Recovery free of heart failure after acute coronary syndrome and coronary revascularization
Abstract
Aims
Previous studies have examined risk factors for the development of heart failure (HF) subsequent to acute coronary syndrome (ACS). Our study seeks to clarify the clinical variables that best characterize patients who remain free from HF after coronary artery bypass grafting (CABG) surgery for ACS to determine novel biological factors favouring freedom from HF in prospective translational studies.
Methods and results
Nova Scotia residents (1995–2012) undergoing CABG within 3 weeks of ACS were included. The primary outcome was freedom from readmission to hospital due to HF. Descriptive statistics were generated, and a Cox proportional hazards model assessed outcome with adjustment for clinical characteristics. Of 11 936 Nova Scotians who underwent isolated CABG, 3264 (27%) had a recent ACS and were included. Deaths occurred in 210 (6%) of subjects prior to discharge. A total of 3054 patients were included in the long-term analysis. During follow-up, HF necessitating readmission occurred in 688 (21%) subjects with a hazard ratio of 12% at 2 years. The adjusted Cox model demonstrated significantly better freedom from HF for younger, male subjects without metabolic syndrome and no history of chronic obstructive pulmonary disease, renal insufficiency, atrial fibrillation, or HF.
Conclusions
Our findings have outlined important clinical variables that predict freedom from HF. Furthermore, we have shown that 12% of patients undergoing CABG after ACS develop HF (2 years). Our findings support our next phase in which we plan to prospectively collect blood and tissue specimens from ACS patients undergoing CABG in order to determine novel biological mechanism(s) that favour resolution of post-ACS inflammation.
Introduction
As developing countries industrialize and people live longer, healthcare systems have observed a growing burden from heart failure (HF).1 Furthermore, HF is not a single entity—rather, this clinical syndrome is characterized by a spectrum of symptoms contingent on diverse patient characteristics (e.g. age, sex, and ethnic origin) and medical history (e.g. hypertension and ischaemic heart disease). The syndrome can be further dichotomized by its dysfunction into diastolic HF with preserved ejection fraction and systolic HF with reduced ejection fraction.1 In addition, HF is associated with increased (i) rates of hospital readmission, (ii) prolonged length of stay, and (iii) significant mortality reaching 50% within 3–5 years of diagnosis.2 Still, despite our current level of understanding of the pathophysiology of HF, we are left questioning why seemingly similar patients may have drastically different prognoses following intervention.
Given the prevalence of ischaemic heart disease, previous studies have largely examined prognostic risk factors for the development of HF subsequent to acute coronary syndrome (ACS).3, 4 To that effect, surgical revascularization in the form of coronary artery bypass grafting (CABG) surgery remains an important treatment modality for patients suffering from ACS.5 However, despite successful revascularization, not all patients remain free from cardiac remodelling (e.g. myocardial fibrotic deposition, cardiomyocyte loss, and cardiomyocyte hypertrophy) and HF symptoms. It is estimated that 10% of all patients require readmission within 30 days of discharge after CABG; the most common reason for this is HF.6, 7 Furthermore, when looking beyond 30 days post-operatively, HF persists in some patients despite optimal medical therapy.2
Studying patients who remain free from HF offers the opportunity to understand why some recover better than others following intervention for ACS. As such, our study adopts a novel approach by seeking to clarify the clinical variables that best characterize patients who remain free from HF after ACS treated with surgical coronary revascularization. Ultimately, and perhaps most importantly, the finding from this study will inform the prospective perioperative collection of blood and tissue samples from ACS patients to examine novel biological factors that may underlie the balance between recovery and the development of HF.
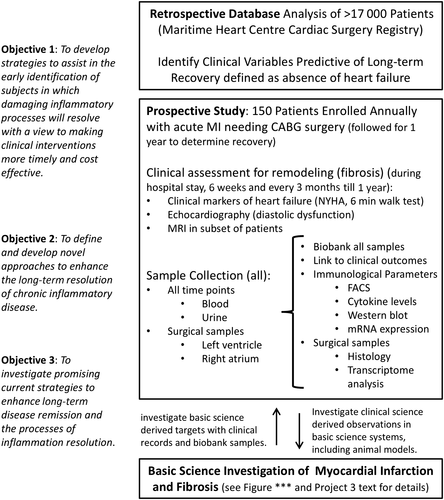
The present investigation represents Phase I of the clinical heart study component in a research programme called Restitution Enhancement in Arthritis and Chronic Heart disease (REACH), in which the objective was to identify clinical variables that favour resolution post-myocardial injury; subsequently, the intent of Phase II (prospective phase) will be to quantify the underlying mechanisms that perpetuate injury or impede resolution focusing on inflammation through analysis of biological specimens. In turn, we aim to eventually identify novel therapeutic targets and strategies for promoting resolution of cardiac injury following ACS.
Methods
Study population
Phase I retrospective study: subjects included all consecutive Nova Scotia residents who underwent an isolated CABG between 1995 and 2012 within 3 weeks of presenting with ACS at the Queen Elizabeth II Health Sciences Center in Halifax, Canada. All patients were identified using the Maritime Heart Center Cardiac Surgery Registry. The Queen Elizabeth II Health Sciences Center is the only cardiac surgical centre for the province of Nova Scotia, serving a population of nearly 1 million. The MHC registry is a detailed prospectively collected clinical database containing pre-operative, intra-operative, and post-operative data on all cardiac surgery cases performed from March 1995 to present at the QEII HSC.
Study objectives
Subjects were followed for 2 years post-operatively for the primary outcome of freedom from readmission due to HF. The secondary objective was to develop a risk predictive model for freedom from developing HF requiring admission after CABG surgery.
Study design
Categorical variables were reported as frequencies and percentages and were analysed by the χ2 or Fisher's exact test as appropriate. Design variables were created for reference level coding of categorical variables with more than two levels. All consecutive patients that underwent CABG within 3 weeks of an ACS were included. Patients undergoing concomitant procedures were excluded. ACS was defined as any presentation with acute myocardial infarction (ST elevation myocardial infarction and non-ST elevation myocardial infarction) and instable angina using standard Society of Thoracic Surgery definitions.8
All data analyses were performed by an experienced Maritime Heart Centre analyst. The primary outcome was readmission to hospital for HF within the first 2 years after the indexed admission. These data were obtained by linking the MHC database with the Canadian Institute for Health Information Discharge Abstract Database through Heath Data Nova Scotia at Dalhousie University using encrypted identifiers, as previously described.9-11 Standard ICD 9 and 10 coding was used to define admission due to HF. Descriptive statistics included discrete variables and χ2 test. Statistical significance was defined as P value less than 0.05. The clinical characteristics of patients who were readmitted for HF were examined univariately.
Kaplan–Meier survival curves were generated to display unadjusted results over time (follow-up time). A Cox proportional hazards model was generated to adjust for differences in clinical presentation between patients who were readmitted to hospital for HF and who continued to be free of HF. A fully adjusted model was then generated, and independent predictors were identified to predict the likelihood of a patient being readmitted to hospital for HF. When any variable did not meet assumptions of Cox proportional hazards, interaction of those variables and log time was introduced in the model. The SAS software package version 8.2 (SAS, Cary, NC, USA) was used to complete all the statistical analyses.
Ethics
This study was conducted with the full approval of the institutional (Nova Scotia Health Authority) Research Ethics Board. The requirement to obtain informed consent was waived under Section 2.1c of the Tri-Council Policy Statement. All personal identifiers were stripped prior to data analysis to ensure patient anonymity and confidentiality.
Results
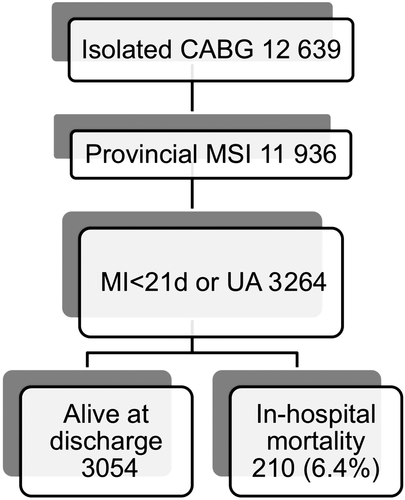
During the chosen study period, a total of 11 936 Nova Scotians underwent isolated CABG surgery. From this group 3264 (27%) patients suffered from an ACS prior to surgery (<21 days) and were included in the study (Figure 1). The characteristics of all ACS patients undergoing CABG surgery are outlined in Table 1. A significant proportion of patients were older than 70 years of age (42.7%), female gender (27.3%), had triple vessel coronary artery disease (84.9%), and required urgent or emergent surgery defined as within 24 h of presentation (43.4%). From this group of patient suffering an acute ACS, a total of 210 died prior to discharge, resulting in a 6.4% unadjusted overall in-hospital mortality.
| n = 3264 (%) | ||
|---|---|---|
| Age (years) | <60 | 902 (27.6) |
| 60–69 | 968 (29.7) | |
| 70–79 | 1048 (32.1) | |
| ≥80 | 346 (10.6) | |
| Female | 890 (27.3) | |
| Metabolic syndrome | 184 (5.6) | |
| Preop Afib | 339 (10.4) | |
| Triple vessel disease/LM > 50 | 2772 (84.9) | |
| Incomplete revascularization | 665 (20.3) | |
| Total arterial revascularization | 383 (11.7) | |
| Renal insufficiency (Cr > 176) | 259 (7.9) | |
| COPD | 493 (15.1) | |
| EF | >60 | 1404 (43.1) |
| 40–60 | 1181 (36.3) | |
| <40 | 672 (20.6) | |
| Status | Elective | 1846 (56.6) |
| Urg/Emer | 1418 (43.4) | |
| CHF | 769 (23.6) | |
| NYHA class | I | 593 (18.2) |
| II–III | 831 (25.5) | |
| IV | 1840 (56.4) |
- CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Cr, serum creatinine; EF, ejection fraction; LM, left main; NYHA, New York Heart Association; Preop Afib, pre-operative atrial fibrillation.
Only patients who survived to discharge were included in the final analysis, which focused on freedom from readmission to hospital after discharge. Follow-up was obtained in all 3054 patients discharged after CABG using administrative data, and a diagnosis of HF was defined using standard ICD9-10 coding and administrative data-linkage. During post-discharge follow-up period (up to 17.5 years), 21% (n = 688) of patients required readmission to hospital for HF. Two year freedom from readmission was 88.2%, or alternatively, the hazard for HF was 11.8% at 2 years (Figure 2). During the same period of time, 234 patients died (Figure 3). Together, the Kaplan–Meier freedom from death and HF at 2 years was 82.7% (Figure 4).
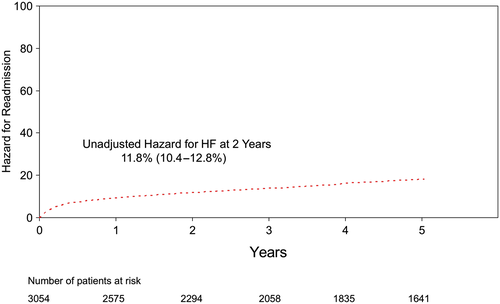
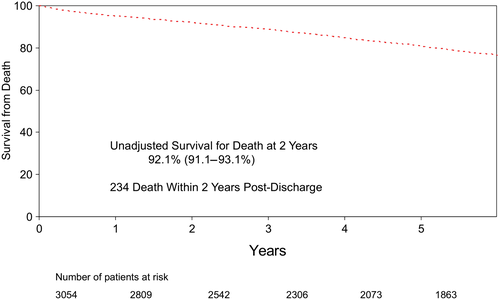
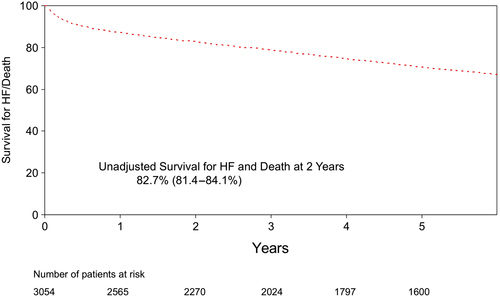
The unadjusted clinical characteristics of patients readmitted to hospital (n = 688) were compared with the remaining patients (n = 2366) (Table 2). Patients who required readmission to hospital for HF were more likely to be older, female, with co-morbidities, lower EF, higher New York Heart Association functional classification, and required urgent surgery at the time of the index admission. The Cox proportional model was used to adjust for clinical differences between the two groups of patients. The model was then used to identify independent predictors of freedom from HF, thus consistent with the goal of the REACH programme: focus on the clinical characteristics of patients remains free of HF. The independent variables that predicted patients who remain free from readmission for HF after CABG are listed in Table 3 with their respective hazard ratio as well as confidence interval. The medical management of the patients included in the study was left to the discretion of treating physician. Furthermore, we have no way to include the exact medical treatment received after discharge based on the data linkage used for the present study that explains why this was not included in the multivariable model. However, we have included the medical management prescribed to these patients at the time of discharge from their index admission (Table 4). Notably, >85% of patients were taking a lipid lowering agent, a Beta blocker, and/or acetyl-salicylic acid.
| Readmission for heart failure | ||||
|---|---|---|---|---|
| Yes = 688 (%) | No = 2366 (%) | P value | ||
| Age (years) | <60 | 124 (18.0) | 759 (32.1) | <0.0001 |
| 60–69 | 207 (30.1) | 714 (30.2) | ||
| 70–79 | 267 (38.8) | 686 (29.0) | ||
| ≥80 | 90 (13.1) | 207 (8.8) | ||
| Female | 236 (34.3) | 583 (24.6) | <0.0001 | |
| Metabolic syndrome | 62 (9.0) | 116 (4.9) | <0.0001 | |
| Renal insufficiency (Cr > 176) | 96 (14.0) | 120 (5.1) | <0.0001 | |
| COPD | 141 (20.5) | 305 (12.9) | <0.0001 | |
| Preop Afib | 101 (14.7) | 199 (8.4) | <0.0001 | |
| Triple vessel disease/LM > 50 | 615 (89.4) | 1963 (83.0) | <0.0001 | |
| Incomplete revascularization | 169 (24.6) | 432 (18.3) | 0.0021 | |
| Total arterial revascularization | 61 (9.0) | 301 (12.7) | 0.006 | |
| EF | >60 | 219 (31.8) | 1135 (48.1) | <0.0001 |
| 40–60 | 257 (37.4) | 858 (36.4) | ||
| <40 | 212 (30.8) | 366 (15.5) | ||
| Status | Elective | 312 (45.4) | 1465 (61.9) | <0.0001 |
| Urg/Emer | 376 (54.7) | 901 (38.1) | ||
| CHF | 278 (40.4) | 385 (16.3) | <0.0001 | |
| NYHA class | I | 49 (7.1) | 526 (22.2) | <0.0001 |
| II–III | 115 (16.7) | 676 (28.6) | ||
| IV | 524 (76.2) | 1164 (49.2) | ||
- CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; Cr, serum creatinine; EF, ejection fraction; LM, left main; NYHA, New York Heart Association; Preop Afib, pre-operative atrial fibrillation.
| Hazard ratio | Confidence interval | ||
|---|---|---|---|
| Age (years) | <60 | 0.62 | 0.49–0.78 |
| 60–69 | 1.0 | ||
| 70–79 | 1.33 | 1.10–1.60 | |
| ≥80 | 1.42 | 1.09–1.83 | |
| Male | 0.75 | 0.64–0.89 | |
| No metabolic syndrome | 0.56 | 0.43–0.74 | |
| Normal renal function | 0.47 | 0.37–0.59 | |
| No COPD | 0.79 | 0.66–0.96 | |
| Ejection fraction | <40 | 1.36 | 1.12–1.66 |
| 40–60 | 1.0 | ||
| >60 | 0.71 | 0.59–0.86 | |
| No history of CHF | 0.57 | 0.48–0.69 | |
| NYHA | I | 0.77 | 0.55–1.08 |
| II or III | 1.0 | ||
| IV | 1.39 | 1.12–1.73 | |
| No atrial fibrillation | 0.71 | 0.57–0.88 | |
| Not urgent status | 0.81 | 0.69–0.95 | |
| No triple vessel disease/LM > 50 | 0.78 | 0.61–1.0 | |
| Total arterial grafting | 0.90 | 0.69–1.19 | |
| Complete revascularization | 0.87 | 0.73–1.04 | |
| Agea | <60 | 1.01 | 0.89–1.14 |
| 60–69 | 1.0 | ||
| 70–79 | 1.12 | 1.02–1.25 | |
| ≥80 | 1.20 | 1.04–1.38 | |
| No metabolic syndromea | 0.84 | 0.72–0.98 |
- CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LM, left main; NYHA, New York Heart Association.
- a Time varying covariates.
| Percent of patients | |
|---|---|
| Lipid lowering agent | 85.6 |
| Beta blocker | 86.4 |
| ACE inhibitor | 30.2 |
| Angiotensin receptor B. | 3.3 |
| Calcium channel B. | 12.9 |
| ASA | 89.4 |
| Plavix | 17.0 |
| Warfarin | 7.3 |
- ACE, angiotensin-converting enzyme; ASA, acetyl-salicylic acid.
The clinical characteristics that significantly and independently predicted HF were older age, female sex, metabolic syndrome (defined as BMI > 35, hypertension, and diabetes mellitus), pre-operative atrial fibrillation, renal insufficiency (Cr > 176), chronic obstructive pulmonary disease, low ejection fraction (EF < 40), urgent/emergent surgery, congestive HF, and advanced New York Heart Association functional classification.
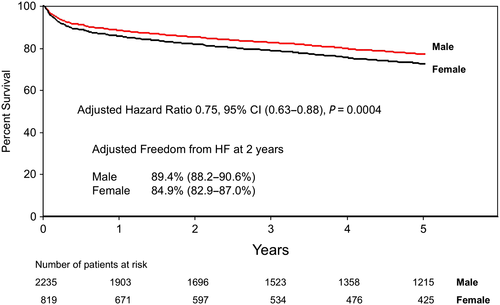
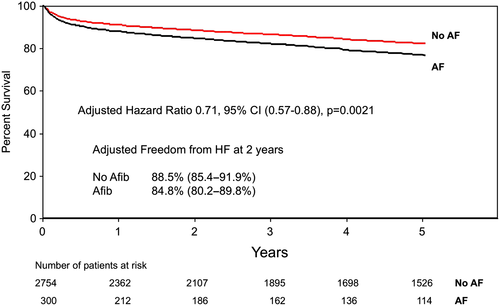
Together, our findings are not surprising in suggesting that healthier patients with fewer co-morbidities are the least likely to develop HF after CABG surgery. However, our findings do identify important variables such as sex and atrial fibrillation, which independently predicted HF by mechanisms that can be explored in a prospective study in which blood and tissue (atrium) will be analysed in greater detail (REACH prospective Phase I; Figure 5). Kaplan–Meier curves adjusted for freedom from HF admissions based on sex (Figure 6) and pre-operative atrial fibrillation (Figure 7) illustrate how these variables affect outcomes over time.
Conclusions
In this paper, we have presented the findings from Phase I of the clinical heart study component of our REACH research programme. This study focused on patients in whom ACS appeared to be followed by clinical resolution of myocardial injury without the development of clinically relevant myocardial remodelling and HF. As such, we aimed to identify key clinical features from these patients, their treatments, and their clinical courses that were associated with successful resolution of injury based on their freedom from HF. Our approach is novel, as most investigators have traditionally focused on variables that predict more severe disease rather than resolution (freedom from HF). Reported variables suggested to be associated with a significant risk for the development of HF in this population have been described but have to date not included biological factors such as inflammation.12 In contrast, factors that have been suggested to reduce the risk of HF have included the use medications such as β-blockers and angiotensin-converting enzyme inhibitors, with little attention to patient characteristics emphasizing that research in these areas is lacking.12, 13
In our study—and not surprisingly—we demonstrated that subjects with a preferred recovery trajectories free of 2 year HF following post-ACS CABG were younger males without metabolic syndrome and no history of chronic obstructive pulmonary disease, renal insufficiency, atrial fibrillation, or chronic HF. We were able to demonstrate that within 2 years of CABG, 12% of all patients required readmission to hospital after developing HF. We believe that 12% likely represents an underestimate of the prevalence of HF given that the threshold here was HF requiring readmission to hospital. This information supports the next phase of our study in which we plan to prospectively follow patients clinically for the development of HF with regular follow-up (3 months, 6 months, and 1 year) and to prospectively collect blood and tissue specimens starting at the time of CABG. We understand that knowledge of the relevant clinical variables associated with recovery will not allow us to predict the trajectory of outcomes of individual patients being enrolled. Population-based probabilities are difficult to extrapolate to individual patients. However, the findings from this study do inform us about important clinical variables that we need to consider and adjust for in our future analysis of the prospective material.
For example, our findings highlight the importance of gender and atrial fibrillation. Through our research programme (REACH), we will have direct access to right atrial tissue from patients undergoing heart surgery allowing direct assessment of the burden of inflammation in circulation and at the cardiac tissue level. Relevant to the present study is the fact that atrial fibrillation has been shown to be associated with significant inflammation (e.g. macrophage infiltration and pro-inflammatory cytokine up-regulation) and atrial fibrosis.14, 15 Cardiac macrophages are increasingly recognized as key regulators of inflammation, tissue injury, and fibrosis in small animal models. We believe that the presence of atrial muscle fibrosis will provide a unique opportunity to characterize myocardial macrophages within human tissue and determine the phenotype associated with fibrosis (Figure 5). Specifically, in phase II of this study, we will be extracting macrophages and other leucocytes from human atria samples through tissue homogenization techniques and processing the single-cell suspension for multi-panel flow cytometry. In doing so, we will be able to characterize differences in the myocardial immune component that may contribute to resolution over the development of HF. Based on prior published work from our group, we predict that ≈10% of patients undergoing heart surgery will have atrial fibrillation pre-operatively and ≈35% post-operatively, which is further supported by the findings from this study.16 The size of the atrial piece to be collected is several-fold larger than the whole heart of mice, suggesting that sufficient tissue will be available to allow for the analysis of inflammatory and repair mediators that may facilitate freedom from HF. In support of our REACH programme, we have recently published prospective data (Phase II) looking at specific markers of macrophages (CD16) and ratios of neutrophils and lymphocytes suggesting some mechanistic links to the development of adverse events like HF.17 While these findings are early, they clearly support our translational approach to link clinical data to biological mechanisms prospectively.
Our original hypothesis (REACH programme) states that closely examining ACS patients who appear to successfully resolve damaging inflammation will allow us to identify novel biological mechanisms involved in the regulation of the myocardial inflammatory response. We acknowledge that the underlying factors responsible for persistence of HF symptoms are likely complex and multifactorial but likely involve structural changes in the myocardium referred to as cardiac remodelling. Cardiac remodelling refers to the manifestation of a complex series of pathophysiological changes in the heart tissue that can occur in the myocardium following injury.18 We acknowledge that our proposed atrial tissue sampling may not reflect fully cardiac ventricular remodelling that ultimately will be responsible for the development of clinical HF. At present, a safe method to sample sufficient ventricular myocardium is not possible. We have addressed this limitation by including in our prospective phase echocardiographic assessment for diastolic function and magnetic resonance imaging for remodelling (ref for MRI). As such, we believe that our REACH programme will allow us to characterize those biological mechanisms that would favour healing without the development of cardiac remodelling.
In this initial phase, we have created a robust predictive model designed to identify major characteristics of patients that do not develop progressive HF and as such, cardiac remodelling after myocardial infarction. Our approach has now set the stage for incorporating biological variables as part of predictive models in order to improve their predictive power. Currently, most predictive models at a population level can predict a desired outcome >70% of the time based on receiver operator curve >0.7. This also means that models entirely based on clinical variables lack yet to be identified components that could improve the receiver operator curve towards 100%. Unfortunately, one important limitation of the data link to administrative data is that we are unable to obtain patient level information such as medication for a particular patient at a particular time to comply with Privacy Law (PHIA Regulation). Thus far, work from this study has defined cardiac patient subsets and created the infrastructure by which we aim to identify relevant biological processes of injury resolution that, in combination with clinical data, will significantly improve the usefulness of our clinical predictive tools.
The novelty of our approach is the focus on patients who are seemingly protected from developing HF post-ACS with intervention, in contrast to the work performed to date, which focused on risk factors predicting development of HF. In this study, we have identified patient characteristics that seemingly protect patients from developing HF post-ACS with intervention, but much remains unknown regarding the underlying mechanisms responsible for these clinical benefits. We believe that our findings in the subsequent, prospective phase of REACH will provide valuable information on the pathophysiology of human HF at the cellular and cytokine level (Figure 5). Importantly, we will be one of the first groups characterizing these processes systematically in human heart tissue, which has the potential to contribute to clinically relevant biomarkers for HF.
Acknowledgements
The Canadian Institute of Health Research (CIHR-THC135230) funded the present work. All authors have read the journal's authorship agreement and policy on disclosure of potential conflicts of interest.
Conflict of interest
None declared.




