTD was significant at level α = 0.05 two sided, with no adjustment for multiple tests because the analysis was exploratory.
Age was dichotomized at age <60 years vs. age ≥60 years. BMI was scored as low (<25 kg/m2), medium (25–30 kg/m2), or high (>30 kg/m2). eGFR was scored as low (<60 mL/min/1.73 m2) and normal (≥60 mL/min/1.73 m2). Haemoglobin was scored as low with haemoglobin <13 g/dL in men and <12 g/dL in women. Stepwise selection procedure was used to develop a multivariable Cox model, accounting for the impact of all other selected variables. The missingness of the baseline characteristics was shown in Supporting Information, Table S1. Missing values for continuous variables were imputed with mean values; those for categorical variables were imputed by mode. Because of the large amount of missing information, the use of aldosterone blocker (39.77%) and statin (25.38%) was not included in the candidates for any adjusted models. Two-sided P < 0.05 was considered significant. Statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Results
Study population baseline characteristics
Of the 2102 patients whose baseline 6MWTD was available, we excluded 10 patients who had either modified Rankin scale <3 and 6MWTD ≤ 10 m or 6MWTD ≥ 999 m, leading to the final study sample of 2092 patients.
The median 6MWTD of the entire cohort was 354 m (mean 351 ± 147 m). Baseline characteristics of the study population according to quartiles of 6MWTD are summarized in Table 1.
Follow-up
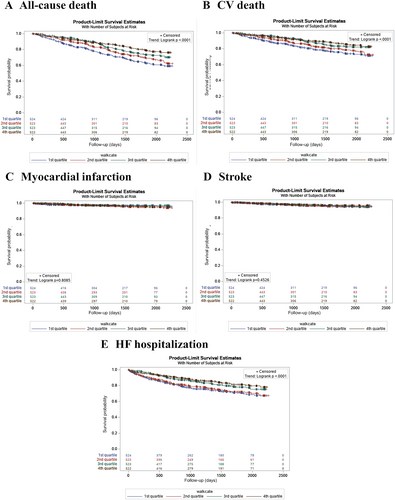
The details of the clinical event distribution are shown in Table 2. The rates of both all-cause and CV death and of HF hospitalization were greatest in patients with the lowest 6MWTD. On the other hand, the risk of MI and stroke was similar among the quartiles of 6MWTD.
| Outcome | 1st quartile (n = 524) | 2nd quartile (n = 523) | 3rd quartile (n = 523) | 4th quartile (n = 522) | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients (%) | Rate of events per 100 patient years | No. of patients (%) | Rate of events per 100 patient years | No. of patients (%) | Rate of events per 100 patient years | No. of patients (%) | Rate of events per 100 patient years | ||
| All-cause death | 163 (31.1) | 9.07 | 127 (24.3) | 7.06 | 100 (19.1) | 5.44 | 82 (15.7) | 4.49 | |
| CV death | 110 (21.0) | 6.12 | 88 (16.8) | 4.89 | 62 (11.9) | 3.37 | 56 (10.7) | 3.07 | |
| Myocardial infarction | 15 (2.9) | 0.85 | 18 (3.4) | 1.02 | 12 (2.3) | 0.66 | 16 (3.1) | 0.89 | |
| Stroke | 21 (4.0) | 1.17 | 19 (3.6) | 1.06 | 18 (3.4) | 0.98 | 17 (3.3) | 0.93 | |
| HF hospitalization | 126 (24.1) | 7.99 | 118 (22.6) | 7.58 | 86 (16.4) | 5.16 | 74 (14.2) | 4.37 | |
- 6MWTD, 6 min walk test distance; CV, cardiovascular; HF, heart failure.
Figure 1 displays the Kaplan–Meier estimated event rates and hazard ratios (HRs) for the 6MWTD quartiles. The log-rank trend test showed that the lower quartile of 6MWTD was associated with significantly higher all-cause death, CV death, and HF hospitalization (all P < 0.0001). The incidence of MI and stroke was not significantly different among the 6MWTD quartiles.
Linear and non-linear association between 6 min walk test distance and the clinical outcomes
The possibility of non-linear relationships was assessed with restricted cubic spline (Supporting Information, Table S2). All-cause death and HF hospitalization exhibited a significant non-linear relationship with 6MWTD (P = 0.023 and 0.032, respectively). There was no evidence of non-linearity in the relationship between 6MWTD and CV death (P = 0.43). Again, 6MWTD showed no association with MI (P = 0.39) and stroke (P = 0.84).
For the outcomes that failed to exhibit non-linear relationship with 6MWTD, we fitted a linear model for each. Table 3 showed the linear association analysis between 6MWTD and those clinical outcomes in unadjusted and adjusted Cox models. There was a significant relationship of 6MWTD with CV death (HR per 10 m increase, 0.989; P = 0.011) in the fully adjusted model, while no association was again observed for 6MWTD with MI (P = 0.70) or stroke (P = 0.73).
| Outcome | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| HR for every 10 m increase in 6MWTD (95% CI) | P-value | HR for every 10 m increase in 6MWTD (95% CI) | P-value | |
| CV death | 0.982 (0.975–0.990) | <0.0001 | 0.989 (0.981–0.998) | 0.011 |
| Myocardial infarction | 1.001 (0.984–1.018) | 0.90 | 1.003 (0.987–1.021) | 0.70 |
| Stroke | 0.998 (0.983–1.013) | 0.79 | 1.003 (0.987–1.018) | 0.73 |
- 6MWTD, 6 min walk test distance; CI, confidence interval; CV, cardiovascular; HR, hazard ratio.
- a The models were adjusted for patient characteristics selected by stepwise forward–backward selection, with entry and removal criteria at P = 0.05. For CV death: blood urea nitrogen, ischaemic cardiomyopathy, diuretics, beta-blocker, New York Heart Association Class III or IV, hypertension, ejection fraction, age, sex, heart rate, systolic blood pressure, and peripheral vascular disease; for myocardial infarction: ischaemic cardiomyopathy, age, and estimated glomerular filtration rate; and for stroke: prior stroke or transient ischaemic attack and blood urea nitrogen.
Linear splines were generated to evaluate the association between 6MWTD and all-cause death and between 6MWTD and HF hospitalization with the best cut-off point at 200 m chosen using Akaike's information criterion (Table 4). Further, Harrell's concordance index (c-index) was calculated to assess the probability of concordance between the observed and the predicted outcome; the cut-off point at 200 m resulted in the highest c-index (Supporting Information, Table S3). For all-cause death, the adjusted HR (per 10 m increase in 6MWTD) for 6MWTD ≤ 200 m was 1.022 [95% confidence interval (CI) 0.993–1.052; P = 0.14], whereas that for 6MWTD > 200 m was 0.987 (95% CI 0.978–0.996; P = 0.0036). For HF hospitalization, the adjusted HR for 6MWTD ≤ 200 and >200 m was 1.021 (95% CI 0.989–1.054; P = 0.21) and 0.986 (95% CI 0.977–0.995; P = 0.0022), respectively.
| Outcome | Unadjusted | Adjusteda | ||
|---|---|---|---|---|
| HR for every 10 m increase in 6MWTD (95% CI) | P-value | HR for every 10 m increase in 6MWTD (95% CI) | P-value | |
| All-cause death | ||||
| Cut-off at 200 m | <0.0001 | <0.0001 | ||
| ≤200 m | 1.020 (0.991–1.049) | 0.19 | 1.022 (0.993–1.052) | 0.14 |
| >200 m | 0.976 (0.968–0.985) | <0.0001 | 0.987 (0.978–0.996) | 0.0036 |
| HF hospitalization | ||||
| Cut-off at 200 m | <0.0001 | <0.0001 | ||
| ≤200 m | 1.021 (0.989–1.054) | 0.21 | 1.021 (0.989–1.054) | 0.21 |
| >200 m | 0.978 (0.969–0.987) | <0.0001 | 0.986 (0.977–0.995) | 0.0022 |
- 6MWTD, 6 min walk test distance; CI, confidence interval; HF, heart failure; HR, hazard ratio.
- a The models were adjusted for patient characteristics selected by stepwise forward–backward selection, with entry and removal criteria at P = 0.05. For all-cause death, the adjustments are age, peripheral vascular disease, diuretics, heart rate, ischaemic cardiomyopathy, body mass index, diabetes mellitus, beta-blocker, New York Heart Association Class III or IV, sex, haemoglobin, intracardiac defibrillator, ejection fraction, education, creatinine, and calcium channel blocker; for HF hospitalization: blood urea nitrogen, diuretics, ejection fraction, Mini-Mental State Examination, systolic blood pressure, diabetes mellitus, heart rate, prior stroke or transient ischaemic attack, peripheral vascular disease, ischaemic cardiomyopathy, and creatinine.
Subgroup analysis
The associations between 6MWTD and CV outcomes were consistent across subgroups defined by sex, eGFR, BMI, haemoglobin, hypertension, diabetes mellitus, and ischaemic cardiomyopathy (Supporting Information, Tables S4–S10). However, there was a significant relationship between 6MWTD and HF hospitalization in patients ≥60 years (HR per 10 m increase, 0.98; P < 0.001; Table 5), whereas no significant association was observed in patients <60 years (HR per 10 m increase, 1.00; P = 0.98; P = 0.02 for the interaction; also Table 5).
| Outcome | Unadjusted | Adjusteda | No. of patients (%) | Rate of events per 100 patient years | ||
|---|---|---|---|---|---|---|
| HR for every 10 m increase in 6MWTD (95% CI) | P-value | HR for every 10 m increase in 6MWTD (95% CI) | P-value | |||
| All-cause death | ||||||
| Age <60 years old | 0.98 (0.97–0.99) | <0.001 | 0.99 (0.98–1.00) | 0.04 | 164 (17.60) | 4.73 |
| Age ≥60 years old | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.98–1.00) | 0.08 | 308 (26.55) | 8.13 |
| Interaction | 0.34 | 0.50 | ||||
| CV death | ||||||
| Age <60 years old | 0.98 (0.96–0.99) | <0.001 | 0.98 (0.97–1.00) | 0.02 | 123 (13.20) | 3.55 |
| Age ≥60 years old | 0.99 (0.98–1.00) | <0.001 | 0.99 (0.98–1.00) | 0.05 | 193 (16.64) | 5.09 |
| Interaction | 0.28 | 0.48 | ||||
| Myocardial infarction | ||||||
| Age <60 years old | 1.00 (0.97–1.03) | 0.87 | 1.00 (0.97–1.03) | 0.81 | 22.0 (2.36) | 0.64 |
| Age ≥60 years old | 1.00 (0.98–1.02) | 0.84 | 1.00 (0.98–1.02) | 0.87 | 39.0 (3.36) | 1.05 |
| Interaction | 0.99 | 0.92 | ||||
| Stroke | ||||||
| Age <60 years old | 1.00 (0.97–1.02) | 0.85 | 1.00 (0.98–1.03) | 0.83 | 32 (3.43) | 0.92 |
| Age ≥60 years old | 1.00 (0.98–1.02) | 0.91 | 1.00 (0.98–1.02) | 0.76 | 43 (3.71) | 1.13 |
| Interaction | 0.93 | 0.98 | ||||
| HF hospitalization | ||||||
| Age <60 years old | 0.99 (0.98–1.00) | 0.18 | 1.00 (0.99–1.01) | 0.98 | 189 (20.28) | 6.16 |
| Age ≥60 years old | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | <0.001 | 215 (18.53) | 6.27 |
| Interaction | 0.04 | 0.02 | ||||
- 6MWTD, 6 min walk test distance; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio.
- a The models were adjusted for patient characteristics selected by stepwise forward–backward selection, with entry and removal criteria at P = 0.05. For all-cause death, the adjustments are blood urea nitrogen, peripheral vascular disease, ischaemic cardiomyopathy, body mass index, heart rate, diabetes mellitus, New York Heart Association Class III or IV, ejection fraction, sex, haemoglobin, intracardiac defibrillator, diuretics, education, creatinine, beta-blocker, and calcium channel blocker; for CV death: blood urea nitrogen, ischaemic cardiomyopathy, diuretics, beta-blocker, New York Heart Association Class III or IV, ejection fraction, sex, systolic blood pressure, heart rate, body mass index, and peripheral vascular disease; for myocardial infarction: ischaemic cardiomyopathy; for stroke: blood urea nitrogen and prior stroke or transient ischaemic attack; and for HF hospitalization: blood urea nitrogen, diuretics, ejection fraction, Mini-Mental State Examination, diabetes mellitus, systolic blood pressure, heart rate, prior stroke or transient ischaemic attack, ischaemic cardiomyopathy, peripheral vascular disease, and creatinine.
Discussion
In this secondary analysis from WARCEF trial, we observed that shorter 6MWTD was significantly associated with higher all-cause death, CV death, and HF hospitalization. All-cause death and HF hospitalization exhibited a significant non-linear relationship with 6MWTD. Moreover, among several prespecified cut-off points, the best cut-off value of 6MWTD for the prediction of those events was 200 m. Finally, the subgroup analyses showed that only age was a significant 6MWT prognostic modifier, and only with respect to HF hospitalization. To the best of our knowledge, this is the first study that has systematically investigated the association between 6MWTD and each CV event, the optimal 6MWTD cut-off point for prognosis, and the potential interaction of clinically relevant patient characteristics. These analyses were conducted in one of the largest international cohorts of patients with HFrEF.
Exercise capacity and tolerance are important factors in the assessment of the clinical status and prognosis of chronic HF. The 6MWT is a submaximal exercise test that identifies the limitation of exercise capacity in daily life.1-3 To date, numerous studies have investigated whether 6MWTD is a prognostic indicator in HF patients. The majority of these studies demonstrated that lower levels of 6MWTD are predictive of mortality in HFrEF.3-16, 18 However, the role of 6MWTD in the prediction of HF hospitalization as an independent event has not been clearly established. The few studies on the topic have been limited by small sample sizes and relatively short follow-up durations.6, 14, 17 Furthermore, 6MWT has the potential to predict other major CV events including MI25 and stroke.26 Although HFrEF is known as a high-risk condition for those atherothrombotic events,21 6MWTD's predictive value for MI and stroke had not been investigated. Our data confirm a significant relationship of 6MWTD with all-cause death, CV death, and HF hospitalization, but not with MI or stroke. Because exercise is beneficial in reducing CV risk factors over time, its beneficial effect on MI and stroke incidence would be expected to occur over a longer follow-up period than that on HF hospitalization. Therefore, the shorter follow-up period in this study compared with those previous studies25, 26 may have weakened the relationship of 6MWTD at baseline with MI and stroke.
Our exploratory analyses identified a distance of 200 m as the best cut-off value of 6MWTD for the prediction of CV events. This value is the lowest among several prespecified cut-offs applied in previous studies.5-7, 10-12, 14, 15 One possible explanation is that our cohort included patients with quite low ejection fraction values, despite optimal medication for HF. Nevertheless, this finding is consistent with previous smaller studies.6, 12 In 541 HFrEF patients from the Digitalis Investigation Group trial, Curtis et al. demonstrated that 6MWT identified patients who walk ≤200 m as being at markedly increased risk of death over a median follow-up of 32 months.6 Moreover, Alahdab et al. showed that a 6MWTD less than 200 m was associated with higher risk of 40 month all-cause mortality and 18 month HF rehospitalization in a study of 198 African American patients with HF.12 The present study extends these findings by including a substantially longer follow-up and a larger patient population. Furthermore, our analysis showed that all-cause death and HF hospitalization exhibited a significant non-linear relationship with 6MWTD and that a progressive protective effect of a longer 6MWTD on all-cause death and HF hospitalization only existed for a distance greater than 200 m. To date, the data on whether the association between 6MWTD and CV outcomes is linear or non-linear are still contradictory.4-8 These inconsistent results may reflect the fact that the specific cut-off points were based on the distribution of 6MWTD in each cohort. It should be noted, however, that our findings are consistent with previous studies in which a much smaller difference in survival was observed among the groups who walked ≤200 m than among those who walked >200 m.7, 12
There are limited data regarding the association between 6MWTD and CV events for subgroups identified by clinically relevant characteristics. Although Alahdab et al. observed that the 6MWT failed to predict mortality or HF hospitalization in women,12 in the present analysis, there was no statistically significant interaction of sex and 6MWTD on the risk of any CV events. Our findings are consistent with those of Curtis et al.6 and Ferreira et al.4 providing further confirmation of the lack of interaction of sex with 6MWTD in a larger cohort. In contrast, the present study found that age group was the only modifier of the prognostic impact of 6MWT and only on HF hospitalization. The 6MWT may identify HF patients with subclinical congestion,17 particularly, among ambulating elderly patients who usually develop symptoms below their maximal exercise capacity27; conversely, 6MWT might not represent a sufficiently challenging test for younger patients with inherent higher exercise capacity. On the other hand, 6MWTD is also a reflection of many factors such as cardiac output, skeletal muscle strength, co-morbidities, and mental status,4, 17 which all may play a role in the link between poor 6MWTD and mortality. This may explain why the close relationship of 6MWTD with all-cause and CV mortality was consistent across all subgroups in the present study, even after multiple adjustments for potential confounders. The study also suggests that no other clinically relevant variable modifies the association between 6MWTD and outcomes; therefore, the results of the present study apply to all HFrEF patients, regardless of other associated cofactors or co-morbidities.
Taken together, our results suggest several conclusions. First, a threshold of 200 m was identified as the best cut-off value for predicting adverse events in HFrEF among several cut-off points suggested by previous studies. This finding may provide insight on better prognostic stratification of HFrEF patients, possibly leading to better management. Also, the positive effect of a longer 6MWTD on prognosis shown in the subgroup with 6MWTD > 200 m suggests that continuation of exercise training, which is associated with improvement of exercise capacity in patients with chronic HF, may be beneficial in terms of outcome.28 Second, our systematic subgroup analyses revealed that the 6MWT for the prediction of HF hospitalization is likely to perform better in older patients, who comprise the largest group of HF patients.27 With this in mind, clinicians should pay more attention to exercise capacity to prevent HF hospitalization in older patients. Third, the present study confirmed the prognostic impact of 6MWT on all-cause death, CV death, and HF hospitalization among major CV events in a large contemporary and international HFrEF population. The 6MWT has been suggested as a safe, simple, and inexpensive test to assess exercise capacity in HF patients.12, 16 In addition, recent studies demonstrated the clinical utility of tele-6MWT29 and 6MWT mobile application30 self-performed by a patient without additional medical staff. Overall, these findings suggest a more widespread use of 6MWT in the stratification of adverse outcome among HFrEF patients.
Several limitations should be acknowledged in our analysis. First, the WARCEF trial enrolled HFrEF in sinus rhythm with a modified Rankin score of 4 or less. Therefore, the results may not be generalizable to other subgroups of HF patients (e.g. those with atrial fibrillation). Second, the incidence of some outcomes, such as MI and stroke, was relatively low, which might decrease the ability to detect significant association between 6MWTD and those events. However, this is the first study to investigate the prognostic impact of 6MWT for the prediction of MI and stroke in HFrEF. Third, several biomarkers, including brain natriuretic peptide, were not systematically recorded in the dataset; however, all multivariable analyses in this study were adjusted for baseline covariables as much as possible. Fourth, neither repeated 6MWT nor information on dyspnoea evaluated by Borg scale was available. Despite the potential for decreased accuracy in 6MWTD assessment in our study, it should be noted that no significant change between tests was observed in the literature for HF patients whose baseline 6MWTD was <300 m.31 Therefore, it is unlikely that the cut-off value of 200 m may have been significantly affected in our study by the lack of a repeat test. Finally, this study is a retrospective analysis from a prospectively designed clinical trial that was not originally designed to evaluate the association between 6MWTD and outcomes. Additionally, the WARCEF trial was conducted when some current strategies of HFrEF such as intravenous iron supplementation, angiotensin receptor–neprilysin inhibitor, and cardiac resynchronization therapy were not widely available. Nevertheless, the study is one of the largest investigations of the relationship between 6MWTD and CV outcomes in HFrEF treated with guideline-recommended medical treatment at the time of the present study.
Conclusions
This study identified 6MWTD as an independent predictor of all-cause death, CV death, and HF hospitalization in one of the largest international HFrEF samples. Moreover, we showed that the 6MWTD of 200 m is the best cut-off point for predicting the adverse events. Finally, the prognostic impact of 6MWT for HF hospitalization was only observed in older HF patients. These findings may provide insights on the prognostic stratification of HFrEF, possibly leading to better HF management strategies.
Acknowledgements
We would like to acknowledge the following members of WARCEF committees and investigators: Arthur J. Labovitz, Douglas L. Mann, Ralph L. Sacco, Patrick M. Pullicino, Ronald S. Freudenberger, John R. Teerlink, Susan Graham, Gregory Y. H. Lip, Bruce Levin, J. P. Mohr, Conrado J. Estol, Dirk J. Lok, and Piotr Ponikowski.
Conflict of interest
S.H. reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim. S.D.A. reports receiving consulting fees from Vifor, Bayer, Janssen, Novartis, Relypsa, ZS Pharma, and Thermo Fisher; grant support from Vifor Pharma and Abbott Vascular; and lecture fees from Vifor, Novartis, and Stealth Peptides. The other authors report no conflicts.
Funding
The study was funded by U01-NS-043975 (S.H.) and U01-NS-039143 (J.L.P.T.) from the National Institute of Neurological Disorders and Stroke (NINDS).




