Association between base excess and mortality in patients with congestive heart failure
Abstract
Aims
The relationship between baseline base excess (BE) and survival outcomes in patients with congestive heart failure (CHF) is unclear. Therefore, we aimed to investigate this relationship based on the Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) database (v1.4).
Methods and results
This retrospective cohort study included 5956 adult patients with CHF from the MIMIC-III database from 2001 to 2012. Using the Cox proportional-hazard analysis and Kaplan–Meier plot, we evaluated the relationship between baseline BE and all-cause death at 1 year after admission to the intensive care unit. At the 1 year follow-up, 2104 participants (35.3%) had died. There was an association between BE and all-cause death (log-rank test P < 0.0001). In the Cox regression model adjusted for demographic and clinical variables, the risk of all-cause death in the first (BE ≤ −8), second (−8 < BE ≤ −3), fourth (2 < BE ≤ 7), and fifth (BE > 7) BE groups was significantly higher than that in the third BE group (−3 < BE ≤ 2) [hazard ratio (HR) 1.99, 95% confidence interval (CI) 1.62–2.43, HR 1.40, 95% CI 1.23–1.60, HR 1.46, 95% CI 1.26–1.69, and HR 1.68, 95% 1.33–2.12, respectively]. Similar results were observed when BE was modelled as a continuous variable using a Cox regression model with a restricted cubic spline.
Conclusions
This study demonstrated the existence of a U-shaped relationship between BE and survival outcome in patients with CHF. Both low and high BE increased the risk of all-cause mortality.
Introduction
Congestive heart failure (CHF) is a common disease with high mortality. An estimated 40 million people were living with CHF worldwide in 2015.1 In the first year after diagnosis, the risk of death is about 35%, while in the second year, those who survive are less than 10%.2
Patients with CHF need diuretics to reduce the burden on the heart.3 Additionally, these patients have pulmonary congestion, decreased blood oxygen partial pressure, and carbon dioxide retention. The long-term existence of these conditions leads to disorders in the acid–base balance.4
Base excess (BE) refers to the amount of acid or alkali needed to adjust the pH value to the normal range, and it is one of the indicators reflecting a disorder in the acid–base balance. Previous studies have shown that BE is a predictor of outcomes in patients with traumatic shock and is a valuable indicator for guiding blood transfusion treatment.5-7 However, the ability of BE values to predict the outcomes of patients with CHF remains unclear.
A recent study suggested that BE has a positive linear relationship with the prognosis of patients with acute heart failure8; high but not low BE values increased the risk of all-cause mortality. However, the sample size of the study was small. Additionally, the authors evaluated the association between different BE intervals (<−2, between −2 and <2, and >2) and survival outcomes, which provides little information when the BE is lower than −2 or higher than 2.
To address this issue, we performed a retrospective study using the open-source Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) database to clarify the association between BE value and survival outcome in CHF patients.
Methods
Data source
Our research was based on the open-source MIMIC-III database, which includes health data for more than 40 000 patients hospitalized in the intensive care unit (ICU) of Beth Israel Deaconess Medical Center from 2001 to 2012.9 We used the most recent version (v1.4) for the research. The database contains comprehensive clinical data, such as patient characteristics, laboratory outcome, medication, International Classification of Diseases 9th revision (ICD-9) disease code, and medical records. We completed the National Institutes of Health online course and passed the Examination for Protecting Human Research Participants and applied for access. The project was approved by Beth Israel Deaconess Medical Center and the institutional review board of the Massachusetts Institute of Technology (Cambridge, Massachusetts). The patients' identification details were hidden to protect their privacy.
Inclusion and exclusion criteria
We included adult patients (≥18 years) with CHF at first ICU admission from the database on the basis of the ICD-9 disease code [4280–4289 and 39891, 4280 = CHF, unspecified; 4281 = left heart failure; 4282 = systolic heart failure; 4283 = diastolic heart failure; 4284 = combined systolic and diastolic heart failure; 4289 = heart failure, unspecified; 39891 = rheumatic heart failure (congestive)]. The exclusion criteria were as follows: (i) non-adult patients; (ii) absence of CHF; and (iii) missing baseline BE value at ICU admission.
Variables of interest
After applying and obtaining permission, we downloaded the database to the local database and used Structured Query Language (SQL) with PostgreSQL (version 9.6) to extract related variables, outcomes, and data. The variables included the subject code, admission code, ICU code, physical characteristics (including age, gender, height, weight, and ethnicity), vital signs (including heart rate, systolic pressure, diastolic pressure, respiration rate, and SPO2), laboratory parameters [including white blood cell (WBC), haemoglobin, total bilirubin, creatinine, C-reactive protein, troponin I, NT-proBNP, sodium, potassium, BE, pH, PO2, PCO2, lactate, and bicarbonate], ejection fraction, medications [including angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), digoxin, beta-blockers, and diuretics], and co-morbidities (including peripheral vascular disease, neurological disease, hypertension, chronic pulmonary disease, diabetes, renal failure, atrial fibrillation/flutter, and hypothyroidism). Missing values were imputed using the random forest imputation method.10
Primary endpoint
Survival information (including survival outcome and time of death) was obtained from the Social Security Death Index records. Our study endpoints were all-cause mortality at 1 year from the date of ICU admission.
Statistical analysis
Descriptive statistics were used to present the differences between characteristics across five groups of BE (BE ≤ −8, −8 < BE ≤ −3, −3 < BE ≤ 2, 2 < BE ≤ 7, and BE > 7). Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as percentages. The Kaplan–Meier method was used to calculate the cumulative incidence of all-cause mortality by BE group. Proportional-hazard analysis was used to examine the association between the baseline BE value with 1 year all-cause mortality. Cox regression analysis was used to calculate the hazard ratio (HR) for all-cause mortality as a function of baseline BE in sequentially adjusted models. The following models were used in the Cox regression analysis: (i) unadjusted model; (ii) adjusted for age, ethnicity, and age × ethnicity interaction term (because ethnicity is more relevant to the mortality of CHF, as previously reported)11; and (iii) adjusted for the variables in Model 2, in addition to height, weight, heart rate, systolic pressure, diastolic pressure, respiration rate, SPO2, WBC, haemoglobin, total bilirubin, creatinine, C-reactive protein, troponin I, NT-proBNP, potassium, ejection fraction, ACEI, ARB, digoxin, beta-blocker, diuretic, peripheral vascular disease, neurological disease, hypertension, chronic pulmonary disease, diabetes, renal failure, and atrial fibrillation/atrial flutter. Because the third BE group is representative of the normal interval of BE, we chose the third group as the reference population. The relationship between baseline BE values (modelled as continuous variables) and the risk of all-cause mortality was evaluated using a Cox regression model with a restricted cubic spline.12 A two-sided P value of <0.05 was considered statistically significant in all analyses. All analyses were performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
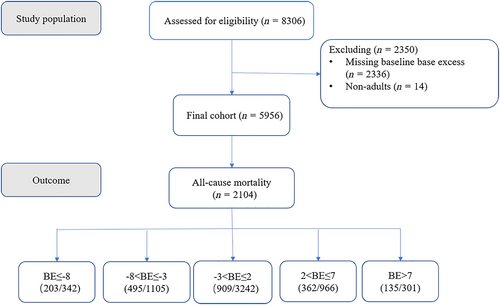
The study flow diagram is shown in Figure 1. A total of 8306 patients with CHF were screened in the database. Fourteen patients were non-adults, and 2336 patients did not have baseline BE values. The final analysis included 5956 participants. A total of 2104 patients (35.3%) died at 1 year from the date of ICU admission (Figure 1). The baseline characteristics of the population across the baseline BE groups are shown in Table 1. When compared with participants with normal BE, those with low BE were more likely to be men and younger; have a lower weight and blood pressure; have a higher heart rate and respiration rate; have a lower SPO2, blood urea nitrogen, C-reactive protein, pH, PO2, and PCO2; have a higher WBC, total bilirubin, creatinine, troponin I, NT-proBNP, potassium, and lactate; have less use of digoxin; and have a lower prevalence of neurological disease, diabetes, and renal failure. When compared with participants with normal BE, those with high BE were more likely to be women; have a lower heart rate; have a higher weight, blood pressure, and respiration rate; have a lower SPO2, WBC, creatinine, BUN, troponin I, NT-proBNP, potassium, PO2, and lactate; have higher haemoglobin, sodium, PCO2, and bicarbonate; have more use of ACEI and digoxin and less use of beta-blocker; have a lower prevalence of peripheral vascular disease, renal failure and acute coronary syndrome; and have a higher prevalence of neurological disease, chronic obstructive pulmonary disease, and diabetes. The baseline characteristics of those with BE and those without are shown in Table 2. Patients without BE had lower levels of NT-proBNP (P < 0.001) and a lower rate of 1 year all-cause mortality (P < 0.001) than did those with BE.
| Variables | Base excess concentration groups | P | ||||
|---|---|---|---|---|---|---|
| First (BE ≤ −8) | Second (−8 < BE ≤ −3) | Third (−3 < BE ≤ 2) | Fourth (2 < BE ≤ 7) | Fifth (BE > 7) | ||
| Number of patients | 342 | 1105 | 3242 | 966 | 301 | |
| Male (%) | 58 | 53 | 54 | 51 | 45 | <0.001 |
| Age | 68.7 ± 12.6 | 70.6 ± 14 | 70.5 ± 12.8 | 70.9 ± 12.1 | 70.3 ± 13.2 | 0.091 |
| Ethnicity (%) | <0.001 | |||||
| White | 72 | 71 | 70 | 71 | 69 | |
| Black | 6 | 11 | 8 | 9 | 12 | |
| Hispanic | 2 | 1 | 2 | 2 | 4 | |
| Asian | 2 | 2 | 3 | 1 | 1 | |
| Other | 18 | 15 | 17 | 17 | 14 | |
| Height (cm) | 167.9 ± 10.8 | 168 ± 11.4 | 168.5 ± 10.8 | 168.5 ± 10.8 | 168.2 ± 11.1 | 0.02 |
| Weight (kg) | 80.5 ± 23.1 | 81.5 ± 25.5 | 82.5 ± 23.2 | 86.1 ± 28 | 94.1 ± 42.2 | <0.001 |
| Heart rate | 90.4 ± 13.7 | 88.8 ± 18.8 | 85.5 ± 16.6 | 84.9 ± 15.1 | 83.3 ± 14.8 | <0.001 |
| Systolic pressure (mmHg) | 107.1 ± 15.3 | 113.2 ± 18.2 | 115.9 ± 16.2 | 119.2 ± 16.6 | 124 ± 17.8 | <0.001 |
| Diastolic pressure (mmHg) | 55.7 ± 9.3 | 57.4 ± 10.8 | 57.5 ± 10.3 | 58.2 ± 10.2 | 60.3 ± 10.9 | <0.001 |
| Respiration rate | 21.4 ± 4 | 20.1 ± 4.8 | 18.9 ± 4.4 | 19.4 ± 4.2 | 19.7 ± 4.3 | <0.001 |
| SPO2 (%) | 95.4 ± 2 | 97 ± 6.7 | 97.3 ± 3.2 | 96.9 ± 2.2 | 95.9 ± 2.5 | <0.001 |
| White blood cell (K/μL) | 15.2 ± 7 | 14.7 ± 9.2 | 13 ± 17.6 | 12.3 ± 8.7 | 10.6 ± 4.8 | <0.001 |
| Haemoglobin (g/dL) | 10.7 ± 1.8 | 10.8 ± 2.1 | 10.6 ± 1.8 | 10.7 ± 1.9 | 11 ± 2 | 0.002 |
| Total bilirubin (mg/dL) | 1.8 ± 1.8 | 1.4 ± 3.2 | 1.1 ± 2.8 | 1.1 ± 1.5 | 1 ± 1.4 | <0.001 |
| Creatinine (mg/dl) | 2.9 ± 1.3 | 2.1 ± 2.3 | 1.4 ± 1.9 | 1.4 ± 1.2 | 1.3 ± 1.2 | <0.001 |
| BUN (mmol/L) | 27.1 ± 18.8 | 52.2 ± 33.9 | 40.2 ± 26.5 | 29.7 ± 20.1 | 29.7 ± 18.3 | <0.001 |
| C-reactive protein (mg/L) | 89.6 ± 76.8 | 91 ± 77 | 105.8 ± 76.1 | 112.9 ± 77.7 | 127.4 ± 72.5 | <0.001 |
| Troponin I (ng/mL) | 12.6 ± 11 | 9.2 ± 13.6 | 8.3 ± 11.4 | 7.3 ± 10.1 | 5.1 ± 7.5 | <0.001 |
| NT-proBNP (pg/mL) | 18719.7 ± 13305.7 | 14049.5 ± 18136.8 | 10416.4 ± 15698.4 | 9140.8 ± 11806.7 | 7401.9 ± 10757.2 | <0.001 |
| Sodium (mmol/L) | 138.1 ± 3.8 | 138 ± 5.1 | 137.8 ± 4.6 | 138.9 ± 4.2 | 139.6 ± 5 | <0.001 |
| Potassium (mmol/L) | 4.6 ± 0.5 | 4.4 ± 0.7 | 4.3 ± 0.6 | 4.1 ± 0.5 | 4.1 ± 0.5 | <0.001 |
| PH | 7.2 ± 0 | 7.3 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | <0.001 |
| PO2 | 68.7 ± 7 | 71.7 ± 10.3 | 72.9 ± 7.8 | 72.8 ± 9.9 | 70.4 ± 14.6 | <0.001 |
| PCO2 | 36.4 ± 17 | 38.8 ± 17.3 | 41.6 ± 17.3 | 46.9 ± 16.4 | 60.5 ± 14.9 | <0.001 |
| Lactate | 4.2 ± 1.2 | 2.7 ± 3.5 | 2.1 ± 1.9 | 1.8 ± 1 | 1.6 ± 0.8 | <0.001 |
| bicarbonate | 17.1 ± 3.7 | 21 ± 5.1 | 24.5 ± 3.8 | 29.4 ± 4.5 | 37 ± 4.8 | <0.001 |
| EF (%) | <0.001 | |||||
| 10–35% | 7 | 10 | 7 | 6 | 6 | |
| 35–55% | 33 | 30 | 34 | 27 | 19 | |
| 55–70% | 26 | 24 | 26 | 29 | 31 | |
| >70% | 6 | 8 | 8 | 6 | 9 | |
| ACEI (%) | 29 | 18 | 24 | 28 | 30 | <0.001 |
| ARB (%) | 2 | 1 | 1 | 3 | 2 | 0.151 |
| Digoxin (%) | 7 | 6 | 9 | 11 | 12 | 0.009 |
| Beta-blocker (%) | 47 | 34 | 45 | 45 | 32 | <0.001 |
| Diuretic (%) | 74 | 57 | 68 | 75 | 69 | <0.001 |
| Peripheral vascular disease (%) | 15 | 11 | 17 | 14 | 11 | 0.012 |
| Neurology (%) | 7 | 10 | 10 | 10 | 12 | 0.004 |
| Hypertension (%) | 60 | 55 | 57 | 59 | 56 | 0.191 |
| Chronic pulmonary disease (%) | 25 | 22 | 23 | 37 | 54 | <0.001 |
| Diabetes (%) | 35 | 34 | 38 | 43 | 43 | <0.001 |
| Renal failure (%) | 18 | 30 | 30 | 21 | 17 | <0.001 |
| Atrial fibrillation/flutter (%) | 47 | 33 | 40 | 49 | 47 | <0.001 |
| Hypothyroidism (%) | 10 | 10 | 10 | 12 | 12 | 0.202 |
| ACS (%) | 29 | 32 | 24 | 17 | 10 | <0.001 |
- The neurology was defined as hypoxic–ischemic encephalopathy, hypoxic–ischemic encephalopathy, unspecified, mild hypoxic–ischemic encephalopathy, moderate hypoxic–ischemic encephalopathy, and severe hypoxic–ischemic encephalopathy.
- ACEI, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blocker; BE, base excess; BUN, blood urea nitrogen; EF, ejection fraction.
| BE | No BE | P value | |
|---|---|---|---|
| Number of patients | 5956 | 2336 | |
| Male (%) | 55.1 | 55.0 | 0.918 |
| Age | 70.45 ± 12.70 | 72.13 ± 12.68 | <0.001 |
| Ethnicity (%) | <0.001 | ||
| White | 71.3 | 73.1 | |
| Black | 7.6 | 9.5 | |
| Hispanic | 2.3 | 2.6 | |
| Asian | 1.7 | 1.8 | |
| Other | 17.1 | 13 | |
| Height (cm) | 168.58 ± 10.75 | 167.80 ± 11.15 | 0.004 |
| Weight (kg) | 83.25 ± 25.35 | 81.88 ± 23.57 | 0.025 |
| Heart rate | 86.17 ± 14.99 | 81.33 ± 16.06 | <0.001 |
| Systolic pressure (mmHg) | 115.82 ± 16.36 | 118.50 ± 18.29 | <0.001 |
| Diastolic pressure (mmHg) | 57.65 ± 9.82 | 58.87 ± 11.65 | <0.001 |
| Respiration rate | 19.37 ± 4.21 | 19.53 ± 3.55 | 0.106 |
| SPO2 (%) | 97.00 ± 2.81 | 96.62 ± 2.08 | <0.001 |
| White blood cell (K/μL) | 13.20 ± 10.18 | 10.65 ± 6.10 | <0.001 |
| Haemoglobin (g/dL) | 10.72 ± 1.82 | 10.94 ± 2.00 | <0.001 |
| Total bilirubin (mg/dL) | 1.15 ± 2.03 | 1.30 ± 2.17 | 0.003 |
| Creatinine (mg/dL) | 1.63 ± 1.53 | 1.75 ± 1.61 | 0.001 |
| BUN (mmol/L) | 31.54 ± 22.82 | 35.89 ± 25.39 | <0.001 |
| C-reactive protein (mg/L) | 99.04 ± 74.46 | 86.13 ± 92.46 | <0.001 |
| Troponin I (ng/mL) | 6.91 ± 9.84 | 11.64 ± 13.01 | <0.001 |
| NT-proBNP (pg/mL) | 10798.29 ± 14204.23 | 7126.32 ± 8004.64 | <0.001 |
| Sodium (mmol/L) | 138.12 ± 4.23 | 138.34 ± 4.49 | 0.031 |
| Potassium (mmol/L) | 4.29 ± 0.56 | 4.23 ± 0.58 | <0.001 |
| EF | <0.001 | ||
| 10–35% | 7.1 | 6.4 | |
| 35–55% | 31.3 | 33.0 | |
| 55–70% | 26.5 | 22.0 | |
| >70% | 6.7 | 5.8 | |
| ACEI (%) | 27.2 | 26.0 | 0.575 |
| ARB (%) | 1.7 | 2.7 | 0.009 |
| Digoxin (%) | 8.6 | 9.8 | 0.167 |
| Beta-blocker (%) | 45.0 | 33.2 | <0.001 |
| Diuretic (%) | 71.8 | 52.7 | <0.001 |
| Peripheral vascular disease (%) | 15.1 | 12.3 | 0.001 |
| Neurology (%) | 8.7 | 7.8 | 0.22 |
| Hypertension (%) | 58.7 | 64.9 | <0.001 |
| Chronic pulmonary disease (%) | 28.0 | 24.0 | <0.001 |
| Diabetes (%) | 37.0 | 39.1 | 0.077 |
| Renal failure (%) | 21.5 | 29.7 | <0.001 |
| Atrial fibrillation/flutter (%) | 45.1 | 44.0 | 0.356 |
| Hypothyroidism (%) | 10.1 | 12.3 | 0.004 |
| 1 year mortality (%) | 35.3 | 31.3 | <0.001 |
- The neurology was defined as hypoxic–ischemic encephalopathy, hypoxic–ischemic encephalopathy, unspecified, mild hypoxic–ischemic encephalopathy, moderate hypoxic–ischemic encephalopathy, and severe hypoxic–ischemic encephalopathy.
- ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; BE, base excess; BUN, blood urea nitrogen; EF, ejection fraction.
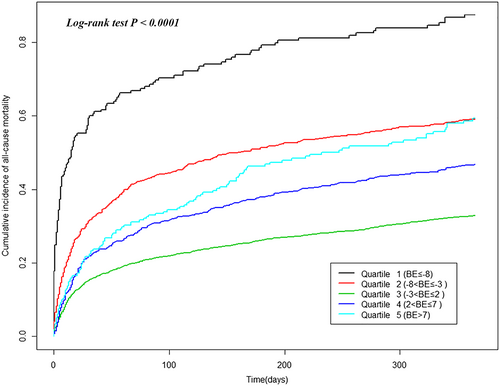
There was a statistically significant association between BE and the risk of all-cause mortality (Figure 2; log-rank test P < 0.0001). The unadjusted and multivariate-adjusted HRs of all-cause mortality by categories of baseline BE are shown in Table 3. In the unadjusted model, when compared with the patients in the third group (−3 < BE ≤ 2), those in the first, second, fourth, and fifth groups had a higher risk of all-cause mortality (HR 3.18, 95% CI 2.73–3.71, HR 1.86, 95% CI 1.67–2.07, HR 1.42, 95% CI 1.25–1.60, and HR 1.75, 95% 1.46–2.09, respectively). In the model adjusted for age, ethnicity, and age × ethnicity interaction, patients in the first, second, fourth, and fifth baseline BE groups also had a higher risk of all-cause mortality than did those in the third group (HR 3.53, 95% CI 3.03–4.11, HR 1.90, 95% CI 1.70–2.12, HR 1.42, 95% CI 1.26–1.61, and HR 1.85, 95% 1.54–2.21, respectively). In the model adjusted for age, ethnicity, age × ethnicity interaction, height, weight, heart rate, systolic pressure, diastolic pressure, respiration rate, SPO2, WBC, haemoglobin, total bilirubin, creatinine, C-reactive protein, troponin I, NT-proBNP, potassium, ejection fraction, ACEI, ARB, digoxin, beta-blocker, diuretic, peripheral vascular disease, neurological disease, hypertension, chronic obstructive pulmonary disease, diabetes, renal failure, and atrial fibrillation/flutter, patients in the first, second, fourth, and fifth baseline BE groups had a higher risk of all-cause mortality than did those in the third group (HR 1.99, 95% CI 1.62–2.43, HR 1.40, 95% CI 1.23–1.60, HR 1.46, 95% CI 1.26–1.69, and HR 1.68, 95% 1.33–2.12, respectively).
| Base excess concentration groups | |||||
|---|---|---|---|---|---|
| First (BE ≤ −8) | Second (−8 < BE ≤ −3) | Third (−3 < BE ≤ 2) | Fourth (2 < BE ≤ 7) | Fifth (BE > 7) | |
| n | 342 | 1105 | 3242 | 966 | 301 |
| events | 203 | 495 | 909 | 362 | 135 |
| Model 1 (HR and 95% CI) | 3.18 (2.73–3.71) | 1.86 (1.67–2.07) | Reference | 1.42 (1.25–1.60) | 1.75 (1.46–2.09) |
| Model 2 (HR and 95% CI) | 3.53 (3.03–4.11) | 1.90 (1.70–2.12) | Reference | 1.42 (1.26–1.61) | 1.85 (1.54–2.21) |
| Model 3 (HR and 95% CI) | 1.99 (1.62–2.43) | 1.40 (1.23–1.60) | Reference | 1.46 (1.26–1.69) | 1.68 (1.33–2.12) |
- BE, base excess; CI, confidence interval; HR, hazard ratio.
- Model 1: unadjusted model. Model 2: adjusted for age, ethnicity, and age × ethnicity interaction. Model 3: adjusted for variables in model 1 plus height, weight, heart rate, systolic pressure, diastolic pressure, respiration, SPO2, WBC, haemoglobin, TB, creatinine, C-reactive protein, troponin I, NT-proBNP, potassium, ejection fraction, ACEI, ARB, digoxin, beta-blocker, diuretic, peripheral vascular disease, neurology, hypertension, chronic pulmonary disease, diabetes, renal failure, and atrial fibrillation/atrial flutter.
The unadjusted spline curve demonstrated a U-shaped relationship between BE and the risk of all-cause mortality (Figure 3). When adjusting for age, ethnicity, height, weight, heart rate, systolic pressure, diastolic pressure, respiration rate, SPO2, WBC, haemoglobin, TB, creatinine, C-reactive protein, troponin I, NT-proBNP, potassium, ejection fraction, ACEI, ARB, digoxin, beta-blocker, diuretic, peripheral vascular disease, neurology, hypertension, chronic pulmonary disease, diabetes, renal failure, and atrial fibrillation/flutter, a U-shaped relationship between BE and all-cause mortality was still observed (Figure 3).

Discussion
Our study, based on the MIMIC-III database, found a U-shaped relationship between BE value and all-cause mortality in patients with CHF. Both high and low BE values increased the risk of all-cause mortality.
BE is one of the most commonly used markers for the management of patients in the ICU. It can be used to diagnose disorders in the acid–base balance, prognosticate, and guide treatment for resuscitation. A study by Dunham et al. suggested that base deficit was superior to vital signs or shock index for predicting outcomes in patients with trauma.13 A continuously negative or increasingly negative standard BE value is considered to predict mortality and shock-related complications (including kidney failure, acute respiratory distress syndrome, acute lung injury, and multiple organ failure).5, 6, 14 The BE value on administration is also a greater indicator for predicting outcomes in patients admitted to the ICU.7 Another study showed that BE can also predict the risk of ICU mortality in patients undergoing cardiac surgery.15 These findings suggest that BE value is a notable indicator of hypoperfusion and potentially unstable conditions in patients without hypotension at admission. However, the ability of BE value to predict outcomes in patients with severe heart failure remains unclear. Nakano et al. conducted an observational study to evaluate the association between BE and survival outcomes in patients with acute heart failure.8 They included 470 patients and divided the participants into three groups (<−2, between −2 and 2, and >2) on the basis of the BE value. The results showed that the rate of all-cause death was higher in the third group than in the second group, while the rates were similar between the first and second groups. The study concluded that high but not low BE values increased the risk of all-cause mortality. However, in this study, there were considerably few patients in the first (BE < −2) and third (BE > 2) groups. It was not possible to subdivide the population into more groups at these intervals. Therefore, the association between BE value and survival outcome is still unclear with BE values of <−2 and >2. The study by Park et al. evaluated the prognostic value of arterial blood gas in high-risk acute heart failure. The results showed that acidosis but not alkalosis was associated with increased mortality in high-risk acute heart failure. However, in this study, the authors did not identify which type of alkalosis was associated with worse outcomes. In another study by Konishi et al., the authors evaluated the clinical profile and management of hypercapnia in acute heart failure. The authors concluded that hypercapnia was possibly involved in the pathophysiology of acute pulmonary oedema. Thus, the hypercapnia may be due to respiratory or metabolic alkalosis, and the type of alkalosis contributing to the prognosis should be identified. Additionally, the authors did not evaluate the association between hypercapnia and survival outcomes in patients with acute heart failure. Our research was based on the open-source MIMIC-III database, which collects the health data of patients in the ICU.9 A total of 5900 CHF patients were included. We divided them into five groups on the basis of the BE value, which is a reflection of the metabolic acid–base imbalance of the body. The results showed that metabolic acidosis or metabolic alkalosis was associated with worse outcomes after adjusting for confounding factors, and this was consistent with the results of the Cox regression model with a restricted cubic spline.
The U-shaped relationship between BE value and all-cause mortality can be explained as follows. Patients with CHF need diuretics to reduce the burden on the heart. However, the long-term or excessive use of diuretics causes insufficient blood volume, which leads to increased aldosterone secretion, increased renal sodium retention, and HCO3− reabsorption and ultimately to alkalosis.16, 17 Previous studies have shown that the base deficit can be used to identify patients at high risk for traumatic shock and to evaluate the need for transfusion.18 Therefore, an increased BE value is a manifestation of hypovolemia. Additionally, a high BE may be a compensatory response to CO2 retention, which reflects worsening heart failure. On the contrary, the deterioration of heart function leads to hypoxia in the tissues and organs, and the anaerobic metabolism of the cells secretes a large amount of acidic substances, which eventually causes acidosis.19, 20 Severe acidosis causes electrolyte disturbances, increases the incidence of malignant arrhythmias, causes myocardial damage, and worsens cardiac function.20, 21 Evidence has shown that BE is a useful marker for evaluating the clearance of acidosis after traumatic shock and that a persistent base deficit suggests a poor prognosis.22
Volume management in patients with heart failure is essential. Excessive or insufficient diuresis may lead to the worsening of heart function. Nevertheless, the early identification of the patient's capacity is challenging, and current methods of identifying insufficient capacity are limited. A study involving 16 000 patients with hypovolemic shock showed that the ability to identify early shock based on the BE value classification was better than that of the current Advanced Trauma Life Support classification of hypovolemic shock (combining heart rate, systolic blood pressure, and Glasgow Coma Scale score).13 Furthermore, BE can be used to identify patients who need early blood transfusions. Therefore, the body's capacity in patients with severe heart failure can be assessed early by combining the symptoms, signs, and BE values.
In this study, PCO2 increased with BE values higher than 2 but the pH was not changed, which showed that the PCO2 increase was a compensatory response to metabolic alkalosis. However, the PCO2 level did not significantly decrease with BE values lower than −3. This demonstrated the absence of a relationship between PCO2 and BE value. Additionally, PCO2 level was not an independent risk factor in the univariate model. Therefore, respiratory acidosis is not a predictor of all-cause mortality.
Our study has several strengths. First, as noted earlier, our study was based on the MIMIC-III database, which collects health information of patients in the ICU. Because blood gas analysis is a critical monitoring indicator in intensive care patients, the BE value can be obtained in most patients. Second, our sample size was large, which allowed us to divide the participants into more groups on the basis of the BE value and to investigate the relationship between BE value and all-cause death. Finally, we evaluated the relationship between the baseline BE value (modelled as a continuous variable) and the risk of all-cause mortality using a Cox regression model with a restricted cubic spline. A U-shaped relationship between BE value and survival outcome was consistent with the results of the multivariate Cox regression analysis.
Limitations
Our research was not without limitations. First, our research was an observational study; therefore, it was impossible to determine a relationship between cause and outcome. However, identifying predictors of survival outcomes helps clinicians to manage patients with CHF. Second, the study was based on a database. Therefore, we could not retrieve all the baseline characteristics of patients via SQL. For instance, it was challenging to extract features stored as text, such as left ventricular end-diastolic diameter. However, we extracted the ejection fraction data of the population, which is also a crucial predictor of outcomes in CHF patients. Third, our study only evaluated the baseline BE value; therefore, we could not evaluate the influence of dynamic changes in BE value on survival outcomes. Fourth, the MIMIC-III dataset mainly collected data for the evaluation of critically ill patients and did not only include those with CHF. In this study, we identified patients with CHF by searching for ICD-9 codes in the ‘d_icd_diagnoses’ table in the database. In this table, the different diagnoses were listed separately. Therefore, it was hard to identify that a patient was admitted because of heart failure. However, the included patients in this study had a notably high NT-proBNP and high diuretic usage, which suggested that uncontrolled heart failure may have been one of the main indications for admission.
Conclusions
Our study showed the existence of a U-shaped relationship between BE value and the risk of all-cause death in patients with CHF. Both high and low BE values increased the risk of all-cause death. These findings reiterate the importance of a strict maintenance of acid–base balance in patients with CHF in order to decrease the risk of poor outcomes.
Conflict of Interest
None declared.
Funding
This work was supported by Shenzhen Fundamental Research Program(JCYJ20160427172544991, JCYJ20180302173854598, 20170502165510880), The Fund of “Sanming” Project of Medicine in Shenzhen(No. SZXJ2017020, SZXJ2017049).




