Comprehensive plasma metabolites profiling reveals phosphatidylcholine species as potential predictors for cardiac resynchronization therapy response
Shengwen Yang, Yiran Hu, and Junhan Zhao, These authors contributed equally to this work.
Abstract
Aims
This study aimed to identify the plasma metabolite fingerprint in patients with heart failure and to develop a prediction tool based on differential metabolites for predicting the response to cardiac resynchronization therapy (CRT).
Methods and results
We prospectively recruited 32 healthy individuals and 42 consecutive patients with HF who underwent CRT between January 2018 and January 2019. Peripheral venous blood samples, clinical data, and echocardiographic signatures were collected before CRT implantation. Liquid chromatography-mass spectrometry was used to perform untargeted metabolites profiling for peripheral plasma under ESI+ and ESI− modes. After 6 month follow-up, patients were categorized as CRT responders or non-responders based on the alterations of echocardiographic characteristics. Compared with healthy individuals, patients with HF had distinct metabolomic profiles under both ESI+ and ESI− modes, featuring increased free fatty acids, carnitine, β-hydroxybutyrate, and dysregulated lipids with heterogeneous alterations such as phosphatidylcholines (PCs) and sphingomyelins. Disparities of baseline metabolomics profile were observed between CRT responders and non-responders under ESI+ mode but not under ESI− mode. Further metabolites analysis revealed that a group of 20 PCs metabolites under ESI+ mode were major contributors to the distinct profiles between the two groups. We utilized LASSO regression model and identified a panel of four PCs metabolites [including PC (20:0/18:4), PC (20:4/20:0), PC 40:4, and PC (20:4/18:0)] as major predictors for CRT response prediction. Among our whole population (n = 42), receive operating characteristics analysis revealed that the four PCs-based model could nicely discriminate the CRT responders from non-responders (area under the curve = 0.906) with a sensitivity of 83.3% and a specificity of 90.0%. Cross-validation analysis also showed a satisfactory and robust performance of the model with the area under the curve of 0.910 in the training dataset and 0.880 in the testing dataset.
Conclusions
Patients with HF held significantly altered plasma metabolomics profile compared with the healthy individuals. Within the HF group, the non-responders had a distinct plasma metabolomics profile in contrast to the responders to CRT, which was characterized by increased PCs species. A novel predictive model incorporating four PCs metabolites performed well in identifying CRT non-responders. These four PCs might severe as potential biomarkers for predicting CRT response. Further validations are needed in multi-centre studies with larger external cohorts.
Introduction
Heart failure (HF), a leading cause of substantial morbidity and mortality, affects about 2% of the adult population worldwide.1 As a complex clinical syndrome, HF is associated with ventricular desynchrony and energy system abnormalities.2-4 Cardiac resynchronization therapy (CRT) is an efficient treatment for patients with drug-refractory HF, which enables an improvement in both heart function and quality of life and prolongs survival time.5-9 Although some patients meet with the latest criteria for implantation, in fact, up to 30% of them may not benefit from CRT.10, 11 It remains a challenge for clinicians to identify high-risk patients with poor outcomes before device implantation.
Metabolomics is defined as the comprehensive analysis of the metabolome of the biological system under defined conditions, reflecting the biochemical reaction.12, 13 As the systematic analysis of small-molecule metabolites, it provides the fingerprint of phenotypic alterations in pathophysiological states. Through the high-throughput technique by liquid chromatography-mass spectrometry (LC-MS/MS), it is possible to identify biomarkers in response to particular pathophysiological changes and explore their correlation to clinical phenotypes of diseases. Recent studies have demonstrated that metabolic alterations in patients with HF can be used as predictors for risk stratification.14, 15 Yet scarce information is available regarding the relationship between metabolites and response of CRT in patients with HF.
The purposes of this prospective study were (1) to assess the specific metabolic profiles of patients with HF compared with healthy individuals; (2) to investigate whether metabolic profiles show differences between CRT responders and non-responders; (3) to develop an applicable predictor with predictive metabolites for evaluating CRT response in patients with HF.
Methods
Study population
We prospectively recruited 42 consecutive patients with HF who had undergone CRT device implantation with defibrillator or not between January 2018 and January 2019 in Fuwai Hospital, Beijing, China. Before device implantation, all patients had received optimal medical therapy for at least 6 months. Inclusion criteria were New York Heart Association II-IV functional class with left ventricular ejection fraction (LVEF) 35% or less and QRS duration more than 120 ms, which were in accordance with guidelines for cardiac resynchronization and defibrillation.16 Exclusion criteria were clinical and haemodynamic instability, tumour with a life expectancy of <1 year, end-stage renal disease, and absence of blood samples. Thirty-two (age 42.0 ± 15.2 and 19 men) healthy participants were recruited as a control group. No documented cardiovascular diseases or history of cardiovascular medication were recorded in the control group.
The Institutional Review Board of our institution approved the protocol, which was in compliance with the Declaration of Helsinki. All participants fully understood the details of the study and provided written informed consent.
Study protocol
All patients underwent clinical and physical examinations. Clinical characteristics were obtained from the electronic health records. All devices were implanted through the cephalic or subclavian left vein into the right atrium, the apex of the right ventricular, and the coronary sinus to pace the left ventricle and into the coronary sinus to pace the left ventricle lateral wall. Transthoracic echocardiography was performed according to the American Society of Echocardiography guidelines,17 and left ventricular end-diastolic diameter and LVEF were measured by using biplane Simpson's method. Echocardiographic signatures were measured by two-dimensional echocardiography (Vivid 7 Dimension/Pro System, GE Healthcare, USA). According to the findings of clinical and echocardiographic alterations after 6 month follow-up, patients were categorized into two groups. Responders were defined as patients with an increase of LVEF more than 5% after CRT implantation. Non-responders were defined as patients with unchanged LVEF or an increase of <5% or who has been hospitalized or had undergone cardiac transplantation or had died due to worsening HF after CRT implantation.18, 19
Blood samples collection and sample preparations for metabolomics
Peripheral venous blood samples were drawn from the cubital vein in the fasting state in the morning. For patients, blood samples were collected 1 day before the CRT implantation. Blood samples were stored in ethylene diamine tetraacetie acid vacutainer tubes (BD Vacutainer, Franklin Lakes, NJ, USA) and then centrifuged at 3000 certrifugal force in g for 15 min at 4°C. Plasma was immediately separated and stored at −80°C until use.
The sample preparation for untargeted metabolomics was according to the manufacturer's instructions. To precipitate the protein, 400 μL of cold methanol was added to 100 μL of the plasma sample, vortexed for 60 s, and then were centrifuged at 14 000 g for 15 min. The supernatant was prepared for LC-MS/MS. Quality control (QC) samples were prepared by mixing equal volumes of each plasma sample. The quality control sample was periodically injected with batches of 10 test samples throughout the analytical run.
Metabolomics data collection and analysis
A Dionex Ultimate 3000 RS UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) was equipped with ACQUITY UPLC BEH C8 column (1.7 μm, 2.1 × 100 mm, Waters Corp, Milford, USA) in positive mode and ACQUITY UPLC BEH C8 column (1.7 μm, 2.1 × 100 mm, Waters Corp, Milford, USA) and UPLC HSS T3 column (2.1 mm × 100 mm, 1.8 μm, Waters Corp, Milford, USA) in negative mode. In positive mode analysis, the binary gradient elution system consisted of (A) water (containing 0.1% formic acid, v/v) and (B) acetonitrile (containing 0.1% formic acid, v/v), and separation was achieved using the following gradient: 5–100% B over 0–24 min, the composition was held at 100% B at 24.1–27.5 min, then 27.5–27.6 min, 100% to 5% B, and 27.6–30 min holding at 5% B. In negative mode analysis, the binary gradient elution system consisted of (A) water (containing 6.5 mM ammonium acetate) and (B) 95% methanol (containing 6.5 mM ammonium acetate), and separation was achieved using the following gradient: 5–100% B over 1–18 min, the composition was held at 100% B at 18.1–22 min, then 22–22.1 min, 100% to 5% B, and 22.1–25 min holding at 5% B. The flow rate was 0.35 mL/min, and the column temperature was 50°C. All the samples were kept at 4°C during the analysis. The injection volume was 5 μL.
Mass spectrometry analysis was performed on a Q-Exactive quadrupole-Orbitrap mass spectrometer equipped with heated electrospray ionization (ESI) source (Thermo Fisher Scientific, Waltham, MA, USA) in both positive and negative ion modes. The mass range was from m/z 70 to 1000. The resolution was set at 70 000 for the full MS scans and 17 500 for HCD MS/MS scans. The collision energy was set at 10, 20, and 40 eV. The mass spectrometer operated as follows: spray voltage, 3800 V (positive) and 3000 V (negative); sheath gas flow rate, 35 arbitrary units; auxiliary gas flowrate, 8 arbitrary units; capillary temperature, 350°C.
Metabolomics data were acquired using the XCMS software (1.50.1 version), which produced a matrix of features with chromatography, accurate mass, and retention time. The variables presented in at least 50% of either group were extracted. All ions were normalized to the total peak area of each sample to achieve a minimum relative standard deviation (SD). Principle component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA) were performed to identify the discrimination of variables. Benjamini–Hochberg false discovery rate procedure was employed for the multiple test adjustments. Adjusted P-values <0.05 were considered statistically significant. Differential metabolites were defined as those with variable importance in the projection (VIP) > 1.0 obtained from PLS-DA and adjusted P-values <0.05. VIP indicates the contribution of each variable to group differences. Heatmaps were obtained based on Spearman correlation and cluster analyses. The significant metabolites were identified by MS/MS fragment through OSI-SMMS (version 1.0, Dalian Chem Data Solution Information Technology Co. Ltd.) with an in-house MS/MS database. MetaboAnalyst 4.0 was used to identify a variety of functional enrichment analyses.
Statistical analysis
Clinical and echocardiographic characteristics were expressed as the mean ± SD for continuous variables and as the number (per cent) for categorical variables. The intensity of metabolites was expressed as median (IQR). Continuous variables of two groups were compared using the Student's t-test (for normally distributed) and the Wilcoxon test (for non-normally distributed and all metabolites). Categorical variables were compared by the χ2 test or Fisher's exact test as appropriate. For the prediction model of CRT response, the least absolute shrinkage and selection operator (LASSO) was used to determine non-zero coefficient features, which were considered as the most important predictors. The tuning parameter (λ) was selected in the LASSO model used 10-fold cross-validation via minimum criteria and the 1 standard error (1-SE). And the model at 1-SE criteria was selected as the final model. The regression model was established by multivariable logistic regression. The performance of the prediction accuracy assessment was conducted by a receiver operating characteristic (ROC) curve with area under curve (AUC). Probability values were yielded from the prediction models, which were subsequently utilized as new input variables for the ROC curve analysis. The optimal cut-off value, which combined the higher value of specifying plus sensitivity, was identified from the ROC curve. A two-sided P value <0.05 was accepted as statistical significance. All data were analysed using the R Programming Language (version 3.3.1; https://www.r-project.org/).
Results
Baseline characteristics
In this study, a total of 42 HF patients who were scheduled for CRT implantation and 32 healthy subjects were included as the HF group and the control group, respectively. Clinical and echocardiographic characteristics of 30 CRT responders and 12 non-responders are presented in Table 1. Age and gender were comparable between the CRT responders and non-responders. The mean left ventricular end-diastolic diameter was 64.7 ± 7.9 mm in the CRT responders group and 71.9 ± 9.6 mm in the CRT non-responders group (P = 0.026). In addition, compared with non-responders, the incidences of hypertension were higher among CRT responders (57% vs. 8%, P = 0.003). Furthermore, no significant differences between these two groups were observed in HF-related biomarkers such as N-terminal pro-brain natriuretic peptide, creatine, high sensitivity C-reactive protein, and creatine kinase isoenzyme-MB (CK-MB). And all the patients received conventional medication for HF, and drug treatment was similar between responders and non-responders, except for trimetazidine (13% vs. 50%, P = 0.014).
| Variable | Responders (N = 27) | Non-responders (N = 11) | P value |
|---|---|---|---|
| Baseline | |||
| Age, years | 58.9 ± 10.6 | 57.0 ± 14.4 | 0.565 |
| Gender, male | 17 (63%) | 8 (73%) | 0.714 |
| BMI, kg/m2 | 26.4 ± 3.3 | 25.3 ± 3.2 | 0.430 |
| Device type, CRT-D | 9 (33%) | 7 (64%) | 0.086 |
| SBP, mmHg | 126.3 ± 20.9 | 113.9 ± 11.8 | 0.075 |
| DBP, mmHg | 72.2 ± 10.7 | 67.6 ± 7.0 | 0.201 |
| HR, b.p.m. | 71.5 ± 9.7 | 69.4 ± 10.4 | 0.562 |
| NYHA functional class | 2.5 ± 0.8 | 2.4 ± 0.7 | 0.577 |
| Alcohol | 7 (26%) | 3 (27%) | 0.932 |
| Smoking | 11 (41%) | 3 (27%) | 0.435 |
| Co-morbidity | |||
| Coronary artery disease | 8 (30%) | 2 (18%) | 0.467 |
| Hypertension | 16 (59%) | 1 (9%) | 0.005 |
| LBBB | 23 (85%) | 11 (100%) | 0.177 |
| Diabetes | 12 (44%) | 2 (18.2%) | 0.128 |
| Atrial fibrillation | 2 (7%) | 0 (0%) | 0.354 |
| Serum biomarkers | |||
| Scr, μmol/L | 88.4 ± 16.2 | 80.2 ± 16.6 | 0.181 |
| hs-CRP, mg/L | 2.8 ± 2.8 | 1.9 ± 1.8 | 0.354 |
| NT-proBNP, pg/mL | 1393.0 ± 1743.0 | 851.6 ± 891.4 | 0.337 |
| Uric acid, μmol/L | 392.9 ± 141.3 | 368.8 ± 155.1 | 0.663 |
| Albumin, g/L | 42.7 ± 5.1 | 43.2 ± 4.7 | 0.776 |
| CK-MB, U/L | 14.8 ± 12.5 | 12.6 ± 3.2 | 0.583 |
| Medication | |||
| ACEI/ARBs | 24 (89%) | 11 (100%) | 0.249 |
| Beta-blockers | 25 (93%) | 10 (91%) | 0.861 |
| Spironolactone | 26 (96%) | 9 (82%) | 0.133 |
| Trimetazidine | 4 (15%) | 5 (45%) | 0.044 |
| Amiodarone | 3 (11%) | 2 (18%) | 0.559 |
| Echocardiography | |||
| LVEDD, mm | 64.7 ± 7.9 | 71.9 ± 9.6 | 0.026 |
| LVEF, % | 35.5 ± 10.0 | 31.5 ± 8.5 | 0.243 |
| Lad, mm | 42.7 ± 5.5 | 43.1 ± 8.3 | 0.854 |
- ACEI-ARBs, angiotensin-converting enzyme-angiotensin receptor blocker; BMI, body mass index; CK-MB, creatine kinase isoenzyme-MB; CRT-D, CRT device implantation with defibrillator; DBP, diastolic blood pressure; HR, heart rate; hs-CRP, high sensitivity C-reactive protein; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
Metabolomics distinctions between the heart failure group and control group
We first evaluated the metabolic signatures between HF and control groups under either ESI+ or ESI− modes of untargeted metabolomics. Obtained by ESI+ metabolome, significant disparities were illustrated between patients with HF and healthy individuals through PCA plot and PLS-DA plot (Figure 1 and B). In the PLS-DA analysis, the cumulative R2Y and Q2 in the ESI+ mode were 0.991 and 0.882, respectively. There were 294 out of 982 metabolites (29.94%) in the ESI+ pattern metabolome showing VIP > 1. Pronounced distinctions were also confirmed by PCA and PLS-DA under ESI− pattern as well (Figure 1D and E), in which the cumulative R2Y and Q2 were 0.981 and 0.847, respectively. Three hundred sixty-one out of 1386 metabolites (26.05%) in the ESI− pattern metabolome had VIP > 1. For visualizing the relationship between the altered metabolites, hierarchical clustering with heatmap was used to arrange the metabolites based on their relative levels across samples as shown in Figure 1 (for ESI+) and Figure 1 (for ESI−). Overall, the metabolic signatures were significantly distinct between HF and control groups identified by either ESI+ or ESI− modes of LC-MS/MS.
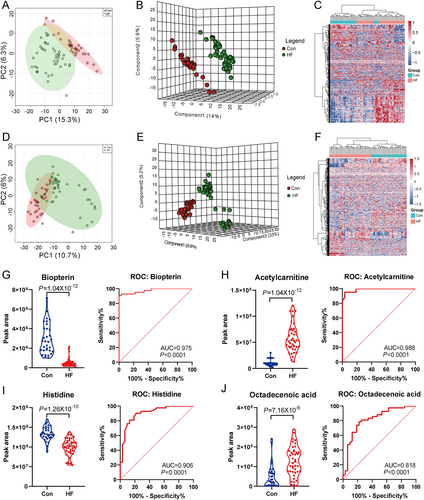
Consistent with previous reports,20-22 the untargeted plasma metabolome could significantly discriminate patients with HF from healthy individuals, independent of ESI positive or negative pattern. Compared with healthy individuals, patients with HF showed increased concentration of long-chain fatty acid, carnitine, β-hydroxybutyrate, and bilirubin. The lipid species, such as phosphatidylcholines (PCs) and sphingomyelins (SMs), exhibited heterogeneous alterations between HF and healthy individuals. Supporting Information Table S1 presented the representative differential metabolites identified between HF patients and healthy controls (with VIP > 2) via ESI+ and ESI− modes, respectively. Furthermore, we selected and plotted the top two metabolites between HF and control groups from ESI+ (biopterin and acetylcarnitine) and ESI− (histidine and octadecenoic acid) modes, respectively (Figure 1). As shown by the ROC curve, the individual metabolite could discriminate against the HF and control individuals very well (all P < 0.001, AUC range: 0.818–0.988) (Figure 1).
Discrimination of cardiac resynchronization therapy responders and cardiac resynchronization therapy non-responders
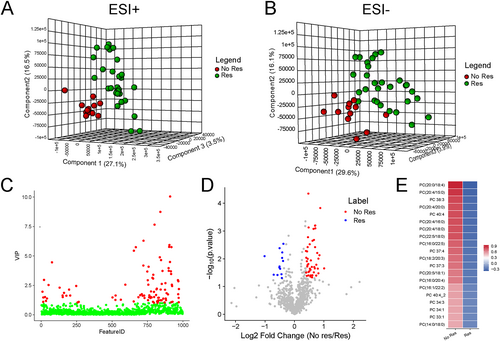
Obtained by ESI+ metabolome, significant disparity was illustrated between CRT responders and non-responders through PLS-DA (Figure 2). The cumulative R2Y and Q2 were 0.858 and 0.591, respectively. However, the metabolic signatures under the ESI− pattern did not show significant difference between CRT responders and non-responders, as the cumulative R2Y and Q2 were 0.733 and −0.260, respectively (Figure 2). Hence, we mainly focused on those metabolites detected by ESI+ pattern for further analysis. A total of 86 metabolites from in the ESI+ pattern metabolome with VIP > 1 in the loading plot were highlighted as biomarker candidates in Figure 2. Through the Mann–Whitney–Wilcoxon test with false discovery rate correction (P < 0.05), differential metabolic features were obtained and highlighted in Figure 2. We combined the criteria of VIP > 1.0 and P < 0.05 to further filter candidate metabolites. Among all the filtered differential metabolic features, a group of PCs species were identified as the top 20 differential variables (Table 2) and summarized as a heatmap (Figure 2). All these 20 PCs were upregulated in the plasma of CRT non-responders compared with CRT responders, with fold change ranging from 1.17 to 1.83. Interestingly, 17 out of 20 PCs were also significantly increased in all these patients HF with CRT compared with healthy controls, except for PC 34:3 (POS_17552), PC 40:4 (POS_18822), and PC (20:5/18:1) (POS_18294). These results suggested that PCs, a sub-species of lipid, may play an essential role in discriminating the CRT response for patients with HF.
| Var ID | RT (min) | m/z | Metabolites | Formula | Fold | P | VIP | QC RSD |
|---|---|---|---|---|---|---|---|---|
| POS_18366 | 23.4135 | 810.5997 | PC (20:0/18:4) | C46H84NO8P | 1.38 | 0.0000 | 10.03 | 3.44 |
| POS_17706 | 22.2982 | 768.5528 | PC (20:4/15:0) | C43H78NO8P | 1.83 | 0.0002 | 2.15 | 6.18 |
| POS_18398 | 23.4129 | 812.6059 | PC 38:3 | C46H86NO8P | 1.29 | 0.0005 | 3.45 | 3.28 |
| POS_18820 | 23.6840 | 838.6237 | PC (20:4/20:0) | C48H88NO8P | 1.62 | 0.0006 | 1.42 | 10.63 |
| POS_18822 | 24.0964 | 838.6311 | PC 40:4 | C48H88NO8P | 1.62 | 0.0006 | 1.05 | 18.33 |
| POS_17910 | 22.6954 | 782.5683 | PC (20:4/16:0) | C44H80NO8P | 1.28 | 0.0007 | 8.73 | 2.28 |
| POS_18365 | 23.1496 | 810.5993 | PC (20:4/18:0) | C46H84NO8P | 1.39 | 0.0008 | 4.47 | 6.27 |
| POS_18797 | 23.6858 | 836.6153 | PC (22:5/18:0) | C48H86NO8P | 1.63 | 0.0008 | 2.83 | 6.09 |
| POS_18325 | 22.8768 | 808.5824 | PC (16:0/22:5) | C46H82NO8P | 1.37 | 0.0017 | 5.75 | 6.81 |
| POS_18140 | 23.0570 | 796.5837 | PC 37:4 | C45H82NO8P | 1.58 | 0.0021 | 2.68 | 5.68 |
| POS_18327 | 22.8614 | 808.5843 | PC (18:2/20:3) | C46H82NO8P | 1.42 | 0.0030 | 5.92 | 7.45 |
| POS_18177 | 23.3154 | 798.5987 | PC 37:3 | C45H84NO8P | 1.36 | 0.0038 | 1.23 | 9.55 |
| POS_18294 | 22.2896 | 806.5683 | PC (20:5/18:1) | C46H80NO8P | 1.34 | 0.0055 | 3.72 | 5.86 |
| POS_17911 | 23.2830 | 782.5662 | PC (16:0/20:4) | C44H80NO8P | 1.19 | 0.0058 | 1.22 | 5.31 |
| POS_18400 | 23.6559 | 812.6155 | PC (16:1/22:2) | C46H86NO8P | 1.28 | 0.0225 | 5.74 | 3.94 |
| POS_18824 | 23.8753 | 838.6308 | PC 40:4 | C48H88NO8P | 1.43 | 0.0229 | 2.17 | 7.14 |
| POS_17552 | 21.8838 | 756.5438 | PC 34:3 | C42H78NO8P | 1.45 | 0.0251 | 1.09 | 9.41 |
| POS_17614 | 23.2671 | 760.5844 | PC 34:1 | C42H82NO8P | 1.17 | 0.0253 | 7.64 | 5.52 |
| POS_17444 | 22.8974 | 746.5686 | PC 33:1 | C41H80NO8P | 1.42 | 0.0283 | 1.46 | 5.61 |
| POS_17283 | 22.5208 | 734.5591 | PC (14:0/18:0) | C40H80NO8P | 1.39 | 0.0303 | 1.47 | 5.15 |
Moreover, we also analysed the differences between ceramide (Cer) and SMs species between CRT response and non-response patients (Supporting Information, Table S2). And it was not observed significant differences in most of these metabolites between these two groups.
Diagnostic potential of phosphatidylcholine species for cardiac resynchronization therapy response
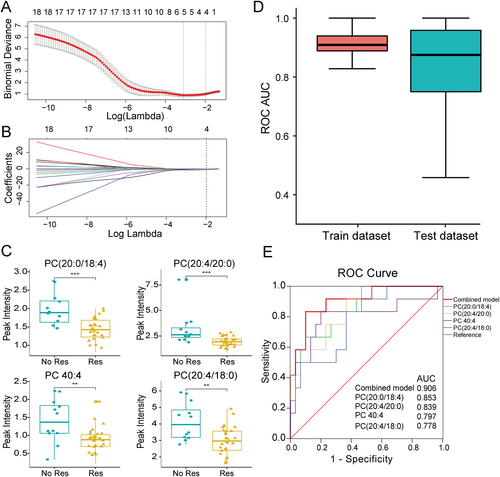
To identify the key metabolites that were essential predictors of CRT response, we used LASSO logistic regression models and selected the optimal λ values at 1-SE criterion (Figure 3A and B). Based on this criterion, we identified four PC metabolites with non-zero coefficients from the 20 differential metabolites observed in the peripheral plasma between CRT responders and non-responders, namely, PC (20:0/18:4), PC (20:4/20:0), PC 40:4, and PC (20:4/18:0), respectively. Each of the four PC metabolites was found to be significant different between CRT responders and non-responders in the univariate analysis, including PC (20:0/18:4) [1.89 (1.56, 2.25) vs. 1.43 (1.20, 1.72), P < 0.001], PC (20:4/20:0) [2.62 (2.21, 3.62) vs. 1.94 (1.57, 2.28), P < 0.001], PC 40:4 [1.37 (1.06, 1.89) vs. 0.88 (0.69, 1.04), P = 0.002], and PC (20:4/18:0) [3.96 (3.16, 5.23) vs. 2.96 (2.38, 3.57), P = 0.005] (Figure 3). We then established the logistic regression model with the combinations of these four biomarkers to predict the CRT response. We randomly split the dataset into training and testing cohort for 500 replications and performed the cross-validation. The median AUC of the ROC for discrimination of CRT response was 0.91 and 0.88 in the training and testing dataset, respectively (Figure 3). Based on the whole population (n = 42), we plotted the ROC curve in predicting CRT response using the logistic regression model incorporating the four biomarkers. Using the optimal cut-off value of 0.275, this model could predict CRT response with a sensitivity and specificity of 83.3% and 90.0%, respectively (AUC = 0.906, P < 0.001, Figure 3). Furthermore, compared with individual PC metabolite, the combination of four PCs had better discrimination performance for predicting CRT response according to the AUC of ROC (Figure 3).
Discussion
Current guidelines recommend CRT implantation for a broad spectrum of patients with systolic dysfunction and wide QRS duration.16 Still, approximately 30% of patients who meet with the implantation criteria failed to benefit from CRT devices.10, 11 Therefore, identifying the non-responders before implantation is urgently needed to avoid excessive treatment. Over the past few years, several studies have proposed multiple parameters to identify subsets of patients with poor prognosis, including clinical features, echocardiographic measurement, and laboratory biomarkers.20-22 However, traditional predictors are unlikely to provide sufficient information for the prognosis of CRT.
Metabolomics can be used to show essential snapshots of pathophysiology mechanisms and may be used to develop novel biomarkers and new potential targets for treatment. The human heart has unique metabolic flexibility and ability. It can switch the major source of energy to adapt to changing physiological or dietary conditions. Several studies have already demonstrated metabolomic profiles in patients with HF.14, 23-25 The main metabolic changes seen in a failing heart is the shift of energy utilization from fatty acids to glucose.26 In the present study, compared with healthy individuals, we found that patients with HF had distinct metabolomic profiles, including increased long-chain free fatty acid, acyl-carnitine, and ketone bodies. This finding is consistent with the previous studies.15, 24, 27 Lipid species such as Cer and SMs are important constituents of cell membranes and participate in several biological activities that may influence the pathophysiology of HF.28, 29 The relationship between Cer/SMs and the risk of HF has been identified.30 In this study, we confirmed that Cer and SMs were different between HF patients and healthy controls as previously reported,30, 31 whereas we did not observe a significant difference in these metabolites between CRT responders and non-responders.
Based on the untargeted LC-MS/MS-based metabolomics analysis, we observed the significant differences in the ESI+ mode rather than ESI− mode between CRT responders and non-responders. This may be related to the different categories of substances detected by the metabolome positive (ESI+) and negative (ESI−) ion mode. In the positive ion mode, lipids such as PCs, Cer, plasmenyl-PCs, and SMs as well as amino acids are detected, whereas the negative ion mode metabolome contains long-chain fatty acid and amino acids, as well as lipids, such as FAHFA, PA, PE and phosphatidylethanolamine, but not containing PCs species. Between CRT non-responders and responders, the dominated differential metabolites were mainly PCs that were mostly upregulated in the plasma of CRT non-responders, compared with responders. Through LASSO regression, we further identified four metabolites among the 20 PCs. And the four PCs-based model displayed excellent performance for predicting CRT response outcome, with high specificity and sensitivity. Because this is a primary discovery study based on a small prospective cohort from a single centre, further validation is needed in multi-centre studies with larger cohorts.
In the present study, PCs species rather than various irrelevant metabolites were found as the distinct plasma metabolites between responders and non-responders to CRT, which is different from previous reports.32, 33 This may be related to changes in myocardial lipid homeostasis, especially PCs metabolism in the failing hearts.34 As a common chronic disease, HF is associated with body consumption. With the development of HF, skeletal muscle loss occurs before fat tissue loss.35, 36 Furthermore, for patients with HF, plasma metabolomics not only represent the alteration in cardiac metabolism but also indicate overall metabolic changes in human body, because the circulating metabolites may involve the interaction between the heart and other organs. As a metabolic organ, the liver also plays an important role in the metabolism of PCs, and its abnormality may be related to the abnormal liver function of the failing heart.24, 37 PCs decomposition may be specifically affected as well during HF progression.
PCs are also key sources for the bioactive eicosanoids, which include leukotrienes, prostaglandins, and lipoxins. The enzyme phospholipases A2 (PLA2) is known to play an important role in the hydrolysis of phospholipids especially PCs, which leads to the accumulation of FFA including (non-esterified) arachidonic acid (AA).38 As a free fatty acid, once being liberated from cell membranes in response to stimulation, AA can be metabolized to form bioactive products including PGs, leukotrienes, and epoxyeicosatrienoic acids, which participate in the inflammation process.39 The amount of AA, as well as AA-containing PCs, depends on an exquisite balance between phospholipid reacylation and hydrolysis reactions.40, 41 Moon et al.42 found that congestive HF is associated with the pathological opening of the mitochondrial permeability transition pore, which activates Ca2+-independent phospholipase A2 (iPLA2) through decreasing the mitochondrial membrane potential. The activation of iPLA2 increased the production of downstream toxic hydroxyeicosatetraenoic acids. Of note, it is iPLA2, which activates AA into hydroxyeicosatetraenoic acids, rather than cPLA2, that predominated in failing human myocardium. In consistent with Moon's research, our study suggests the important value of AA-PCs in CRT response, indicating that the metabolism of AA and AA-PCs may play an important role in failing heart. In addition, disturbances and abnormal membrane phospholipids in the ratio of phosphatidylcholine to phosphatidylethanolamine in failing heart altered the interaction of membrane-associated protein complexes, thereby affecting the myocardial metabolism and cellular signalling.43 Nevertheless, the mechanisms of potential correlation of cardiac lipid metabolism with response to CRT were rarely studied. Our novel findings, despite clinical association study, will provide a promising clue to study the potential pathophysiology of failing heart in response to CRT in the future. The potential mechanism between phosphatidylcholine metabolism and CRT responses still remain to be further illustrated in the future.
Study limitations
In this study, we used patients with an increase of LVEF more than 5% after CRT implantation as the definition of response to CRT. Thus, the performance using this panel to predict other definitions of responses to CRT is required. Moreover, the precise mechanisms association between PCs species and CRT response remains unclear, which should be explored in future studies. Additionally, the current study was performed in a single centre with a relatively small sample size. Multi-centre studies with larger populations as external validation groups are currently in progress by our team.
Conclusions
Heart failure is associated with a significantly altered metabolomics profile compared with healthy individuals. Within the HF group, the non-responders had a distinct plasma metabolic profile featuring alterations of 20 PC metabolites, compared with the responders to CRT. A novel predictive model incorporating four PCs performed well in identifying CRT non-responders. These four PCs might severe as potential biomarkers for predicting CRT response. Further validation is needed in multi-centre studies with larger samples.
Conflict of interest
None declared.
Funding
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2017-I2M-1-009) and the National Natural Science Foundation of China (81570370).




