Comparable outcomes between genders in patients undergoing surgical ventricular reconstruction for ischaemic heart failure
Abstract
Aims
Female sex and heart failure (HF) are considered poor prognostic factors for surgery. We aimed to investigate the association between sex and surgical outcomes in patients with ischaemic HF undergoing surgical ventricular reconstruction and coronary artery bypass grafting.
Methods and results
From July 2001 to June 2017, 648 patients [111 women (17%) and 537 men (83%)] were referred to our centre. Follow-up continued through June 2018. All patients underwent surgical ventricular reconstruction; coronary artery bypass grafting was performed in 582 patients (90%). Primary outcome was defined as all-cause mortality. Secondary outcome included all-cause mortality or all-cause hospitalization. Women were older (70 vs. 65 years, P < 0.0001) with lower body surface area (1.70 vs. 1.86 m2, P < 0.0001). Women had more diabetes (36% vs. 24%, P = 0.005) and a higher New York Heart Association classification (Class III/IV 65.7% vs. 47.8%, P = 0.0006), without any significant difference in medical therapy except for a higher use of oral antidiabetic agents in women (P = 0.029). At baseline, the left ventricular (LV) end-diastolic volume index was significantly lower in women [median 107.06 (80.6–127.81) vs. 113. 04 (94.33–135.52) mL/m2, P = 0.0078] but not the LV end-systolic volume index (ESVI) [median 73.45 (51.93–96.79) vs. 77.03 (60.33–95.71) mL/m2, P = 0.1393] and the ejection fraction (median 31% vs. 32%, P = 0.150). Women had a higher rate of anterior remodelling (90.9% vs. 79.1%, P = 0.0129), without evidence of differences in mitral valve insufficiency (P = 0.761 for Grade 0 to 4) and mitral surgery (P = 0.810). After surgery, the percentage of reduction in LV ESVI was higher in women than in men (median ΔLV ESVI −42.06 vs. −31.99, P = 0.0003). Mortality within 30 days occurred in 43 patients (6.64%): 12 women (10.81%) and 31 men (5.77%, P = 0.0522). Over a median follow-up of 9.8 years, all-cause mortality occurred in 269 patients (41.64%), without significant difference between women (45.9%) and men (40.7%). There was no evidence of difference of all-cause death between sexes (log-rank = 0.2441). When considering mortality and first hospitalization as competing events, Gray's test showed no difference of cumulative incidence functions (all-cause hospitalization, all-cause death, and combined endpoint) according to sex (P = 0.909, P = 0.445, and P = 0.429, respectively).
Conclusions
In this study, long-term outcomes for women and men with ischaemic HF undergoing complex cardiac surgery were equivalent. Albeit older and more symptomatic, women should not be denied this type of cardiac surgery.
Introduction
Ischaemic heart disease (IHD) is the leading cause of death in women. Heart failure (HF) of ischaemic aetiology, although less likely to occur in women, is considered a poor prognostic factor.1-3 Women have their first presentation of IHD approximately 10 years later than men. However, they have more co-morbidities that are likely to reduce survival after the first myocardial infarction or to increase the risk of HF development.4-7 This leads to a higher risk profile, which may preclude women from getting aggressive therapeutic strategies, including surgical revascularization for IHD or heart transplantation for advanced HF.8, 9 However, the influence of patient sex on outcomes following cardiac surgery is proving controversial, and the current guidelines are lacking in providing specific indications. A series of trials and registries conducted in the last century included a small number of women, mostly with preserved ejection fraction (EF) and under suboptimal medical therapy.10-12 In this regard, the most robust and recent contributions stem from a post hoc analysis of the STICH (Surgical Treatment for Ischaemic Heart Failure) Hypothesis 1 population, showing that women with ischaemic HF and low EF who underwent surgical revascularization had lower all-cause mortality and cardiovascular mortality compared with men.13 The STICH Hypothesis 2 study was designed to specifically investigate the role of coronary artery bypass grafting (CABG) combined or not with surgical ventricular reconstruction (SVR) in patients with left ventricular (LV) systolic dysfunction.14 The trial failed to show an additional survival benefit in the SVR group, although the combined procedure resulted in greater reverse remodelling.15 At the same time, the trial did not demonstrate any detrimental effects from the procedure, making the use of SVR in select patients still justifiable.16 However, a sex-related analysis on the STICH Hypothesis 2 is lacking.
We aimed to investigate the association between sex and surgical outcomes in patients with ischaemic LV dysfunction and HF undergoing SVR and CABG with or without mitral valve surgery in a large, single-centre population. Primary outcome was defined as all-cause mortality. Secondary outcome included all-cause mortality or all-cause hospitalization.
Methods
Study design
This was a retrospective study at IRCCS Policlinico San Donato for patients with ischaemic HF referred for cardiac surgery. The study protocol was approved by the local ethics committee, according to Italian regulatory law for observational retrospective studies. All the patients admitted to the study gave their informed consent for the anonymous use of their clinical data for research purposes.
Study population
From July 2001 to June 2017, 648 patients [111 women (17%) and 537 men (83%)] with previous myocardial infarction and LV remodelling were referred to our centre for cardiac surgery. Follow-up continued through June 2018. All patients underwent SVR. CABG was performed in 582 patients (89.8%) and mitral valve surgery in 200 patients (30.1%). Indications for surgery were HF, angina, and/or a combination of the two.
Echocardiography
A complete echocardiographic assessment (GE Vivid 7, Healthcare, Waukesha, WI) was performed before and after surgery at a median time from surgery of 12 months. All four-chamber, two-chamber, long-axis, and short-axis views were analysed. The following parameters were collected: LV diastolic diameter (mm), LV systolic diameter (mm), end-diastolic volume (EDVI) index (mL/m2), end-systolic volume index (ESVI) (mL/m2), EF (%), stroke volume index (mL/m2), LV mass index (g/m2), tricuspid annular plane systolic excursion (mm), systolic pulmonary artery pressure (mmHg), left atrial diameter (mm), E wave velocity (cm/s), E/A ratio, deceleration time (mm), mitral annulus dimension (mm), inter-papillary distance (mm) at the end of diastole and the end of systole, diastolic and systolic sphericity index (calculated as the short-axis to long-axis ratio in apical four-chamber view), and systolic and diastolic conicity index (calculated as the ratio between the apical and the short axis to assess the shape of the apex).17 All measurements were done in triplicate and displayed as an average value.
Follow-up
Follow-up was conducted either at the hospital during a routine clinical evaluation or by telephone contact with the patients, their relatives, or family doctors and was 100% complete. If the patient was not seen in the hospital or telephone interview was not possible, we contacted the National Registry of Death.
Surgical technique
The surgical technique has been previously reported in detail.18 Briefly, the procedure was performed under total cardiac arrest with antegrade crystalloid cardioplegia. After completion of coronary grafting when indicated, the left ventricle was opened with an incision parallel to the left anterior descending artery, starting at the middle scarred region and ending at the apex. SVR was performed using a mannequin (TRISVR, Chase Medical Richardson, TX) filled at 50–60 mL/m2 to optimize the size and shape of the new ventricle. When needed, the mitral valve was repaired through the ventricular opening.
Statistical analysis
Categorical variables are presented as proportions and continuous variables as mean (standard deviation) and median [interquartile range], as appropriate. Changes over time between preoperative and post-operative echocardiographic characteristics were compared with McNemar paired test. The differences in baseline demographic and clinical characteristics between the sexes were compared with the χ2 test; continuous variables were compared by the non-parametric Kruskal–Wallis test for non-normally distributed data.
Overall mortality (including operative mortality defined as death occurring within 30 days after surgery) was analysed using the Kaplan–Meier method and compared between the sexes using the log-rank test.
The cumulative incidence function (CIF) was used to assess the probability of hospitalization and death from all causes during the follow-up period.
Gray's test was used to evaluate the hypotheses of no difference of crude CIFs between the sexes. Median follow-up time was calculated according to the reverse Kaplan–Meier method.
A propensity score based on unbalance baseline characteristics listed in Table 1 was created using a multivariable logistic regression model. The propensity score considered only variables associated with both sexes and surgical outcomes (age, creatinine, diabetes, and New York Heart Association classification). The score was used for appropriate fixed ratio 1:1 matching without replacement using 0.25 calliper width of the logit of the standard deviation.
| n = 648 | F (111) | M (537) | P-value | |
|---|---|---|---|---|
| Demographic | ||||
| Age, years | 66 [58–72] | 70 [64–75] | 65 [57–71] | <0.0001 |
| BSA, m2 | 1.83 [1.74–1.92] | 1.70 [1.60–1.78] | 1.86 [1.77–1.96] | <0.0001 |
| Risk score | ||||
| ACEF score | 2.06 [1.70–2.59] | 2.25 [1.77–2.89] | 2.03 [1.67–2.52] | 0.0020 |
| Laboratory | ||||
| Creatinine | 1.10 [0.91–1.39] | 1 [0.81–1.34] | 1.11 [0.94–1.40] | 0.0043 |
| Medical history, n (%) | ||||
| Family history of CAD | 271 (41.82) | 41 (36.94) | 230 (42.83) | 0.2518 |
| Smoker or ex-smoker | 439 (67.75) | 42 (37.84) | 397 (73.93) | <0.0001 |
| Hypertension | 381 (58.80) | 69 (62.16) | 312 (58.10) | 0.4287 |
| Atrial fibrillation | 91 (14.04) | 15 (13.51) | 76 (14.15) | 0.8955 |
| Stroke | 55 (8.49) | 13 (11.71) | 42 (7.82) | 0.1806 |
| Angina | 230 (35.49) | 38 (34.23) | 192 (35.75) | 0.7606 |
| Ventricular arrhythmias | 106 (16.36) | 19 (17.12) | 87 (16.20) | 0.8123 |
| Chronic renal failure | 47 (7.25) | 8 (7.21) | 39 (7.26) | 0.9837 |
| Diabetes mellitus | 166 (25.66) | 40 (36.04) | 126 (23.51) | 0.0059 |
| Hypercholesterolaemia | 381 (58.80) | 64 (57.66) | 317 (59.03) | 0.7889 |
| NYHA Class III/IV | 330 (50.93) | 73 (65.77) | 257 (47.86) | 0.0006 |
| No previous procedures | 358 (55.25) | 57 (51.35) | 301 (56.05) | 0.7974 |
| PCI | 183 (28.24) | 34 (30.63) | 149 (27.75) | |
| PCI + ICD | 37 (5.21) | 7 (6.31) | 30 (5.59) | |
| ICD | 40 (6.17) | 6 (5.41) | 34 (6.33) | |
| Other | 30 (4.63) | 7 (6.31) | 23 (4.28) | |
| Medication at baseline, n (%) | ||||
| ACE inhibitor | 532 (82.61) | 94 (84.68) | 438 (82.18) | 0.5259 |
| Beta-blockers | 482 (74.84) | 78 (70.27) | 404 (75.80) | 0.2221 |
| Aspirin | 525 (81.52) | 90 (81.08) | 435 (81.61) | 0.8954 |
| Digitalis | 46 (7.14) | 8 (7.21) | 38 (7.13) | 0.9769 |
| Statins | 434 (67.29) | 72 (64.86) | 362 (67.79) | 0.5500 |
| Diuretic | 537 (83.26) | 93 (83.78) | 444 (83.15) | 0.8699 |
| Warfarin Ω | 73 (11.34) | 12 (10.81) | 61 (11.44) | 0.8480 |
| Amiodarone | 146 (22.67) | 27 (24.32) | 119 (22.33) | 0.6474 |
| Nitrates | 205 (31.83) | 41 (36.94) | 164 (30.77) | 0.2044 |
| Insulin | 56 (8.6) | 13 (11.7) | 43 (8.6) | 0.2151 |
| Oral antidiabetic agent | 106 (16.35) | 26 (23.42) | 80 (14.89) | 0.0296 |
- ACE, angiotensin-converting enzyme; ACEF, age, creatinine, and ejection fraction; BSA, body surface area; CAD, coronary artery disease; F, female; ICD, implantable cardioverter defibrillator; M, male; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
- Data are mean (standard deviation)/median [Q1–Q3] or number (%).
All P-values are two-tailed and considered significant if <0.05. Statistical analyses were done with SAS software, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
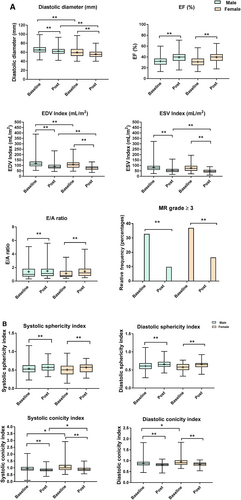
In this cohort of patients, women were generally older [70 years old (64–75 years old) vs. 65 years old (57–71 years old), P < 0.0001] with lower body surface area [1.70 m2 (1.60–1.78 m2) vs. 1.86 m2 (1.77–1.96 m2), P < 0.0001]. Women had more diabetes [40 (36%) vs. 126 (23.5%), P = 0.006], were less likely to be smokers [42 (37.8%) vs. 397 (73.9%), P < 0.0001], and had a higher New York Heart Association classification [Class III/IV, 73 (65.8%) vs. 257 (47.9%), P = 0.0006], without any significant difference in medical therapy except for oral antidiabetic agents [26 (23.42%) vs. 80 (14.89%), P = 0.0296] (Table 1). Baseline echocardiographic, angiographic, and operative variables of the total study cohort overall and by gender are listed in Table 2. The diastolic diameter [59 (52–67) vs. 65 (60–71) mm, P < 0.0001], the systolic diameter [47 (38–54) vs. 52 (45–59) mm, P < 0.0001], and the LV EDVI index [107.06 (80.6–127.81) vs. 113.04 (94.33–135.52) mL/m2, P = 0.0078] were statistically significantly lower in women, while the LV ESVI [73.45 (51.93–96.79) vs. 77.03 (60.33–95.71) mL/m2, P = 0.1393] and the EF [31% (25–37) vs. 32% (26–37), P = 0.150] were not statistically significantly different. LV mass index was lower in women [152.51 (127.75–178.18) vs. 169.71 (139.89–201.79) g/m2, P = 0.0002], while the relative wall thickness was higher [0.34 (0.28–0.40) vs. 0.31 (0.27–0.38), P = 0.04]. Indeed, in respect to the shape of the LV chamber, the sphericity index either in diastole or in systole was not different between the sexes [SId: 0.58 (0.50–0.66) vs. 0.61 (0.53–0.69), P = 0.060, in women and men, respectively; SIs: 0.51 (0.39–0.61) vs. 0.53 (0.44–0.63), P = 0.204, in women and men, respectively], but women showed a higher conicity index [CId 0.91 (0.82–1.02) vs. 0.87 (0.79–0.95), P = 0.005; CIs 1.00 (0.86–1.21) vs. 0.92 (0.82–1.06), P = 0.006] demonstrating a remodelling that mainly affected the apex. Furthermore, women had a higher rate of anterior remodelling [101 (90.9%) vs. 425 (79.1%)], while the rate of posterior remodelling and anterior/posterior remodelling was lower [7 (63.1%) vs. 89 (16.57%) and 3 (2.70%) vs. 23 (4.28%), respectively, all P = 0.0129], although without differences in the degree of mitral valve insufficiency (P = 0.761 for Grade 0 to 4) and mitral surgery [34 (43.04%) vs. 173 (41.59%), P = 0.810, in women and men, respectively]. The rate of single vessel disease was higher in women [35 (31.53%) vs. 124 (23.09%)] unlike the rate of multi-vessel disease [67 (60.36%) vs. 369 (68.72%)], although not statistically different (P = 0.163 for all groups).
| N | Pre | n | F (n = 111) | n | M (n = 537) | P-value | |
|---|---|---|---|---|---|---|---|
| Diastolic diameter (mm) | 630 | 65 [58–71] | 107 | 59 [52–67] | 523 | 65 [60–71] | <0.0001 |
| Systolic diameter (mm) | 625 | 51 [44–59] | 107 | 47 [38–54] | 518 | 52 [45–59] | <0.0001 |
| EDVI index (mL/m2) | 645 | 112.12 [92.34–134.30] | 110 | 107.06 [80.6–127.81] | 535 | 113.04 [94.33–135.52] | 0.0078 |
| ESV index (mL/m2) | 646 | 76.08 [59.17–95.81] | 110 | 73.45 [51.93–96.79] | 536 | 77.03 [60.33–95.71] | 0.1393 |
| EF (%) | 648 | 32 [26–37] | 111 | 31 [25–37] | 537 | 32 [26–37] | 0.1509 |
| SV index (mL/m2) | 644 | 35.20 [29.38–41.69] | 110 | 31.11 [26.09–36.72] | 534 | 36.13 [30.39–42.49] | <0.0001 |
| TAPSE (mm) | 609 | 20 [18–23] | 101 | 20 [17–22] | 508 | 20 [18–23] | 0.0413 |
| PAPs (mmHg) | 556 | 38 [30.5–48] | 98 | 38 [30–50] | 458 | 38 [31–47] | 0.7919 |
| LVMI (g/m2) | 561 | 164.29 [137.88–199.48] | 94 | 152.52 [127.75–178.18] | 467 | 169.71 [139.89–201.79] | 0.0002 |
| RWT | 597 | 0.32 [0.27–0.38] | 103 | 0.34 [0.28–0.40] | 494 | 0.31 [0.27–0.38] | 0.0402 |
| Left atrial diameter (mm) | 594 | 46 [41–51] | 99 | 44 [40–49] | 495 | 47 [42–51] | 0.0018 |
| E/A ratio | 458 | 0.96 [0.66–1.71] | 76 | 0.81 [0.63–1.48] | 382 | 1 [0.69–1.80] | 0.0653 |
| DT (mm) | 435 | 185 [149–239] | 75 | 181 [155–234] | 360 | 186 [146–240] | 0.9096 |
| Mitral annulus (mm) | 367 | 34 [30–37] | 64 | 32 [29–36.50] | 303 | 34 [30–38] | 0.1502 |
| Sphericity index, diastole | 395 | 0.60 [0.50–0.68] | 66 | 0.58 [0.50–0.66] | 329 | 0.61 [0.53–0.69] | 0.0606 |
| Sphericity index, systole | 394 | 0.53 [0.43–0.62] | 66 | 0.51 [0.39–0.61] | 328 | 0.53 [0.44–0.63] | 0.2047 |
| Conicity index, diastole | 385 | 0.88 [0.80–0.96] | 65 | 0.91 [0.82–1.02] | 320 | 0.87 [0.79–0.95] | 0.0055 |
| Conicity index, systole | 385 | 0.94 [0.83–1.09] | 65 | 1.00 [0.86–1.21] | 320 | 0.92 [0.82–1.06] | 0.0060 |
| MR grade | 0.7612 | ||||||
| 0 | 80 (12.35) | 15 (13.51) | 65 (12.10) | ||||
| 1 | 209 (32.25) | 35 (31.53) | 174 (32.40) | ||||
| 2 | 142 (21.91) | 20 (18.02) | 122 (22.72) | ||||
| 3 | 119 (18.36) | 21 (18.92) | 98 (18.25) | ||||
| 4 | 98 (15.12) | 20 (18.02) | 78 (14.53) | ||||
| Site of remodelling | 0.0129 | ||||||
| Posterior | 96 (14.81) | 7 (6.31) | 89 (16.57) | ||||
| Anterior | 526 (81.17) | 101 (90.99) | 425 (79.14) | ||||
| Anterior and posterior | 26 (4.01) | 3 (2.70) | 23 (4.28) | ||||
| Coronary angiography | 0.1632 | ||||||
| Single vessel disease | 159 (24.54) | 35 (31.53) | 124 (23.09) | ||||
| Multi-vessel disease | 436 (67.28) | 67 (60.36) | 369 (68.72) | ||||
| No significant stenosis | 53 (8.18) | 9 (8.11) | 44 (8.19) | ||||
| Mitral valve surgery | 207 (41.82) | 34 (43.04) | 173 (41.59) | 0.8105 | |||
| SVR + CABG | 582 (89.81) | 96 (86.49) | 486 (90.50) | 0.2028 | |||
| SVR | 66 (10.19) | 15 (13.51) | 51 (9.50) | ||||
| Number of distal anastomosis | 0.0276 | ||||||
| 0 | 66 (10.19) | 15 (13.51) | 51 (9.50) | ||||
| 1 | 144 (22.22) | 33 (29.73) | 111 (20.67) | ||||
| ≥2 | 438 (67.59) | 63 (56.76) | 375 (69.83) |
- CABG, coronary artery bypass grafting; DT, deceleration time; EDVI, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; F, female; LVMI, left ventricular mass index; M, male; MR, mitral regurgitation; PAPs, systolic pulmonary artery pressure; RWT, relative wall thickness; SV, stroke volume; SVR, surgical ventricular reconstruction; TAPSE, tricuspid annular plane systolic excursion.
- Data are mean (standard deviation)/median [Q1–Q3] or number (%).
Figure 1 shows changes after surgery in echocardiographic parameters in women and men in respect to the baseline values. The LV internal diameters, as well as the EDVI index and ESVI, decreased, while the EF increased in both sexes, all statistically significant. However, considering the percentage of reduction in the LV ESVI (Δpercentage = {[ESVI at follow-up ESVI at baseline]/ESVI at baseline}*100) as a measure of reverse remodelling, the reduction was significantly greater in women compared with men [median ΔESVI −42.06 (−62.13 to 26.39) vs. −31.99 (−45.72 to 17.60), P = 0.0003].
Overall, 43 patients (6.6%) suffered operative mortality, defined as mortality within 30 days from surgery [12 (10.81%) women vs. 31 (5.77%) men, P = 0.0522]. Hospital length of stay was not significantly different between women and men [14 (10–20) vs. 12 (9–17), P = 0.0571]. The age, creatinine, and ejection fraction (ACEF) score [age (years)/left ventricular ejection fraction (%) + 1 (if baseline serum creatinine was >2 mg/dL)]19 for operative mortality was statistically different between the sexes [2.25 (1.77–2.89) vs. 2.03 (1.67–2.52) in women and men, respectively, P = 0.0020].
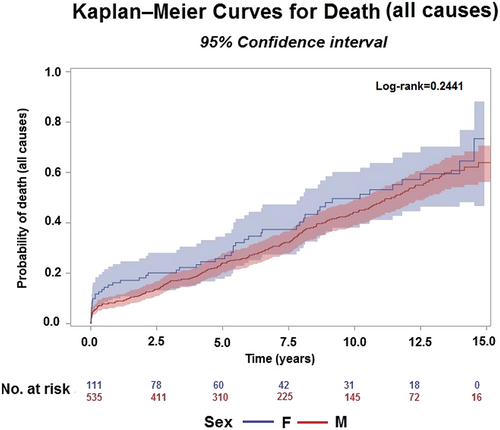
Over a median follow-up of 9.8 years (range: 0 days to 16 years), all-cause death occurred in 269 (41.64%) patients: 51 (45.95%) women and 218 (40.75%) men. The median follow-up was 10.3 years (range: 0 days to 15 years) and 9.9 years (range: 0 days to 16 years), respectively.
The Kaplan–Meier estimated mortality for the entire population was 13% (10.5–15.8%) at 2 years, 19.2% (16.2–22.5%) at 4 years, and 36.6% (32.4–40.9%) at 8 years.
The estimated mortality was 19.1% (12.4–27.0%) in women and 12.2% (9.5–15.11%) in men at 2 years, 23.4% (19.8–37.4%) in women and 18.8% (15.5–22.4%) in men at 4 years, and 41.8% (31.2–52.1%) in women and 36.2% (31.6–40.9%) in men at 8 years.
The probability of all-cause death was not different between the two genders (log-rank = 0.2441) (Figure 2). In addition, hospitalizations (247, 41.6% overall) were not statistically different between the genders (40.40% vs. 41.9%, P = 0.7825).
Within 8 years after surgery, the crude cumulative incidence of all-cause hospitalization as the first event was 37.8% (27.6–47.7%) in women and 35.8% (31.4–40.2%) in men (Figure 3), whereas the crude cumulative incidence of death as the first event was 28.1% (19.2–37.7%) in women and 21.9% (18.1–25.9%) in men (Figure 3).
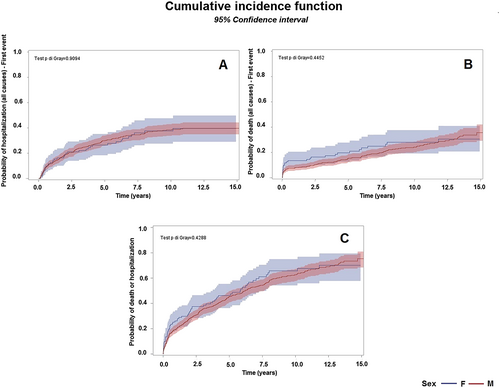
Considering mortality or first hospitalization as competing events, the estimated probability of experiencing one of the adverse events is 43.9% (34.2–53.2%) during the first 4 years in women and 39.4% (35.1–43.7%) in men and 65.7% (54.2–75.02%) within 8 years after surgery in women and 57.4% (52.5–61.9%) in men (Figure 3).
Gray's test showed no difference of CIFs (all-cause hospitalization, all-cause death, and combined endpoint) and sex (P = 0.909, P = 0.445, and P = 0.429, respectively).
Upon application of the matching algorithm, 111 women were matched to 111 men, yielding a total matched sample size of 222 patients. Similar to the results produced using crude proportional Cox hazard model for overall death [1.20 (0.88–1.63), female vs. male], the results for propensity score matching indicated that there are no differences in mortality between the two genders [1.03 (0.70–1.54)]. This was confirmed also for death or first hospitalization [crude hazard ratio 1.13 (0.86–1.49) vs. matching hazard ratio 1.09 (0.76–1.56)] (see Data S1).
Discussion
Comprising 111 women out of 648 patients with ischaemic LV remodelling, systolic LV dysfunction, and HF, all undergoing complex cardiac surgery, this is the largest single-centre study demonstrating the equivalent outcome between the sexes at long-term follow-up. Women had a significantly higher risk of operative death, but among surviving patients, there was no difference in terms of all-cause death or all-cause hospitalization.
The type of surgery (SVR mostly combined with CABG and/or mitral valve surgery when indicated), the time interval of recruitment, and the baseline clinical and echocardiographic characteristics of the population do not allow direct systematic comparison with previous surgical studies. However, considering the low inclusion of women in clinical trials and registries, our data set included a reasonable percentage of female participants [17% vs. 5% in the CASS trial, 15.7% in the CABG Patch trial, 27% (only 14% with HF) in the BARI registry, and 12% and 14% in the STICH Hypothesis 1 and Hypothesis 2, respectively].11, 12, 15, 20, 21 Regrettably, in the RESTORE registry, the most extensive study of SVR outcomes, including 1198 HF patients from 12 centres on four continents between the years 1998 and 2003, the number or percentage of female patients is missing.22 Conversely, Hernandez et al., looking at 731 patients undergoing SVR at US hospitals participating in the Society of Thoracic Surgeons National Cardiac Database from January 2002 to June 2004, reported the highest percentage of women (27%), with the female sex being a major predictor of operative mortality and morbidity, in agreement with the majority of studies.23, 24 Not surprisingly, in our analysis, we observed a higher operative mortality in women compared with men, as predicted from the ACEF score. In fact, despite no difference in terms of EF and renal function (creatinine value < 2) in both sexes, women were significantly older (either compared with men or with other surgical series) and were more symptomatic for HF.25
Notably, among surviving patients, there was no significant difference between the sexes with regard to long-term mortality. Although women had a higher event rate than men for all-cause mortality (45.95% vs. 40.75%) at a median follow-up of 9.8 years, the probability of death was not statistically different between the sexes. The same trend was observed for all-cause hospitalization as the first event, although the event rate was more similar for both women and men with more paired curves (Figure 3). A different trend for fatal events has been recently reported by Piña et al. in a paper focused on the long-term outcome of STICH Hypothesis 1 patients.13 The authors showed that when receiving CABG, women had a lower all-cause mortality rate (43.8% vs. 60.9%) over a median follow-up of 9.8 years. The difference in the event rate between the genders is likely related to the different type of surgery (CABG alone in the STICH or SVR plus CABG in the present study); the opposite trend between the sexes is likely explained by the older age of our female population (median 70 vs. 63.4), which showed a persistent, even if not statistically significant, poor outcome over time.
The reasons, besides age, for our results are more complex. Considering that the 4 year all-cause mortality for the entire population was lower in the present study (19.2%) than in STICH Hypothesis 2 for CABG with SVR (28%), it should be remarked that the percentage of reduction in LV ESVI was greater in this study (37%) than in the STICH (19%).15 Furthermore, in our data set, the reduction was significantly greater in women (median ΔESVI −42.06 vs. −31.99), despite similar ESVI and EF at baseline. The matter of (RR) and related sex differences has been explored in medical trials and registries that have reported a higher incidence of RR among women, regardless of the cause and severity of LV dysfunction.26-29 Indeed, experimental and clinical studies have found myocardial remodelling in response to different types of cardiac stress or injury to be more favourable in women.30 In agreement with these observations, we found that women, at baseline, exhibited a different shape of the LV chamber, in that the left ventricle was spherical, but in women, it was more conical. The higher conicity is related to a remodelling process mainly involving the apex.17 In this case, the surgical procedure, aimed to exclude the scar tissue and to rebuild the apex, allows a more effective volume reduction.31 These interesting findings regarding the phenotype of remodelling in women could represent a benchmark for novel, less invasive therapeutic strategies for ischaemic HF.32 Whether the more extensive reverse remodelling confers a survival benefit in women, partially counterbalanced by an older age, remains to be established.
Limitations
Several limitations because of the descriptive nature of this observational study must be taken into account in interpreting the results. We are a tertiary referral centre for cardiovascular care, so we treated patients for whom diagnosis and therapy for HF had been already established elsewhere. Furthermore, the observation time is extremely long, and changes in the patient profiles, medical therapy, or surgical treatment might preclude robust conclusions. The diagnostic tools are changed as well. In the last decade, the use of cardiac magnetic resonance for the assessment of myocardial scar tissue has become almost mandatory, when not contraindicated, and it might have contributed to the optimization of the patient selection.33 However, in the present study, we used only echocardiographic parameters because of more completeness of the data, including geometric variables, which indeed were useful in characterizing the LV remodelling between the sexes.
Hospitalizations were not classified on the basis of the cause. However, considering that not all patients were seen in the clinic for the follow-up and in the absence of some written medical reports, we preferred to consider all-cause hospitalizations for the analysis.
Nevertheless, to the best of our knowledge, this study provides the largest cohort of women affected by HF of ischaemic aetiology undergoing cardiac surgical procedures and observed over a long time interval.
Conclusions
In this large, single-centre study on a ‘real-world’ population of patients with ischaemic HF undergoing complex cardiac surgery, the long-term outcome for women and men was equivalent. Considering that women are less likely to receive non-pharmacological therapies, including heart transplantation, we believe that despite the initial high risk, women should not be denied cardiac surgery, including SVR.
Acknowledgements
We would like to thank Dr Federica Cervini for her valuable contribution in database management and Dr Michelle Monasky for the English language editing.
Conflict of interest
All authors declare no competing interests.
Funding
This study was partially supported by the Ricerca Corrente funding from the Italian Ministry of Health to IRCCS Policlinico San Donato.




