Myocarditis and coronary aneurysms in a child with acute respiratory syndrome coronavirus 2
Abstract
A 6-year-old African boy with multi-viral infection including parvovirus B19 and severe acute respiratory syndrome coronavirus 2 was admitted for persistent fever associated with respiratory distress and myocarditis complicated by cardiogenic shock needing ventilatory and inotropic support. Coronary aneurysms were also documented in the acute phase. Blood tests were suggestive of macrophage activation syndrome. He was treated with intravenous immunoglobulins, aspirin, diuretics, dexamethasone, hydroxychloroquine, and prophylactic low molecular weight heparin. Normalization of cardiac performance and coronary diameters was noticed within the first days. Cardiac magnetic resonance imaging, performed 20 days after the hospitalization, evidenced mild myocardial interstitial oedema with no focal necrosis, suggesting a mechanism of cardiac stunning related to cytokines storm rather than direct viral injury of cardiomyocytes.
Introduction
Coronavirus disease 2019 (COVID-19), also known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a major world health issue. Approximately 1% of COVID-19-affected patients are less than 18 years old.1, 2 Major concerns have recently been raised about severe manifestations in the paediatric age group.3, 4 A multisystem inflammatory syndrome (MIS), potentially needing intensive care support, has been described as well as a wide spectrum of cardiovascular anomalies, particularly a Kawasaki-like disease (KD).5
Case report
A 6-year-old African boy, with an uneventful clinical history, was hospitalized for persistent fever. A contact with a SARS-CoV-2-positive subject (mother) was referred. At admission, he was febrile (39.9°C) and eupneic with normal oxygen saturation and had no signs of mucocutaneous inflammation. The nasopharyngeal swab for SARS-CoV-2 was negative. Blood tests showed increased C-reactive protein and procalcitonin and hyponatraemia (Table 1). A wide-spectrum antibiotic therapy was started.
| 24 April, admission | 29 April | 2 May | 6 May | |
|---|---|---|---|---|
| WBC (× 109/L) | 9.18 | 4.21 | 7.23 | 23.7 |
| Neutrophils (%) | 76 | 69.6 | 50 | 46 |
| Lymphocytes (%) | 18 | 22.3 | 14.4 | 46.1 |
| Hb (g/dL) | 10.5 | 7.5 | 10.7 | 10.5 |
| PLT (× 109/L) | 183 | 101 | 353 | 982 |
| d-Dimer (mg/L FEU) | 3.75 | 2 | 1 | |
| CRP (mg/dL) | 14.8 | 23.5 | 9 | 0.8 |
| Procalcitonin (ng/mL) | 13 | 17.2 | 1 | 0.2 |
| Ferritin | 358 | 1515 | 357 | 321 |
| Fibrinogen (mg/dL) | 368 | 289 | ||
| Triglycerides (mg/dL) | 295 | 200 | 149 | |
| ALT (U/L) | 371 | 93 | 98 | |
| AST (U/L) | 492 | 78 | 64 | |
| Creatinine (mg/dL) | 0.26 | 0.32 | 0.31 | 0.26 |
| Blood urea nitrogen (mg/dL) | 40 | 38 | 34 | 39 |
| Na (mmol/L) | 129 | 132 | 136 | 140 |
| IL-6 (pg/mL) | 398 | 30.8 | <2 | |
| IL-10 (pg/mL) | 10.3 | |||
| IL-1 (pg/mL) | <2 | |||
| TNF-α (pg/mL) | <2 | |||
| High-sensitivity troponin (ng/L) | 98 | 65 | 16 | |
| BNP (pg/mL) | 1170 | 18 | ||
| CPK (U/L) | 552 | 537 | 189 | |
| Parvovirus B19 (couples/mL) | 14 298 | <600 |
- ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; CPK, creatine phosphokinase; CRP, C-reactive protein; FEU, fibrinogen equivalent units; Hb, haemoglobin; IL-1, interleukin 1; IL-6, interleukin 6; IL-10, interleukin 10; Na, sodium; PLT, platelets; TNF-α, tumour necrosis factor-α; WBC, white blood cells.
Five days after the onset of fever, he showed worsening of clinical conditions with shock, marked tachypnoea, desaturation, fever persistence, and hepatosplenomegaly. The laboratory tests (Table 1) were suggestive of macrophage activation syndrome (MAS)6 with pancytopenia, hypertriglyceridaemia, hyperferritinaemia, hypocalcaemia, and increased values of aspartate aminotransferase, alanine aminotransferase, interleukin 6, d-dimer, C-reactive protein, procalcitonin, and cardiac biomarkers (Table 1). The nasopharyngeal swab for SARS-CoV-2 was repeated and resulted positive. A coinfection with parvovirus B19 was documented. A thoracic high-resolution computed tomography showed bilateral consolidations and pleural effusion (Figure 1–1). The electrocardiogram showed low voltage in the limb leads and minimal ST-segment depression with T-wave inversion in the anterior leads.
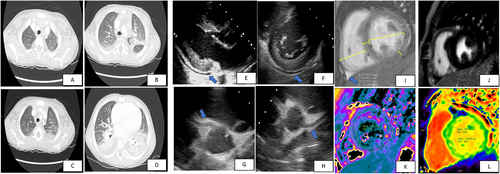
Echocardiography showed normal left ventricular dimensions, trivial increased septal thickness (interventricular septal thickness at end-diastole 8 mm, Z score +2.0; Figure 1), an impaired left ventricular systolic function (left ventricular ejection fraction 48%), and a mild circumferential pericardial effusion (10 mm: Figure 1 and 1). Multi-coronary aneurysms were detected: left main coronary artery was 3.9 mm, Z score +2; left anterior descending artery was 3.8 mm, Z score +3.9 (Figure 1); left circumflex coronary artery was 3.6 mm, Z score +3.0; and right coronary artery was 4.3/3.3 mm, respectively, Z score +3.8/+2.4, in the proximal/medial segment (Figure 1).
A non-invasive ventilatory support and inotropic therapy were started. Intravenous immunoglobulins (IVIGs, 2 g/kg), aspirin (5 mg/kg), diuretics, dexamethasone, hydroxychloroquine, and prophylactic low molecular weight heparin were added as target therapy. A rapid recovery was observed with a complete normalization of left ventricular function and weaning of ventilatory and inotropic support in less than 72 h. Seven days after IVIG inflammation marker and plasmatic interleukin levels normalized, DNA polymerase chain reaction parvovirus B19 couples significantly reduced (Table 1).
Ten days after onset, chest radiography, lung echography, abdominal echography, and echocardiography did not show any abnormalities. Twenty days after onset, a cardiac magnetic resonance imaging (CMRI) showed normal biventricular diameters, volumes, and systolic function (Figure 1). Phase-sensitive inversion recovery sequences showed absence of late gadolinium enhancement (Figure 1). T2-mapping sequences showed mild myocardial interstitial oedema (Figure 1). Moreover, T1 mapping showed increased values, which confirmed the presence of oedema (Figure 1).
The patient was discharged on aspirin therapy. The 1 month echocardiographic follow-up was normal.
Discussion
Herein, we describe the case of a 6-year-old boy admitted for persistent fever who rapidly progressed to respiratory distress, ventricular dysfunction, and shock. Coronary aneurysms were documented in the acute phase. He tested positive for multi-viral infection including SARS-CoV-2. His blood tests were suggestive of MAS.6
SARS-CoV-2-associated myocardial dysfunction has been described in adults and children3-5, 7-9: the combination of the direct viral insult to cardiomyocytes and myocardial dysfunction due to hyperinflammation with cytokine release is thought to be the mechanism leading to cardiac dysfunction.7, 9 SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2) as its entry receptor: in addition to the heart and lung, ACE2 is localized in the vascular endothelium.10 More than 7.5% of myocardial cells have positive ACE2 expression, which could mediate SARS-CoV-2 entry into cardiomyocytes and cause direct cardiotoxicity.11 Based on post-mortem biopsies, the pathological features of SARS-CoV-2 in the cardiac tissue vary from minimal change to interstitial inflammatory infiltration and myocyte necrosis. Moreover, microthrombosis and vascular inflammation were found.12 The increased values of troponin may be caused by the myocardial injury either viral or due to the cytokine-driven myocardial damage or by the presence of vasculitis.13 While elevated troponin levels in adults with COVID-19 are associated with a poorer outcome, in children, the prognostic value in COVID-19 setting has to be investigated; however, as in our case, a normalization of cardiac biomarkers is associated with a good prognosis, and serial testing could be useful in detecting patients at risk. 3-5 IVIGs are known to modulate immune and inflammatory response by a variety of mechanisms and are used in several systemic autoimmune diseases with no major side effects,14, 15 and they are the standard treatment for KD and also the standard local protocol for myocarditis.3, 5, 14-16 Therefore, the patient was treated using the IVIGs, and corticosteroids were added for MAS with subsequent clinical, laboratory, and instrumental improvement.
An important increase of KD incidence was noticed in the regions severely affected by the SARS-CoV-2 epidemic,5 mostly the patients with Kawasaki disease shock syndrome and MAS.6 Because both KD and MIS are systemic inflammatory syndromes, they share clinical, laboratory features and pathological pathways with consequences such as cardiac involvement, elevation of inflammatory markers, and coagulopathy in the absence of other microbial causes.
Notably, despite a rapidly evolving critical clinical presentation with high inflammatory markers, multisystem dysfunction, polyserositis with pleural and pericardial effusion, myocardial dysfunction, and coronary aneurysms, treatment with IVIG and steroid therapy were remarkable, as in most cases of KD. Moreover, aspirin was started given the presence of coronary lesions and according to previous studies that described endothelial dysfunction, microthrombosis, and vascular inflammation.7 To date, no recommendations for the duration of anti-aggregating therapy have yet been published, but it seems reasonable to follow the same suggested for KD.
Coinfections in children with COVID-19 are described: our patient had a coinfection with parvovirus B19.14 Positive viral serology does not imply myocardial infection but rather indicates the interaction of the peripheral immune system with an infectious agent, which we believe happened in our case; the presence of MIS was associated with transitory myocardial injury. In addition, the interaction between parvovirus B19 and SARS-CoV-2 might maintain myocardial (low-grade) inflammation and consequently the myocardial depression. Furthermore, the optimal response to treatment and the evidence of isolated septal oedema more than necrosis at the CMRI make cardiac stunning more conceivable.
A relatively recent review showed no difference in parvovirus B19 prevalence between myocarditis/dilated cardiomyopathy and healthy control hearts.17 However, findings suggest that parvovirus B19 can be aetiologically relevant either in the presence of other cardiotropic viruses or when parvovirus B19 load is high and/or actively replicating, which both may maintain myocardial (low-grade) inflammation. If it was true, combination of COVID/parvovirus B19 also could be a possible aetiological factor in developing myocardial depression.
In addition, in line with the previous series,3 cardiac, pulmonary, and laboratory manifestations of our patient completely regressed without sequelae.
Because hydroxychloroquine can inhibit replication of SARS-CoV-2 in vitro, its use has been promoted for treatment and prevention of COVID-19 despite conflictual data have been published about its efficacy18 and its side effects on QT prolongation.19, 20 At the time of patient's hospitalization, the hydroxychloroquine was recommended empirically by the local/national guidelines in patients with SARS-CoV-2 infection and pulmonary involvement and was administered under regular electrocardiogram and blood test monitoring without adverse reactions.
In conclusion, our case underlines the presence of MIS in paediatric patients with SARS-CoV-2 infection with a multi-organ involvement and a rapidly progressive clinical impairment. As previously described, shock might complicate the clinical presentation, and cardiac involvement is not exceptional. Our findings evidenced a reversible myocardial injury probably related with cytokine storm rather than direct viral damage considering the rapid recovery and the absence of myocardial necrosis at CMRI. Moreover, IVIG results were remarkable and safe in the acute stage. There are also other unanswered questions such as the long-term therapy, especially when coronaries are involved, and the timing of the follow-up.
Conflict of interest
None declared.




